To the Editor,
In some recently published papers researchers reported “coinfection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and Influenza virus.” 1 , 2 , 3 They described the development of clinical symptoms of pneumoniae in patients and discussed about the importance of other respiratory viruses that cause symptoms similar to coronavirus disease 2019 (COVID‐19).
We evaluated 600 samples of suspected patients to acute respiratory syndrome due to COVID‐19 in the local screening and therapeutic program. We also assessed these samples for detection of some important respiratory viruses such as influenza virus, para influenza virus, bocavirus, metapneumovirus, respiratory syncytial virus, and adenovirus. In agreement with Wu et al 1 we found two cases of coinfection of COVID‐19 with Influenza A.
The first case was 78 years old woman that hospitalized on 9 April 2020 under ventilation with the symptoms of respiratory distress syndrome, dyspnea, fever 38.8°C, confusion, headache, joints pain, and history of chronic lung disease and died on the 12 April 2020. Laboratory findings showed total white blood cells of 16.4 × 103, lymphopenia (lymphocyte 5%, neutrophil 86%, monocyte 9%), Estimated sedimentation rate of 28 mm/h and CRP 3+. Other hematological and biochemical factors were normal except sodium (119 mmol/L) and potassium (3.4 mmol/L). Apart from that chest CT scan showed Bilateral multi focal ground glass opacities with peripheral distribution and mild interalobular septal thickening (Figure 1).
Figure 1.
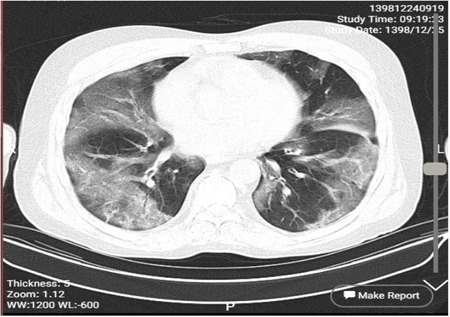
The chest CT scan of case 1. CT, computed tomography
The nasopharyngeal and throat swab was positive for COVID‐19 (LightMix Modular SARS‐CoV, TIB molbiol, Berlin, Germany). She was treated with combination hydroxychloroquine and kaletra (lopinavir/ritonavir) under oxygen therapy but finally she died at the intensive care unit after 3 days. Evaluation of sample revealed that it was positive for Influenza A virus type H1N1 based on Wu et al 4 and Poon et al 5 method. Her tests were negative for other respiratory viruses.
The second case was 75 years old man hospitalized on 15 March 2020 with fever 37.9°C, severe cough, dyspnea, joint pain and gastrointestinal symptoms include nausea and diarrhea, and died on the 22 March 2020. His signs begin from 6 days before hospitalization. Laboratory findings showed total white blood cells of 3.5 × 103, lymphopenia (lymphocyte 15%, neutrophil 80%, mixed 5%), estimated sedimentation rate of 30 mm/h and CRP 4+. Other hematological and biochemical factors were normal except platelet count (125 × 103/µL). The chest computed tomography (CT) scan demonstrated centri acinar ground glass opacity with peripheral distribution and intera lobular septal thickening (Figure 2).
Figure 2.
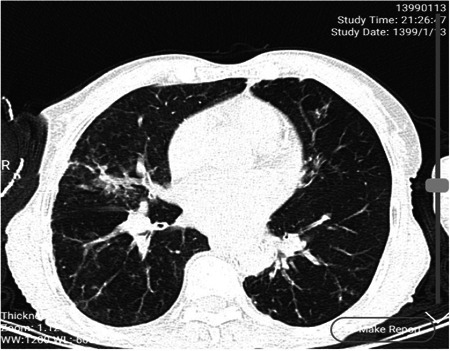
The chest CT scan of case 2. CT, computed tomography
Like case 1, this patient treated with combination hydroxychloroquine and kaletra (lopinavir/ritonavir) under oxygen therapy after obtaining positive result of reverse transcription polymerase chain reaction for COVID‐19. Despite the drug and oxygen therapy, he died after 1 week at the intensive care unit. Evaluation of sample revealed that it was also positive for Influenza A virus type H1N1. The test for other respiratory viruses was negative. As said by Wu et al Influenza A and other respiratory viruses may have symptoms like COVID‐19 and lead to complication of identification and treatment of respiratory syndrome caused by this virus.
In summary, our findings suggest that we should consider other respiratory viruses in COVID‐19 suspected patients because it seems that the coinfection with other viruses can lead to the complication of patients' condition and even their deaths.
AUTHOR CONTRIBUTIONS
SAH was involved in primary hypothesis and data collection. SS assisted in manuscript writing and english edition. MG and MRT collected data. MSMZH collected CT scan data. HNAA and HGZM were helped in laboratory data collection. AA primary was involved in hypothesis and perform reverse transcription real‐time polymerase chain reaction tests.
ACKNOWLEDGMENTS
This study is the part of national COVID‐19 screening program of Iran. The primer and probes were supported by Ministry of Health of Iran and other laboratory kits provided by North Khorasan University of Medical Sciences.
REFERENCES
- 1. Wu X, Cai Y, Huang X, et al. Co‐infection with SARS‐CoV‐2 and influenza A virus in patient with pneumonia, China. Emerging Infect Dis. 2020;26:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khodamoradi Z, Moghadami M, Lotfi M. Co‐infection of coronavirus disease 2019 and Influenza: a report from Iran. Arch Iran Med. 2020;23(4):239‐243. [DOI] [PubMed] [Google Scholar]
- 3. Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients co‐infected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu L‐T, Thomas I, Curran MD, et al. Duplex molecular assay intended for point‐of‐care diagnosis of influenza A/B virus infection. J Clin Microbiol. 2013;51(9):3031‐3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poon LLM, Chan KH, Smith GJ, et al. Molecular detection of a novel human influenza (H1N1) of pandemic potential by conventional and real‐time quantitative RT‐PCR assays. Clin Chem. 2009;55(8):1555‐1558. [DOI] [PMC free article] [PubMed] [Google Scholar]


