Abstract
Background
The COVID‐19 pandemic poses a critical global public health crisis. Operating room (OR) best practice in this crisis is poorly defined. This systematic review was performed to identify contemporary evidence relating to OR practice in the context of COVID‐19.
Methods
MEDLINE was searched systematically using PubMed (search date 19 March 2020) for relevant studies in accordance with PRISMA guidelines. Documented practices and guidance were assessed to determine Oxford Centre for Evidence‐Based Medicine (OCEBM) levels of evidence, and recommendations for practice within five domains were extracted: physical OR, personnel, patient, procedure, and other factors.
Results
Thirty‐five articles were identified, of which 11 met eligibility criteria. Nine articles constituted expert opinion and two were retrospective studies. All articles originated from the Far East (China, 9; Singapore, 2); eight of the articles concerned general surgery. Common themes were identified within each domain, but all recommendations were based on low levels of evidence (median OCEBM level 5 (range 4–5)). The highest number of overlapping recommendations related to physical OR (8 articles) and procedural factors (13). Although few recommendations related to personnel factors, consensus was high in this domain, with all studies mandating the use of personal protective equipment.
Conclusion
There was little evidence to inform this systematic review, but there was consensus regarding many aspects of OR practice. Within the context of a rapidly evolving pandemic, timely amalgamation of global practice and experiences is needed to inform best practice.
This rapid systematic review was performed to identify contemporary evidence relating to operating room (OR) practice during the COVID‐19 pandemic. Evidence underpinning the review was sparse; however, consensus regarding many aspects of OR practice was apparent.
High quality evidence needed
Antecedentes
La pandemia por COVID‐19 plantea una crisis crítica de salud pública a nivel mundial. La mejor práctica en el quirófano en esta crisis está mal definida. Esta revisión sistemática se realizó para identificar la evidencia contemporánea relacionada con la práctica en el quirófano en el contexto del COVID‐19.
Métodos
Se realizó una búsqueda sistemática en Medline usando PubMed (fecha de búsqueda, 19 de marzo de 2020) para seleccionar estudios relevantes de acuerdo con las directrices PRISMA. Las prácticas documentadas y las guías se evaluaron para determinar los niveles de evidencia según la normativa Oxford (Oxford levels of evidence, OLE), y se extrajeron las recomendaciones para la práctica en el entorno de cinco ámbitos: físico en el quirófano, personal, paciente, procedimiento y otros factores.
Resultados
Se identificaron 35 artículos, de los cuales 11 cumplían con los criterios de elegibilidad. Nueve artículos constituían opinión de expertos y dos fueron estudios retrospectivos. Todos los artículos provenían del Lejano Oriente (nueve de China, dos de Singapur); ocho de los artículos estaban relacionados con cirugía general. Se identificaron temas comunes en cada ámbito, pero todas las recomendaciones se basaron en bajos niveles de evidencia, mediana de 5 OLE (rango 4 ‐ 5). El mayor número de recomendaciones superpuestas se relacionaban con el ámbito físico del quirófano (n = 8) y con factores del procedimiento (n = 13). Aunque hay pocas recomendaciones relacionadas con factores del personal, el consenso fue alto en este ámbito, y todos los estudios exigieron el uso de equipos de protección personal.
Conclusión
Hubo poca evidencia para que esta revisión sistemática proporcionara información, pero existió consenso con respecto a muchos aspectos de la práctica en el quirófano. En el contexto de una pandemia en rápida evolución, es preciso aglutinar a tiempo real las estrategias y experiencias globales a fin de poder informar acerca de las mejores prácticas.
Introduction
Coronavirus has killed thousands since emerging in China in December 2019, and compelled many governments to lock down populations 1 .
Preliminary data from China and Italy regarding the spectrum of severity and fatality vary. China reported that 80 per cent of those infected reported no or mild, 15 per cent severe, and 5 per cent critical symptoms, with a mortality rate ranging from 0·25 to 3·0 per cent 2 , indicating demand for intensive medical intervention for one in five patients. Case fatality is much greater in the vulnerable: patients aged 80 years or more (mortality rate above 14 per cent) and those with coexisting conditions, such as cardiovascular disease (10 per cent) and diabetes (7 per cent) 3 .
From an acute surgical perspective, although some patients may present with an acute abdomen secondary to the viral infection, it is more likely that general surgeons will encounter patients with common acute abdominal pathology and undiagnosed COVID‐19 infection, or those who develop nosocomial COVID‐19 infection while an inpatient with a surgical diagnosis. Guidance on perioperative care of surgical patients with suspected or proven coronavirus infection is slender, and operating room (OR) best practice remains unknown.
The aim of this study was to perform a systematic review of the literature to identify and collate global experience, practice and recommendations relating to OR practice in the context of the COVID‐19 pandemic.
Methods
A rapid systematic review of published work was conducted using standard rapid review methodology, as outlined by Schünemann and Moja 4 and in accordance with the PRISMA guidelines 5 .
MEDLINE was searched via PubMed on 19 March 2020 (no date restriction), for articles describing specific practices, or providing recommendations or guidance relating to OR practice, in the context of the COVID‐19 pandemic. No limitation was placed on language or publication type, but non‐English‐language articles without extractable data were excluded. Relevant articles were identified using terms in any field relating to coronavirus (for example, coronavirus, COVID‐19, SARS‐CoV‐2) and the OR (such as operating room, theatre, surgery), and operating room practice (such as preparation, procedures, guidance, advice, practice, recommendations). The full search algorithm is shown in Appendix S1 (supporting information). Further articles were identified by hand search of references and using the PubMed related articles function. Levels of evidence were determined as described by the Oxford Centre for Evidence‐Based Medicine (OCEBM) 6 .
Data extraction
Nine authors extracted data independently, and two verified a random subsample of seven articles. The following details were extracted: first author, date of publication, study design, country, region, and any description of specific practices, recommendations or guidance in relation to OR practice in the context of the COVID‐19 pandemic. Five domains for data capture were identified a priori: the physical OR factors, personnel factors, patient factors, procedure factors, and other considerations.
Inclusion and exclusion criteria
Articles reporting specific practices, experience, recommendations or guidance in relation to emergency OR practice, in the context of the COVID‐19 pandemic, were included.
Articles were excluded if they did not meet the inclusion criteria, if the full‐text article was unavailable, or if no English version was extractable.
Results
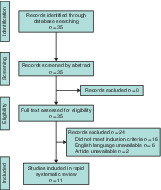
The MEDLINE search yielded a total of 35 articles. Full manuscripts were obtained for all of these, of which 11 articles 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 met the eligibility criteria for inclusion. Nine 7 , 9 , 10 , 11 , 12 , 13 , 15 , 16 , 17 of these 11 articles constituted expert opinion in the form of reviews or journal correspondence, and two 8 , 14 were observational studies, median OCEBM level 5 (range 4–5). All originated in the Far East (9 from China, 2 from Singapore), with the majority related to general surgery (8 articles). The inclusion pathway is illustrated in the PRISMA diagram (Fig. 1 ).
Figure 1.
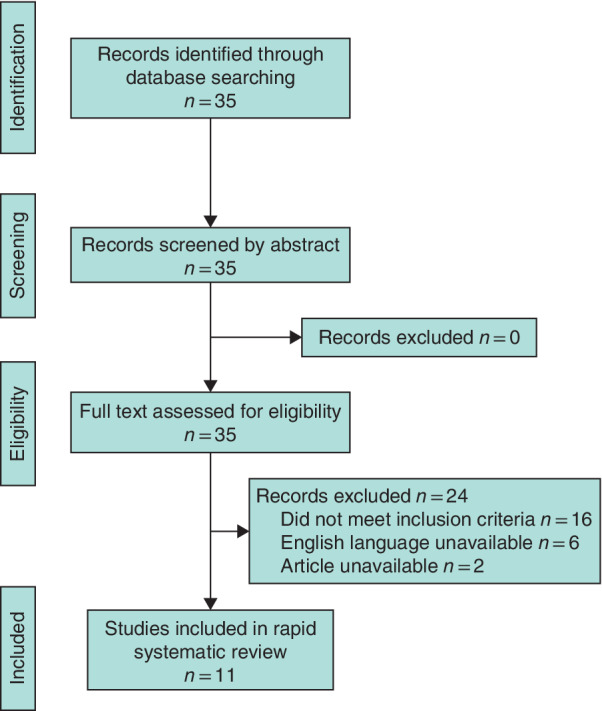
PRISMA diagram for the review
Table 1 gives an overview of the evidence grading, and Table 2 outlines which of the five specified domains were covered in each paper.
Table 1.
Characteristics of included studies
| Reference | Country | Design | OCEBM level of evidence | Subspecialty | Cohort size |
|---|---|---|---|---|---|
| Tao et al. 7 | China | Expert opinion/experience | 5 | General surgery | – |
| Gou et al. 8 | China | Case report/series (retrospective) | 4 | HPB | 4 |
| Wong et al. 9 | Singapore | Expert opinion/experience | 5 | – | – |
| Ti et al. 10 | Singapore | Expert opinion/experience | 5 | – | – |
| Wu et al. 11 | China | Expert opinion/experience | 5 | HPB | – |
| Li et al. 12 | China | Expert opinion/experience | 5 | Upper GI | – |
| Luo and Zhong 13 | China | Expert opinion/experience | 5 | Colorectal | – |
| Chen et al. 14 | China | Case report/series (retrospective) | 4 | Obstetrics | 17 |
| Li et al. 15 | China | Expert opinion/experience | 5 | HPB | – |
| Hu et al. 16 | China | Expert opinion/experience | 5 | Colorectal | – |
| Chen and Peng 17 | China | Expert opinion/experience | 5 | Upper GI | – |
OCEBM, Oxford Centre for Evidence‐Based Medicine; HPB, hepatopancreatobiliary; GI, gastrointestinal.
Table 2.
Domains covered in each study
Physical operating room factors
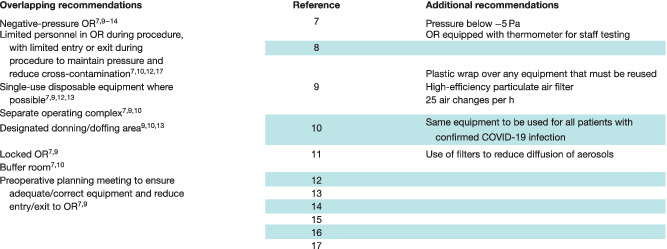
All 11 papers contained recommendations relating to the physical OR. Despite wide‐ranging specialty coverage, common themes for preparation of the OR were identified as shown in Table 3 .
Table 3.
Domain 1: recommendations relating to physical operating room factors
|
OR, operating room.
Both of the case series reported data from patients treated within a single centre. One study 14 involved 17 pregnant women who tested positive for COVID‐19 and had a caesarean section in Renmin University Hospital, Wuhan, China. Recommendations for the physical OR specified the use of a negative‐pressure OR, alongside strict separation of clean and contaminated areas, and a buffer system to ensure that clean rooms remain as such, avoiding cross‐contamination. The other study 8 , from Tongji Medical College in Wuhan, also acknowledged and discussed the theme of separation of patients with COVID‐19 from those without the disease.
The remaining nine papers made suggestions derived from local operative experience during the pandemic. Use of a negative‐pressure OR, along with a number of other practices to minimize intraoperative viral load, was recommended in six 7 , 9 , 10 , 11 , 12 , 13 of these articles (Table 3 ).
The use of single‐use/disposable equipment for COVID‐19‐positive patients was recommended in four communications 7 , 9 , 12 , 13 . Other units recommended use of the same anaesthetic equipment for COVID‐19‐positive patients only 10 , or covering equipment with disposable plastic wrapping when required for use in multiple patients 9 . In two 7 , 9 of these papers, the need for adequate preoperative planning was highlighted, to ensure availability of equipment that may be required, and to minimize store cupboard visits and consequent risks of cross‐contamination and disturbance of the pressure within the OR. One article 11 , from Beijing National Cancer Centre, referred to the use of suction or filters to diffuse aerosols, but without offering specific instruction.
Personnel factors
Of the 11 communications, eight 7 , 8 , 9 , 10 , 12 , 13 , 14 , 17 specified measures directly related to staff members involved in the operative care of patients (Table 4 ). One 14 reported that all staff involved in the operative care of COVID‐19‐positive pregnant patients undergoing caesarean delivery were swabbed after surgery to establish their own COVID‐19 status, and had thoracic CT every 2 weeks. The number of postoperative CT scans performed per member of staff and the level of preoperative screening undertaken was not reported. Appropriate personal protective equipment (PPE), including N95 or powered air‐purifying respirator (PAPR) masks, was used for all staff. Of the 38 staff involved in the care of these patients, none tested positive for the virus subsequently 14 .
Table 4.
Domain 2: recommendations relating to personnel factors
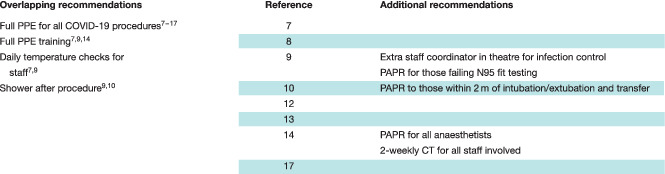
|
PPE, personal protective equipment; PAPR, powered air‐purifying respirator.
Indications for the use of PAPR masks varied (Table 4 ), although full PPE, and formal training in its use, was recommended in all articles 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 for patients with suspected or confirmed COVID‐19 infection. An extra staff coordinator in theatre, assigned to provide guidance in staff members' new roles and aid with unfamiliarity in new infection prevention procedures, was described at a tertiary centre in Singapore 9 .
Two articles 9 , 10 mandated staff showering after an operation, and two hospital units undertook temperature checks at least daily for all staff 7 , 9 .
Patient factors
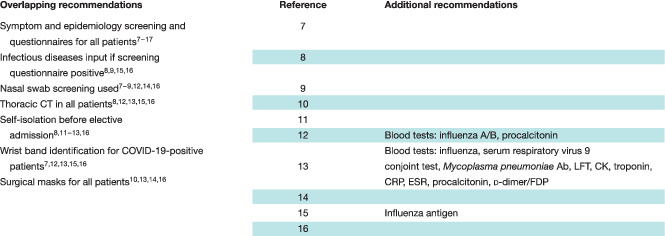
Ten articles 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 highlighted patient‐related considerations (Table 5 ). In patients for whom surgery was advocated, the importance of screening was a common theme; nine articles described practices ranging from questionnaires and swabs to thoracic CT 7 , 8 , 9 , 11 , 12 , 13 , 14 , 15 , 16 , and recommended self‐isolation before any elective admission 8 , 11 , 12 , 13 , 16 .
Table 5.
Domain 3: recommendations relating to patient factors
|
Ab, antibodies; LFT, liver function tests; CK, creatinine kinase; ESR, erythrocyte sedimentation rate; FDP, fibrin degradation products.
A host of routine blood tests were recommended by Li and colleagues 12 and Luo and Zhong 13 before hospital admission, which included procalcitonin, and both type A and type B influenza screening (Table 5 ).
Four articles 10 , 13 , 14 , 16 recommended surgical masks for all patients, especially on transfer, with special routes and elevators used for infected patients. The use of wrist bands, issued to all patients testing positive for COVID‐19, was described in five articles 7 , 12 , 13 , 15 , 16 as a method to promote ready identification.
Communication with patients and families was highlighted. Patients with cancer were described as needing special consideration, with four articles 7 , 11 , 12 , 13 recommending nutritional support, and two 11 , 12 additional psychological support.
Procedural factors
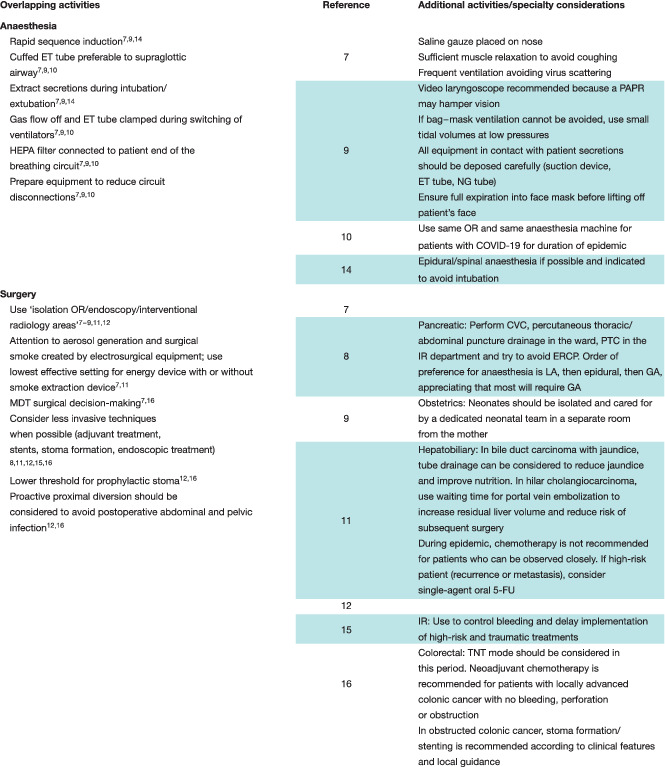
Nine articles 7 , 8 , 9 , 10 , 11 , 12 , 14 , 15 , 16 outlined OR procedural details. Of these, four 7 , 9 , 10 , 14 focused on technical adjustments to provide safer anaesthetic protocols. The remainder 8 , 9 , 11 , 12 , 15 , 16 reported modifications made to surgical planning or interventions.
Seven focused on individual subspecialty practice, including preoperative considerations. Although each of these concentrated on different surgical circumstances, overlapping themes were apparent, as described in Table 6 . A common theme was to avoid surgery where possible. In the emergency setting, temporizing measures such as stenting, endoscopic drainage or embolization were considered alternatives. Three articles 7 , 9 , 16 recommended that emergency patients testing negative for COVID‐19 could be treated as normal and undergo surgery if necessary. No study had been published focusing solely on the management of general surgical emergencies in the OR during the COVID‐19 crisis.
Table 6.
Domain 4: recommendations relating to anaesthetic and surgical procedures (procedural factors)
|
ET, endotracheal; PAPR, powered air‐purifying respirator; HEPA, high‐efficiency particulate air; NG, nasogastric; OR, operating room; CVC, central venous catheter; PTC, percutaneous transhepatic cholangiography; IR, interventional radiology; ERCP, endoscopic retrograde cholangiopancreatography; LA, local anaesthesia; GA, general anaesthesia; MDT, multidisciplinary team; 5‐FU, 5‐fluorouracil; TNT, total neoadjuvant therapy.
Other considerations
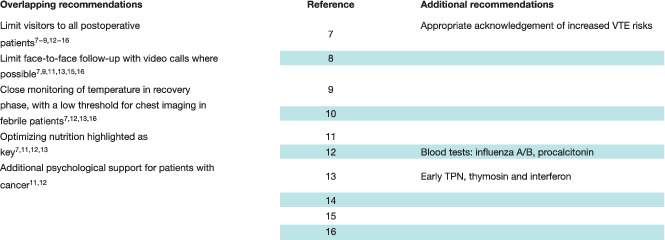
The main additional area considered was postoperative management. Most described the importance of altered postoperative practice, eight articles 7 , 8 , 9 , 12 , 13 , 14 , 15 , 16 recommending limiting visitors, with any visitors regularly checked for fever (Table 7 ). Six articles 7 , 9 , 11 , 13 , 15 , 16 recommended limiting in‐person contact with physicians, replacing this with video calls where possible.
Table 7.
Domain 5: recommendations relating to other considerations
|
VTE, venous thromboembolism; TPN, total parenteral nutrition.
Recognizing the risks of significant complications, four articles 7 , 12 , 13 , 16 describing practice in general surgery recommended that any postoperative pyrexia should be considered an indication for chest CT to look for possible COVID‐19 infection.
Nutrition was again highlighted in four articles 7 , 11 , 12 , 13 , one of which advised early parenteral nutrition, supplemented with thymosin and interferon 13 . A single communication 7 specifically raised the risk of venous thromboembolism in the postoperative period, given the increased risks associated with reduced mobility in patients with COVID‐19.
Discussion
This systematic review identified and evaluated evidence of OR best practice in the face of the COVID‐19 pandemic. The research question was deliberately kept broad, to optimize the volume of clinical practice and consequent literature reports available. At the time of the review (19 March 2020), COVID‐19 had been reported in 156 countries 18 .
Although the epidemiology of COVID‐19 has been well reported 19 , 20 , this systematic review found little robust evidence regarding safe and best surgical practice. Nine of the 11 papers provided OCEBM level 5 evidence, generating grade D recommendations consistent with very low quality and inconclusive evidence 6 . Consensus regarding perioperative practice was apparent, from institutions exposed to high COVID‐19 caseloads. Within the operating suite, common recommendations focused on room design, in particular geographic segregation of COVID‐19‐positive areas and the use of negative‐pressure ventilation. Such practices were reported previously 21 , 22 during the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) epidemics, in an attempt to minimize the risk of cross‐contamination from areas of negative to normal air pressure. Evidence regarding actions to minimize viral load was sparse, but such general measures were pragmatic and achievable in most, if not all, hospitals.
The importance of appropriate PPE, both before and during surgery, to protect staff and minimize viral transmission was highlighted in all articles, supported by evidence in reports from previous outbreaks of SARS and MERS 23 , 24 , 25 . Other studies have emphasized the importance of adequate training in the correct use of PPE, where poor protocol compliance has been shown to have a significant association with viral transmission 26 , 27 . In the present review, guidance regarding the use of PAPR and N95 masks was inconsistent, with only two10,14 of the 11 articles mandating the use of PAPR during high‐risk procedures such as intubation. The filtering ability of PAPR masks is reported to be marginally better than that of N95 masks (99 versus 95 per cent respectively filtration of particles smaller than 5 μm in diameter), but the clinical significance of this is unknown 28 .
The level of screening necessary for surgical patients differed between hospital units. All recommended health questionnaires or nasopharyngeal swabs as a minimum requirement, with additional thoracic CT recommended by almost half. Evidence‐based consensus regarding patient screening is therefore a priority, based on the balance of risk and sensitivity of individual screening test modalities.
The issue of a deliberate pause in elective surgical practice was a common theme, with the justification cited being to minimize further viral transmission, strategically to ration a stretched health resource threatened with an overwhelming burden, and as a response to the risk of unknown potential adverse clinical outcomes of patients developing COVID‐19‐related morbidity and complications after surgery. To mitigate potential surgical and respiratory morbidity, consideration of less invasive procedures such as interventional radiology or endoscopy was recommended, and in those requiring surgical resection the formation of proximal diversion or end stomas to avoid the risk of operative sepsis associated with anastomotic leakage 8 , 11 , 12 , 15 , 16 . Such recommendations seem reasonable and have subsequently been published in guidance documents produced by international professional associations 29 , 30 , 31 .
Minimizing intraoperative aerosol generation and the potential for dissemination of viral particles was addressed in a number of the papers included in this review. The use of rapid sequence induction with cuffed endotracheal tubes and limiting energized dissection, as reported, are recognized techniques in the literature; however, high‐quality evidence‐based practices are lacking 32 , 33 , 34 , 35 , 36 , 37 .
There are several limitations to this review. The number of publications available for review was modest. Rapid evaluation of evidence, which has been essential in informing early recommendations in an emerging pandemic, can result in false assumptions and conclusions. The results are subject to the risk of significant bias. The study relied on the inclusion of expert opinion and low‐quality observational studies, owing to the absence of high‐quality clinical trials. In exceptional circumstances, such as the current coronavirus pandemic, a conventional strong evidence‐based approach to healthcare policy development is neither feasible nor safe.
Within the constraints of time and resource, clinical practice must be driven by a pragmatic approach to developing evidence‐based practice, and arguably a novel scientific approach to collating global evidence when rigorous research evidence is not available. The widespread professional use of social networking platforms represents a powerful way of sharing such information internationally.
This review has highlighted the need for novel methodological approaches. There is a need to assimilate ‘real‐time’ preliminary experiences and evidence, in advance of publication, while recognizing the heightened potential for bias. An ability to transcend geographical and language barriers is essential to facilitate international knowledge‐sharing. The encouragement of wide participation across a range of disciplines, not limited to healthcare professionals, would help to capture the full spectrum of viewpoints and potential solutions. Finally, the ability to recruit a pool of engaged stakeholders, ready to assist, would permit iterative development and re‐evaluation as a situation unfolds.
Collaborators
Members of the WSRI Collaborative: T. Abdelrahman, J. Ansell, C. Brown, R. Egan, T. Evans, E. Ryan Harper, R. L. Harries, L. Hopkins, O. James, S. Lewis, W. G. Lewis, O. Luton, K. Mellor, A. G. Powell, D. Robinson, R. Thomas, A. Williams, A. J. Beamish.
Supporting information
Appendix S1: Supporting information
Acknowledgements
The authors acknowledge support from Cardiff University in the form of funding for open access publication.
Disclosure: The authors declare no conflict of interest.
Contributor Information
Welsh Surgical Research Initiative (WSRI) Collaborative:
T. Abdelrahman, J. Ansell, C. Brown, R. Egan, T. Evans, E. Ryan Harper, R. L. Harries, L. Hopkins, O. James, S. Lewis, W. G. Lewis, O. Luton, K. Mellor, A. G. Powell, D. Robinson, R. Thomas, A. Williams, and A. J. Beamish
References
- 1. WHO . Coronavirus Disease 2019 (COVID‐19) Situation Report – 81; 2020. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200410‐sitrep‐81‐covid‐19.pdf?sfvrsn=ca96eb84_2 [accessed 11 April 2020]. [Google Scholar]
- 2. Wilson N, Kvalsvig A, Barnard LT, Baker MG. Case‐fatality risk estimates for COVID‐19 calculated by using a lag time for fatality. Emerg Infect Dis 2020; 26: 10.3201/eid2606.200320 [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 10.1001/jama.2020.2648 [online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4. Schünemann HJ, Moja L. Reviews: Rapid! Rapid! Rapid! … and systematic. Syst Rev 2015; 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A et al The 2011 Oxford CEBM Evidence Levels of Evidence (Introductory Document). http://www.cebm.net/index.aspx?o=5653 [accessed 11 April 2020].
- 7. Tao KX, Zhang BX, Zhang P, Zhu P, Wang GB, Chen XP; General Surgery Branch of Hubei Medical Association; General Surgery Branch of Wuhan Medical Association. [Recommendations for general surgery clinical practice in novel coronavirus pneumonia situation.] Zhonghua Wai Ke Za Zhi 2020; 58: 10.3760/cma.j.issn.0529-5815.2020.0001 [online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Gou SM, Yin T, Xiong JX, Peng T, Li Y, Wu HS. [Treatment of pancreatic diseases and prevention of infection during outbreak of 2019 coronavirus disease.] Zhonghua Wai Ke Za Zhi 2020; 58: 10.3760/cma.j.cn112139-20200224-00123 [online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9. Wong J, Goh QY, Tan Z, Lie SA, Tay YC, Ng SY et al Preparing for a COVID‐19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth 2020; 10.1007/s12630-020-01620-9 [online ahead of print]. [DOI] [PMC free article] [PubMed]
- 10. Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID‐19 patient needs an operation: operating room preparation and guidance. Can J Anaesth 2020; 10.1007/s12630-020-01617-4 [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu F, Song Y, Zeng HY, Ye F, Rong WQ, Wang LM et al [Discussion on diagnosis and treatment of hepatobiliary malignancies during the outbreak of novel coronavirus pneumonia.] Zhonghua Zhong Liu Za Zhi 2020; 42: 10.3760/cma.j.cn112152-20200227-00137 [online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Qin JJ, Wang Z, Yu Y, Wen YY, Chen XK et al [Surgical treatment for esophageal cancer during the outbreak of COVID‐19.] Zhonghua Zhong Liu Za Zhi 2020; 42: 10.3760/cma.j.cn112152-20200226-00128 [online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13. Luo Y, Zhong M. [Standardized diagnosis and treatment of colorectal cancer during the outbreak of novel coronavirus pneumonia in Renji Hospital.] Zhonghua Wei Chang Wai Ke Za Zhi 2020; 23: 10.3760/cma.j.cn441530-20200217-00057 [online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Chen R, Zhang Y, Huang L, Cheng B‐H, Xia ZY, Meng Q‐T. Safety and efficacy of different anesthetic regimens for parturients with COVID‐19 undergoing Cesarean delivery: a case series of 17 patients. Can J Anaesth 2020; 10.1007/s12630-020-01630-7 [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li LH, Zhang G, Dang XW, Li L. [Treatment strategies of Budd–Chiari syndrome during the epidemic period of 2019 coronavirus disease]. Zhonghua Wai Ke Za Zhi 2020; 58: 10.3760/cma.j.cn112139-20200221-00109 [online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Hu XH, Niu WB, Zhang JF, Li BK, Yu B, Zhang ZY et al [Thinking of treatment strategies for colorectal cancer patients in tumor hospitals under the background of coronavirus pneumonia.] Zhonghua Wei Chang Wai Ke Za Zhi 2020; 23: 10.3760/cma.j.cn441530-20200217-00058 [online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17. Chen YH, Peng JS. [Treatment strategy for gastrointestinal tumor under the outbreak of novel coronavirus pneumonia in China.] Zhonghua Wei Chang Wai Ke Za Zhi 2020; 23: I–IV. [DOI] [PubMed] [Google Scholar]
- 18. WHO . Coronavirus Disease 2019 (COVID‐19) Situation Report – 60; 2020. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200320‐sitrep‐60‐covid‐19.pdf?sfvrsn=8894045a_2 [accessed 11 April 2020].
- 19. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China.] Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41: 145–151.32064853 [Google Scholar]
- 20. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun 2020; 109: 102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect 2006; 64: 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park J, Yoo SY, Ko JH, Lee SM, Chung YJ, Lee JH et al Infection prevention measures for surgical procedures during a Middle East respiratory syndrome outbreak in a tertiary care hospital in South Korea. Sci Rep 2020; 10: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim JY, Song JY, Yoon YK, Choi S‐H, Song YG, Kim S‐R et al Middle East respiratory syndrome infection control and prevention guideline for healthcare facilities. Infect Chemother 2015; 47: 278–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. Novel Coronavirus (2019‐nCoV) Technical Guidance: Infection Prevention and Control; 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/technical‐guidance/infection‐prevention‐and‐control [accessed 11 April 2020].
- 25. Nicolle L. SARS safety and science. Can J Anaesth 2003; 50: 983–988, 985–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loeb M, McGeer A, Henry B, Ofner M, Rose D, Hlywka T et al SARS among critical care nurses, Toronto. Emerg Infect Dis 2004; 10: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chee VW, Khoo ML, Lee SF, Lai YC, Chin NM. Infection control measures for operative procedures in severe acute respiratory syndrome‐related patients. Anesthesiology 2004; 100: 1394–1398. [DOI] [PubMed] [Google Scholar]
- 28. Roberts V. To PAPR or not to PAPR? Can J Respir Ther 2014; 50: 87–90. [PMC free article] [PubMed] [Google Scholar]
- 29.Royal College of Surgeons of England. Updated Intercollegiate General Surgery Guidance on COVID‐19; 2020. https://www.rcseng.ac.uk/coronavirus/joint‐guidance‐for‐surgeons‐v2/ [accessed 11 April 2020].
- 30. Society of American Gastrointestinal and Endoscopic Surgeons, European Association for Endoscopic Surgery. SAGES and EAES Recommendations Regarding Surgical Response to COVID‐19 Crisis; 2020. https://www.sages.org/recommendations‐surgical‐response‐covid‐19/ [accessed 11 April 2020]. [Google Scholar]
- 31. Faculty of Intensive Care Medicine, Intensive Care Society, Association of Anaesthetists, Royal College of Anaesthetists. ICM Anaesthesia COVID‐19 – Clinical Guidance; 2020. https://icmanaesthesiacovid‐19.org/clinical‐guidance [accessed 11 April 2020]. [Google Scholar]
- 32. Mowbray N, Ansell J, Warren N, Wall P, Torkington J. Is surgical smoke harmful to theater staff? A systematic review. Surg Endosc 2013; 27: 3100–3107. [DOI] [PubMed] [Google Scholar]
- 33. Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med 1998; 23: 172–174. [DOI] [PubMed] [Google Scholar]
- 34. Hensman C, Baty D, Willis RG, Cuschieri A. Chemical composition of smoke produced by high‐frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc 1998; 12: 1017–1019. [DOI] [PubMed] [Google Scholar]
- 35. Johnson GK, Robinson WS. Human immunodeficiency virus‐1 (HIV‐1) in the vapors of surgical power instruments. J Med Virol 1991; 33: 47–50. [DOI] [PubMed] [Google Scholar]
- 36. Hahn KY, Kang DW, Azman ZAM, Kim SY, Kim SH. Removal of hazardous surgical smoke using a built‐in‐filter trocar: a study in laparoscopic rectal resection. Surg Laparosc Endosc Percutan Tech 2017; 27: 341–345. [DOI] [PubMed] [Google Scholar]
- 37. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth 2020; 124: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


