Abstract
Objectives
Novel coronavirus (COVID‐19) is a global pandemic currently spreading rapidly across the United States. We provide a comprehensive look at COVID‐19 epidemiology across the state of Georgia, which includes vast rural communities that may be disproportionately impacted by the spread of this infectious disease.
Methods
All 159 Georgia counties were included in this study. We examined the geographic variation of COVID‐19 in Georgia from March 3 through April 24, 2020 by extracting data on incidence and mortality from various national and state datasets. We contrasted county‐level mortality rates per 100,000 population (MRs) by county‐level factors.
Results
Metropolitan Atlanta had the overall highest number of confirmed cases; however, the southwestern rural parts of Georgia, surrounding the city of Albany, had the highest bi‐weekly increases in incidence rate. Among counties with >10 cases, MRs were highest in the rural counties of Randolph (233.2), Terrell (182.5), Early (136.3), and Dougherty (114.2). Counties with the highest MRs (22.5–2332 per 100,000) had a higher proportion of: non‐Hispanic Blacks residents, adults aged 60+, adults earning <$20,000 annually, and residents living in rural communities when compared with counties with lower MRs. These counties also had a lower proportion of the population with a college education, lower number of ICU beds per 100,000 population, and lower number of primary care physicians per 10,000 population.
Conclusions
While urban centers in Georgia account for the bulk of COVID‐19 cases, high mortality rates and low critical care capacity in rural Georgia are also of critical concern.
Keywords: COVID‐19, epidemiology, geographic distribution, Georgia, geospatial, incidence, novel coronavirus, SARS‐CoV‐2, social determinants
1. INTRODUCTION
1.1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the virus responsible for the novel coronavirus disease (COVID‐19) and has led to a global pandemic with >2.9 million confirmed cases, including over 964,000 confirmed cases and 54,840 deaths in the United States. 1 In the state of Georgia, the first case of the novel coronavirus (COVID‐19) was detected on March 3, 2020, and the first death attributed to COVID‐19 was about 10 days later on March 12. As of April 24, there were 22,147 confirmed cases, 4221 patients hospitalized, and 892 deaths attributed to COVID‐19. 2 Furthermore, it was forecasted that Georgia will have >1620 deaths attributed to COVID‐19 between March 3 and June 1, 2020. 3
1.2. Importance
Hospital critical care capacity represents the most important medical care factor for preventing deaths from COVID‐19. Understanding the geographic areas that have the highest disease burden and morbidity will allow policy makers, public health professionals, and critical care providers to appropriately allocate resources and adequately prepare for the disease pandemic for specific populations.
1.3. Goal of this investigation
Here, we present the overall prevalence of confirmed COVID‐19 cases, overall mortality rates attributed to COVID‐19, and the bi‐weekly incidence rates of confirmed COVID‐19 cases in Georgia, stratified by counties.
2. METHODS
2.1. Study design and setting
Using counties as the units of analysis, we examined the geographic variation of COVID‐19 in the state of Georgia from March 3 through April 24, 2020. Since the first case was confirmed on March 2, the Georgia Department of Public Health (GADPH) has provided a daily update of confirmed cases of COVID‐19, tested both by commercial labs as well as the state department using the CDC 2019‐nCoV Real‐Time Reverse‐Transcription‐Polymerase Chain Reaction (RT‐PCR) diagnostic tests (https://dph.georgia.gov/covid-19-daily-status-report). From Limit of Detection studies sensitivity has been estimated to range from 65% to 100% depending on RNA concentrations. 4 We obtained the county‐level data on COVID‐19 confirmed cases and deaths from the Johns Hopkins 2019 Novel Coronavirus Data Repository, (https://github.com/CSSEGISandData/COVID-19) which imports the GADPH daily status report file for each county, allowing for investigators to examine time‐series (ie, day‐by‐day cumulative cases of cases and deaths). We linked the county‐level COVID‐19 data with the county‐level data on socio‐demographic, access to healthcare, and hospital critical care infrastructure factors, derived from the 2014 County Health Rankings, 2014 American Community Survey, 2010 Census, and 2017–2019 Centers for Medicare & Medicaid Services hospital reports. This study was considered exempt by Institutional Review Board review because we used publicly available, de‐identified secondary data.
The Bottom Line
This descriptive epidemiologic study sheds light on the burden of COVID‐19 in Georgia with a report of higher mortality rates and reduced access to critical care among African‐American communities in rural Georgia.
2.2. Statistical analysis
We calculated 3 measures of disease morbidity at the county level: (1) the bi‐weekly confirmed case incidence rate per 100,000 population, (2) overall prevalence of confirmed cases per 100,000 population, and (3) mortality rate per 100,000 population among confirmed cases. We compared the county‐level characteristics of the population such as demographic factors by mortality rate quintiles using Kruskal‐Wallis tests. In secondary analysis, we examined county‐level differences in incidence and mortality rates, comparing: (1) counties with <50% non‐Hispanic Black residents versus those with ≥50% NH‐Black residents and (2) counties with <50% male residents versus those with ≥50% male residents. These results are presented within Figures S1 and S2. We performed all statistical analysis using SAS version 9.4 and executed all mapping using ArcGIS version 10.5.
3. RESULTS
3.1. Incidence rates in Georgia, March 3 through April 24, 2020
Figure 1 presents a bi‐weekly progression and county‐level incidence rates (IR) for COVID‐19 per 100,000 population. From March 3 through 16, northwest Georgia counties surrounding the metropolitan Atlanta area had the highest incidence rates, specifically, in the following counties (presenting counties with >5 cases): (1) Bartow (IR: 9.0; 95% confidence interval [95% CI]: 4.7–17.3), (2) Dougherty (IR: 6.3; 95% CI: 2.9–14.1), (3) Fayette (IR: 4.7; 95% CI: 2.0–11.3), and Cherokee (IR: 3.3; 95% CI: 1.6–6.9). During the second period, March 17 through 29, Fulton County experienced the highest number of cases (n = 407), but the pandemic spread dramatically to the southwestern portion of the state surrounding the Albany area. In addition, the overall incidence for the most affected area had more than a 20‐fold increase. The highest incidence rates were observed in the following counties: (1) Dougherty (IR: 246.4 95% CI: 216.7–280.2), (2) Early (IR: 145.3; 95% CI: 89.0–237.3), (3) Lee (IR: 144.9; 95% CI: 106.7–196.8), (4) Bartow (IR: 108.8; 95% CI: 90.2–131.3). During the third period, March 30 through April 11, Fulton County had the highest number of new cases with 959 confirmed cases. However, the Albany area had the highest increase with the following incidence rates: (1) Randolph (IR: 1295.5; 95% CI: 1064.9–1576.0), (2) Terrell (IR: 987.7; 95% CI: 805.1–1211.6), (3) Dougherty (IR: 855.5; 95% CI: 798.5–916.5), and (4) Early (IR: 817.6; 95% CI: 665.0–1005.2). During the fourth bi‐weekly period, April 12 through April 24, Fulton County still had the highest number of new cases with an additional 1033 confirmed cases. However, southwestern Georgia experienced the highest increase with the following incidence rates: (1) Mitchell (IR: 646.9; 95% CI: 551.8–758.3), (2) Terrell (IR: 622.7; 95% CI: 481.4–805.4), (3) Calhoun (IR: 612.5; 95% CI: 451.0–831.8), and Randolph (IR: 608.9; 95% CI: 457.5–810.4). By April 24, counties with ≥50% NH‐Black residents had 79% higher incidence rates (incidence rate ratio = 1.79; 95% CI = 1.74–1.85) when compared with counties with <50% NH‐Black residents (Figure S1A). Counties with ≥50% male residents had 19% higher incidence rates (incidence rate ratio = 1.19; 95% CI = 1.13–1.25), when compared with counties with <50% male residents (Figure S2A).
FIGURE 1.
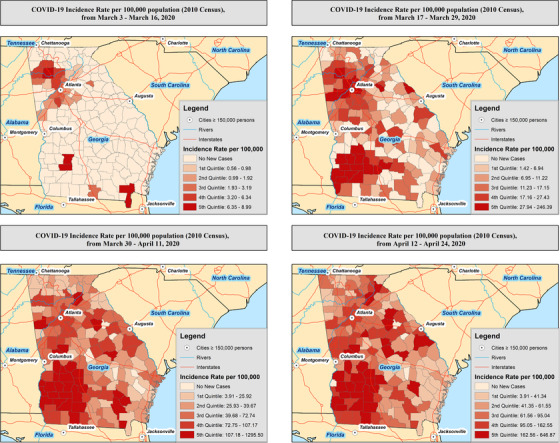
Bi‐weekly incidence of COVID‐19 within the state of Georgia after first confirmed case on March 3, 2020. Highest incidence for March 3 to March 16: Polk county, IR: 1.0; 95% CI: 0.1–7.4. Highest incidence for March 17 to March 29: Bartow county, IR: 19.0; 95% CI: 12.1–29.7. Highest incidence for March 30 to April 11: Dougherty county, IR: 143.8; 95% CI: 121.6–170.1. Highest incidence for April 12 to April 24: Terrell county, IR: 472.4; 95% CI: 351.5–634.7. CI, confidence interval; IR, incidence rate
3.2. Prevalence in Georgia through April 24, 2020
Figure 2A presents the prevalence of confirmed COVID‐19 cases in Georgia through the beginning of April, the first 7 weeks of community spread. There were a total of 22,147 confirmed cases corresponding to a prevalence rate (PR) of 228.6 (95% CI: 225.6–231.6) per 100,000 population. Fulton County experienced the highest total number of confirmed cases with 2500 people testing positive for through April 24, 2020. However, the highest PRs where in 4 counties located in southwest Georgia: (1) Randolph (PR: 1969.2; 95% CI: 1679.7–2308.5), (2) Terrell (PR: 1771.3; 95% CI: 1520.7–2063.3), (3) Early (PR: 1707.9; 95% CI: 1480.4–1970.3), and (4) Dougherty (PR: 1549.2; 95% CI: 1471.9–1630.6).
FIGURE 2.
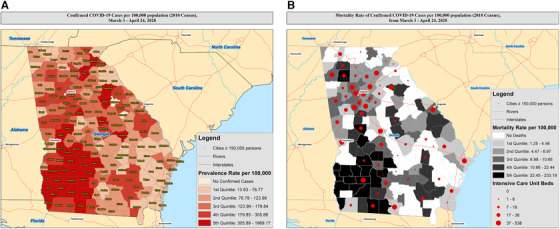
(A) The prevalence of confirmed COVID‐19 cases in Georgia up through April 24, 2020. (B) Mapping of mortality rates per 100,000 population, overlaid with estimated number of available ICU beds
3.3. Mortality rates in Georgia through April 24, 2020
Among the 22,147 confirmed cases, 4221 (19.1%) were hospitalized and 892 died (4.0% confirmed case‐fatality rate; 95% CI: 3.8–4.3%) due to complications from COVID‐19. We present the mortality rates of confirmed COVID‐19 per county in Figure 2B. Among counties with >10 cases, the highest mortality rates (MR) per 100,000 population were in: (1) Randolph (MR: 233.2; 95% CI: 228.0–238.5), (2) Terrell (MR: 182.5; 95% CI: 178.8–186.2), (3) Early (MR: 136.3; 95% CI: 133.7–138.8), and (4) Dougherty (MR: 114.2; 95% CI: 113.5–114.9). We compared the distribution of county‐level demographics and community resources by quintiles of COVID‐19 MRs (Table 1), including counties with no deaths, in order to understand similarities or dissimilarities between counties yet to experience mortality from the disease. We observed that counties with the highest mortality rates (22.5–233.2 deaths per 100,000 population) had a lower proportion of non‐Hispanic White residents (median: 48.8%; interquartile range (IQR): 40.6–59.6, P = 0.01), a higher proportion of non‐Hispanic Black residents (median: 45.3%; IQR: 34.7–51.6, P < 0.01), a higher proportion of adults aged 60 and older (median: 22.5%; IQR: 19.3–24.6, P < 0.01), lower number of ICU beds per 100,000 population (median: 0.0; IQR: 0.0–0.0), lower number of primary care physicians per 10,000 population (median: 2.7; IQR: 1.1–6.1), a higher proportion of adults earning <$20,000 annual (median: 34.2; IQR: 28.4–35.3), a lower proportion of the population that attained a college education (median: 11.1; IQR: 9.4–15.1), and a much higher proportion of residents living in rural communities (median 54.5; IQR: 46.9–73.1), when compared with counties with lower mortality rates. By April 24th, counties with ≥50% NH‐Black residents had 2‐fold higher mortality rates (mortality rate ratio = 2.08; 95% CI = 1.80–2.41) when compared with counties with <50% NH‐Black residents (Figure S1B). Counties with ≥50% male residents had 47% higher mortality rates (mortality rate ratio = 1.47; 95% CI = 1.18–1.84) when compared with counties with <50% male residents (Figure S2B).
TABLE 1.
County‐Level Characteristics Comparisons by COVID‐19 Mortality Rates per 100,000 population, Among All 159 Georgia Counties March 3 through April 24, 2020
| Presented as median (IQR) b | |||||||
|---|---|---|---|---|---|---|---|
| Mortality rate per 100,000 | 0.0 (no deaths) | 1.3–4.5 | 4.5–7.0 | 7.0–10.7 | 10.7–22.4 | 22.5–233.2 | |
| No. of counties | 52 | 21 | 22 | 21 | 22 | 21 | P value a |
| Race | |||||||
| % NH‐White | 65.2 (57.4, 83.4) | 72.5 (59.7, 80.8) | 64.2 (51.7, 79.6) | 62.5 (51.1, 69.2) | 60.1 (55.7, 72.5) | 48.8 (40.6, 59.6) | 0.01 |
| % NH‐Black | 25.7 (8.4, 34.1) | 17.3 (11.2, 35.7) | 20.4 (5.6, 39.5) | 27.4 (20.4, 43.6) | 27.4 (19.2, 39.7) | 45.3 (34.7, 51.6) | <0.01 |
| % Hispanic | 3.9 (2.1, 9.0) | 5.1 (3.0, 6.5) | 5.5 (3.2, 9.6) | 6.3 (3.3, 9.6) | 4.0 (2.4, 9.6) | 2.9 (1.9, 4.4) | 0.04 |
| % Female sex | 50.9 (49.6, 51.6) | 51.1 (50.4, 51.3) | 50.9 (50.3, 51.8) | 51.4 (50.0, 51.6) | 50.7 (50.2, 51.6) | 51.0 (49.6, 52.4) | 0.90 |
| % Age 60+ | 21.6 (19.8, 25.1) | 18.4 (16.4, 21.6) | 18.9 (14.7, 22.7) | 18.6 (16.4, 20.6) | 21.0 (19.5, 22.5) | 22.5 (19.3, 24.6) | <0.01 |
| ICU c beds per 100,000 population | 0.0 (0.0, 0.0) | 14.9 (0.0, 33.0) | 6.5 (0.0, 19.6) | 23.4 (0.0, 49.9) | 0.0 (0.0, 22.0) | 0.0 (0.0, 0.0) | <0.01 |
| PCP d per 10,000 population | 3.1 (1.5, 4.2) | 5.7 (3.4, 6.5) | 5.1 (3.0, 6.1) | 6.3 (3.1, 8.7) | 3.7 (2.2, 4.8) | 2.7 (1.1, 6.1) | <0.01 |
| % Uninsured | 23.1 (21.1, 24.6) | 21.1 (18.8, 22.6) | 21.1 (18.3, 23.8) | 22.4 (20.6, 24.4) | 23.6 (21.0, 25.0) | 22.1 (21.0, 23.5) | 0.04 |
| % Income <$20,000 | 28.8 (23.6, 32.9) | 21.0 (17.0, 26.0) | 17.6 (13.3, 26.5) | 24.6 (21.2, 30.2) | 29.0 (23.0, 32.2) | 34.2 (28.4, 35.3) | <0.01 |
| % Attained college education | 13.4 (10.7, 15.2) | 18.6 (15.1, 23.7) | 23.5 (16.2, 27.8) | 18.1 (12.1, 20.6) | 13.3 (12.7, 15.3) | 11.1 (9.4, 15.1) | <0.01 |
| % Adult obesity | 31.3 (29.2, 32.9) | 31.3 (28.4, 32.2) | 28.5 (27.5, 31.7) | 30.8 (28.9, 33.0) | 32.2 (31.0, 34.0) | 32.6 (31.3, 33.6) | <0.01 |
| % Adult smoking | 23.8 (19.7, 27.5) | 21.2 (18.0, 24.6) | 17.8 (14.8, 21.2) | 17.0 (14.1, 20.6) | 23.8 (17.0, 24.6) | 21.5 (19.0, 22.9) | <0.01 |
| % Rural | 75.0 (60.7, 100.0) | 42.7 (27.2, 58.6) | 55.6 (17.1, 73.1) | 41.8 (14.9, 66.7) | 68.8 (51.6, 99.0) | 54.5 (46.9, 73.1) | <0.01 |
Significance determined using Kruskal‐Wallis tests, P values <0.05.
IQR, interquartile range.
ICU, intensive care unit, ICU bed tally does not include Veterans Affairs hospitals, which are sure to play a role in treating COVID‐19 patients, because VA hospitals do not file cost reports to CMS.
PCP, primary care physicians.
4. LIMITATIONS
Our analysis is limited by several factors. First, as with all early surveillance studies of COVID‐19, our analysis suffers from underreporting of all morbidity values, as asymptomatic or slightly symptomatic cases may not be tested and subsequently diagnosed. Therefore, our analysis is based on confirmed COVID‐19 cases only. Measures of confirmed prevalence, incidence, and mortality rates may be an underestimate of the true burden of disease, as most COVID‐19 patients will recover before being tested, and thus may not be included in numerator if disease is not severe enough to warrant testing. A statewide surveillance study which includes mandatory and available testing for all Georgia residents is the most precise method to estimate the true incidence of cases. Nevertheless, all novel respiratory virus infections (including COVID‐19) are diseases that need to be immediately notifiable to the Georgia Department of Public Health by law. In addition to geography, mortality is likely to be a function of many factors arising from the accumulation of specific high‐risk populations in various locations. For example, places with large elderly populations or a high prevalence of pre‐existing respiratory, cardiovascular, or immunocompromised conditions would have even higher case‐fatality rates. Our observed increases of county‐level incidence rates are both a function of true incidence and more widespread testing. We reported on the hospital critical care infrastructure by county using the number of ICU beds and primary care physicians as proxies. We were unable to account for the number of available ventilator supplies. Additionally, hospital critical care infrastructure is not static, and hospitals may have the capacity to increase staffing and ICU bed space during the COVID‐19 pandemic. However, urban centers may be more equipped to increase their critical care capacity than rural centers. A further limitation is that we lacked data on transfers of critically ill patients from rural areas to facilities in large urban areas.
5. DISCUSSION
To date and to our knowledge, this is one of the first studies to describe the epidemiology of COVID‐19 in the state of Georgia, with geographic trends and elucidating possible county‐level differences that are correlated with higher mortality rates. As expected, we observed that metropolitan Atlanta had the overall highest number of confirmed cases in Georgia. However, incidence growth rates show that the southwestern rural portion of Georgia, surrounding the city of Albany, had the highest bi‐weekly increases in incidence rates. Further, the counties of Randolph, Dougherty, Terrell, Early, and Lee all experienced PRs for COVID‐19 well above 1000 per 100,000 population. Among counties with >10 cases, mortality rates were highest in the rural counties of Randolph (233.2 per 100,000), Terrell (182.5 per 100,000), Early (136.3 per 100,000), and Dougherty (1142 per 100,000), all of which are associated with having very limited health care resources, including relatively few intensive care unit beds and active primary care physicians. When compared with counties with lower proportions (<50%) of NH‐Black residents and male residents, those counties with a higher proportion of non‐Hispanic Black residents and male residents were associated with higher incidence and mortality of COVID‐19 through April 24, 2020.
While Georgia has an estimated 8196 hospital beds available for critically ill patients, we observed that mortality rates were higher in counties with low to no ICU beds available. A shortage of ICU beds is important because prior reports have shown that the vast majority of COVID‐19 patients admitted to the ICU are critical and only 20% of those who were critical has survived, mostly due to ICU care. 5 It is likely that mortality rates are higher in rural Georgia communities due to delays in treatment and care because of the need to transport rural patients to other centers with available ICU beds and/or ventilators. Further, the southwest Georgia counties identified as hot spots for COVID‐19 mortality and prevalence have been previously identified as hot spots for mortality of other diseases including sepsis and stroke, indicating that these communities may be experiencing prolonged disparities in health outcomes attributed to reduced healthcare services, preventive care, and social inequities. 6 , 7 Our findings showing higher rates of incidence and mortality among counties with higher percentage of NH‐Black residents and males, indicate that racial and gender disparities may play a role in COVID‐19 and recent evidence among the first 393 COVID‐19 patients in New York City are consistent with this finding. 8 In order to mitigate the burden and excess deaths due to COVID‐19, varying stakeholders including policymakers, politicians, medical professionals, and community leaders will need to strategize and appropriately allocate resources to these under‐resourced areas.
In Georgia, the peak of the COVID‐19 pandemic was estimated to arrive in late April 2020. The state and local governments should strongly support rural counties and the southwestern Georgia area by supplying increased ICU beds, ventilators, and emergency medical staff. While urban centers in Georgia account for the bulk of COVID‐19 cases, the high mortality rates and low critical care capacity in rural Georgia is becoming a highly detrimental factor in fighting this pandemic. This is especially concerning, given hospital critical care capacity may be the most important medical system stopgap in terms of preventing/limiting deaths associated with COVID‐19.
CONFLICTS OF INTEREST
There are no conflicts to declare.
AUTHORS CONTRIBUTION
Justin Xavier Moore is responsible for conception, design, data acquisition, analysis, and drafting of this manuscript. Marvin E. Langston is responsible for the conception, design, and drafting of this manuscript. Varghese George is responsible for the conception and critical revision for important intellectual content. Steven S. Coughlin is responsible for the conception and critical revision for important intellectual content. All authors give final approval of this work, take public responsibility for the content, and agree to be accountable for all aspects of the work.
Supporting information
Supplementary information
Biography
Justin Xavier Moore, PhD, MPH, is an Assistant Professor in the Division of Epidemiology, Department of Population Health Sciences at Augusta University at the Medical College of Georgia.

Moore JX, Langston ME, George V, Coughlin SS. Epidemiology of the 2020 pandemic of COVID‐19 in the state of Georgia: Inadequate critical care resources and impact after 7 weeks of community spread. JACEP Open. 2020;1:527–532. 10.1002/emp2.12127
Funding and support: ByJACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Alexander Lo, MD, PhD.
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Georgia Department of Public Health COVID‐19 Daily Status Report. 2020; https://dph.georgia.gov/covid-19-daily-status-report. Accessed April 4, 2020, 2020.
- 3. Murray C. Forecasting COVID‐19 impact on hospital bed‐days, ICU‐days, ventilator‐days and deaths by US state in the next 4 months. BMJ: Yale; 2020.
- 4. CDC 2019‐Novel Coronavirus (2019‐nCoV) Real‐Time RT‐PCR Diagnostic Panel. 2020; https://www.fda.gov/media/134922/download. Accessed April 27, 2020, 2020.
- 5. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore JX, Donnelly JP, Griffin R, Howard G, Safford MM, Wang HE. Defining sepsis mortality clusters in the United States. Crit Care Med. 2016;44(7):1380‐1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karp DN, Wolff CS, Wiebe DJ, Branas CC, Carr BG, Mullen MT. Reassessing the stroke belt: using small area spatial statistics to identify clusters of high stroke mortality in the United States. Stroke. 2016;47(7):1939‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


