To the Editor,
Di Giambenedetto et al. 1 described their experience with intravenous tocilizumab (TCZ) in treating severe coronavirus disease 2019 (COVID‐19). This adds to several data emerging from case reports and uncontrolled studies. 2 , 3 , 4 , 5 However, to the best of our knowledge data on subcutaneous (SC) use of TCZ are lacking. We have treated three patients with this drug at a single dose of 162 mg given subcutaneously, in line with data showing similar efficacy compared to intravenously given TCZ for different diseases. 6 Treatment course for these patients is described below.
The first patient was a 61‐year‐old woman who had only hypercholesterolemia in her past medical history. She was admitted to our hospital on 31 March 2020, with cough, fever, and shortness of breath. She was already on treatment with azithromycin, lopinavir/ritonavir, and hydroxychloroquine from 28 March 2020. Oxygen was administered with high flow nasal cannula at 60 L/min with FiO2 = 75%. An antibiotic coverage for pneumonia with piperacillin/tazobactam was added. For persistence of dyspnoea and on the basis of radiologic findings and IL‐6 serum level (106.1 pg/mL), SC TCZ was administered on 1 April 2020. No major adverse events were reported apart from mild increase of liver function tests 2 days following SC TCZ (ALT 201 UI/L; AST 108 UI/L), with subsequent rapid normalization. Fever disappeared 2 days after and oxygen support was progressively decreased and stopped 12 days after TCZ administration. Significant reduction of IL‐6 was observed in 1 week (from 106.1 to 6.32 pg/mL) with a clear improvement of radiological findings at high‐resolution computerized tomography from 1st April to 18th April 2020 (see Figure 1A,B, respectively).
Figure 1.
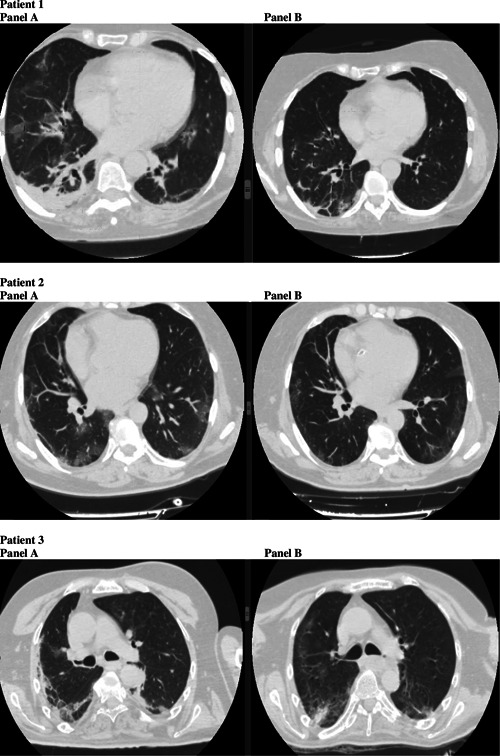
Panel A and B for each patients show evolution of lung disease at high‐resolution computerized tomography before and after subcutaneously tocilizumab
The second patient was a 57‐year‐old woman who suffered from hypertension, diabetes, obesity, and depression. She was admitted on 29th March with fever, cough, tachypnoea, and occasional shortness of breath. Antiviral treatment with hydroxychloroquine and azithromycin was started. The day after admission, nasal flow oxygen therapy was prescribed (2 L/min), followed by Venturi mask (at 15 L/min, FiO2 = 60%) for worsening of SpO2 from 98% to 94%. Plasma concentration of IL‐6 was 72.65 pg/mL after 3 days from admission, with worsening of symptoms and persistence of fever. So, SC TCZ was given on 3 April 2020, with no adverse events recorded. Two days after SC TCZ administration, fever healed and oxygen therapy was no longer needed. Also, improvement of IL‐6 was observed in 1 week (from 72.65 to 5.55 pg/mL). Figure 1A,B shows computed tomography (CT) scan sections before and after treatment (1st April and 30th April 2020, respectively).
The third patient was a 56‐year‐old man with multiple comorbidities. He suffered from neurological aftermaths due to previous stroke and meningoencephalitis during his childhood, diabetes, hyperthyroidism, chronic kidney disease, fatty liver disease, and hypertension. The patient was admitted on 1st April, for persistent fever and shortness of breath, requiring Venturi mask (at 15 L/min, FiO2 = 60%). Antiviral treatment with hydroxychloroquine and azytromycin was started. Fever continued and respiratory parameters worsened (PaO2‐to‐FiO2 ratio from 110 to 96). Chest X rays showed pneumonia, so piperacillin/tazobactam was added to treatment. Respiratory function continued to worse, so on 9th April SC TCZ was administered with no side effects recorded. Mild elevation of liver function tests was already present at baseline. After administration of SC TCZ, fever disappeared on 9 April 2020, and lung function progressively improved, with reduction of oxygen support and positioning of nasal oxygen flow with 4 L/min. IL‐6 plasma concentration decreased from 64.3 ng/mL before TCZ to 40.5 1 week after TCZ. Figure 1A,B shows CT scan sections before (3 April 2020) and after (27 April 2020) treatment.
Results of this case series appear to support efficacy and tolerability of TCZ given subcutaneously in patients with COVID‐19, at least in those with pneumonia not requiring mechanical ventilation. Indeed, favourable clinical outcome and improvement of inflammation biomarkers were observed. While randomised controlled clinical trials are needed to demonstrate the effectiveness of TCZ per se, more investigations are required to clarify any possible differences in terms of efficacy and adverse effects between the two different routes of administration (intravenous or SC). Particularly, it has to be seen whether SC TCZ at single dose in early stages of COVID‐19 is equally effective to intravenous TCZ given at standard doses. Moreover, although tolerability and toxicity profiles of TCZ seem quite good, since side effects have however been reported, 7 , 8 these aspects merit more attention in future studies and compared across different dosages and modes of administration. In particular, since SC TCZ was not administered further for rapid improvement of patient conditions, this suggest the possibility of reducing the dosage of TCZ, at least in patients with less severe COVID‐19.
CONFLICT OF INTERESTS
All authors declare to have not financial or nonfinancial interests in the publication of this study.
ACKNOWLEDGMENTS
We want to thank all our patients and our nurses. We also thank the Infectious Diseases and Tropical Medicine of the University “Magna Graecia” (UMG) COVID‐19 Group, which is composed, besides the main authors, by the following: Giorgio Settimo Barreca, Bernardo Bertucci, Maria Teresa Busceti, Anna Cancelliere, Chiara Davoli, Paolo Fusco, Luigia Gallo, Aida Giancotti, Amerigo Giudice, Giuseppe Greco, Valentina La Gamba, Angelo Lamberti, Maria Carla Liberto, Nadia Marascio, Maria Petullà, Graziella Perri, Angela Quirino, Giada Procopio, Marco Ricchio, Vincenzo Scaglione.
REFERENCES
- 1. Di Giambenedetto S, Ciccullo A, Borghetti A, et al. Off‐label use of tocilizumab in patients with SARS‐CoV‐2 infection [published online ahead of print April 16, 2020]. J Med Virol. 2020. 10.1002/jmv.25897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020;1‐5. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang X, Song K, Tong F, et al. First case of COVID‐19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(7):1307‐1310. 10.1182/bloodadvances.2020001907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michot JM, Albiges L, Chaput N, et al. Tocilizumab, an anti‐IL6 receptor antibody, to treat Covid‐19‐related respiratory failure: a case report [published online ahead of print July, 2020]. Ann Oncol. 2020. 10.1016/j.annonc.2020.03.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mihai C, Dobrota R, Schröder M, et al. COVID‐19 in a patient with systemic sclerosis treated with tocilizumab for SSc‐ILD. Ann Rheum Dis. 2020;79(5):668‐669. 10.1136/annrheumdis-2020-217442 [DOI] [PubMed] [Google Scholar]
- 6. Abdallah H, Hsu JC, Lu P, et al. Pharmacokinetic and pharmacodynamic analysis of subcutaneous tocilizumab in patients with rheumatoid arthritis from 2 randomized, controlled trials: SUMMACTA and BREVACTA. J Clin Pharmacol. 2017;57(4):459‐468. 10.1002/jcph.826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennardo F, Buffone C, Giudice A. New therapeutic opportunities for COVID‐19 patients with tocilizumab: possible correlation of interleukin‐6 receptor inhibitors with osteonecrosis of the jaws [published online ahead of print July, 2020]. Oral Oncol. 2020. 10.1016/j.oraloncology.2020.104659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrison AR, Johnson JM, Ramesh M, Bradley P, Jennings J, Smith ZR. Letter to the editor: acute hypertriglyceridemia in patients with COVID‐19 receiving tocilizumab [published online ahead of print April 21, 2020]. J Med Virol. 2020. 10.1002/jmv.25907 [DOI] [PMC free article] [PubMed] [Google Scholar]


