Abstract
Background
The current outbreak of COVID‐19 has spread rapidly all over the world. Respiratory droplets and contaction with infected patients are the two major transmission routes. However, the value of tear virus nucleic acid is still not clear. We dynamic detected the SARS‐CoV‐2 in eye sample of one COVID‐19 patient with obstruction of common lacrimal ducts.
Methods
Besides the routine examination, nasopharyngeal and eye swab were continuously measured by polymerase chain reaction assay and next‐generation sequencing (NGS). Gene detection was performed for drug use guidance, and flow cytometry was performed to analyse the lymphocyte subsets.
Results
Nasopharyngeal swabs were positive for 22 days, but eye swabs were still continuously positive for 2 weeks after nasopharyngeal swabs turned negative. The low level of lymphocyte and the high level IL‐6 lasted for almost 4 weeks, then became near normal. Next‐generation sequencing (NGS) confirmed the existing of SARS‐CoV‐2, HSV1 and HHV6B virus nucleic acid. The gene detection for drug use guidance showed the genetic locus ABCB1 (3435T>C) rs1045642 belonged to type CC and it mean the efficiency of lopinavir–ritonavir would be significantly decreased. The flow cytometry of lymphocyte subsets showed PD‐1+ CD95+ cells was accounting for 94.8% in CD3+ CD8+ T subset and for 94.8% in CD3+ TCRγδ+ T subset.
Conclusions
As obstruction of common lacrimal duct, positively detection in one eye for 2 weeks more after nasopharyngeal swab became negative. More eye swabs should be collected from COVID‐19 patients, especially from those immunocompromised, those with eye symptoms and those had a history of ocular diseases.
The current outbreak of Coronavirus Disease 2019 (COVID‐19) has spread rapidly all over the world. More than 80 000 cases in China and 300 000 cases in the other countries have been reported until March 24th. Respiratory droplets and contaction with infected patients are the two major transmission routes. However, the value of tear virus nucleic acid is still not clear. We reported a case that positive detection of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) combined herpes simplex virus type 1 (HSV1) and human herpesvirus type 6B (HHV6B) virus nucleic acid in tear and conjunctival secretions of a non‐conjunctivitis COVID‐19 patient with obstruction of common lacrimal ducts in order to understand the ocular change of COVID‐19 and to be a basis for clinical strategy.
Methods
The patient’s agreement was obtained for each sample collection. Tear and conjunctival secretion samples were collected by eye swab. Eyelids were everted, and samples were obtained by sweeping the inferior fornices of both eyes with sterile cotton swabs with topical anaesthesia. The swabs were placed into Hank’s balanced salt solution. Samples were transported in ice to the laboratory in our hospital and centre for disease control (CDC). The samples were tested for SARS‐CoV‐2 in our hospital by use of polymerase chain reaction (PCR) with the CDC recommended Kit. Routine blood test and flow cytometry are operated according to routine methods.
Case Report
A 70‐year‐old, native resident of Xi’an city (Shaanxi Province, China) was admitted to the First Affiliated Hospital of Xi’an Jiaotong University complicated with a fever, fatigue, cough and sputum for one day. Just one week ago, he has contacted with some relatives that came from Wuhan Province (China).
After being treated in quarantine ward, a series of laboratory examinations including nasopharyngeal swab were detected for SARS‐CoV‐2. According to the records, the initial physical examination revelled T 37.7°C, and SpO2 92% without oxygen supplement. The blood routine examination showed white blood cells (6.92 × 109/l), lymphocyte per cent (5.8%), neutrophil per cent (88.7%) and C‐reactive protein (15.4 mg/l). IL‐6 in serum was 40.90 pg/ml. The changes of granulocyte (A) and IL‐6 (B) in the whole course were shown in Fig. 1. The test for influenza A and B showed negative. Computerized tomography (CT) of lung showed significant ground‐glass opacities. The CDC confirmed that both the twice nasopharyngeal swab detections were all positive for SARS‐CoV‐2. Sure enough, the man suffered COVID‐19 (mild type).
Fig. 1.
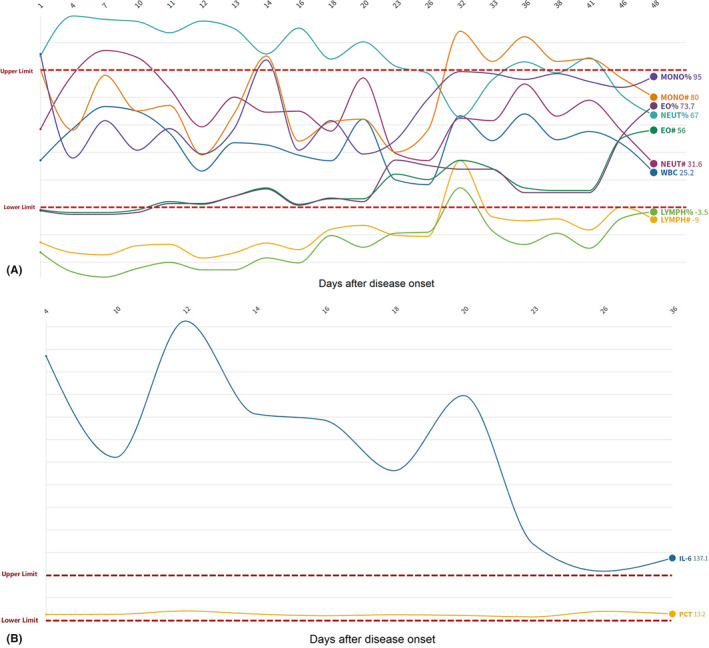
The change of granulocyte (A) and IL‐6 (B) in the whole course. Each datum was relative to the normal value, respectively.
The whole clinical course was shown in Fig. 2A. After combined treatment, (lopinavir and ritonavir tablets per os for 6 days, moxifloxacin per os for 7 days, interferon nebulization for 16 days, traditional Chinese medicine per os for 7 days), the man’s body temperature returned to normal after onset 12 days. After 23 days, his chief complaint recovered, and the ground‐glass opacities decreased in CT of lung were detected with the negative nasopharyngeal swabs for several times continuously (24 hr apart). Before discharged, nasopharyngeal swab and eye swab were collected again at the same time. Surprisingly, the nasopharyngeal swab was negative and the eye swab was positive on Day 25 of the course. Reexamination was the same results and next‐generation sequencing (NGS) confirmed PCR results (Fig. 2B). Besides, HSV1 and HHV6B virus nucleic acid were also detected by NGS (Fig. 2B). However, any symptoms and signs of conjunctivitis were not found in his eyes. Only the patient had a history of obstruction of common lacrimal duct in his left eye with mild tearing and without any secretion. Through lacrimal duct irrigation, obstruction of common lacrimal duct in his left eye was diagnosed, and levofloxacin with Arbidol eye drops was taken. The left eye swab quickly turned undetectable. The whole changes of C t value in swabs were shown in Fig. 2D.
Fig. 2.
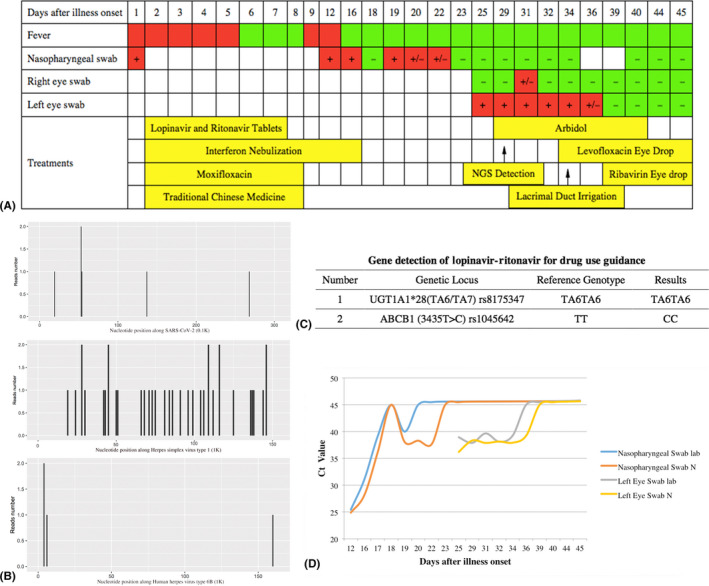
(A) The whole clinical course and results of swabs and treatments of this patient. (B) The NGS detection of SARS‐CoV‐2 (n = 6), HSV1 (n = 36) and HHV6B (n = 4). (C) The results of gene detection of lopinavir–ritonavir for drug use guidance. (D) The whole changes of C t value in swabs.
The gene detection of lopinavir and ritonavir for drug use guidance showed the genetic locus ABCB1 (3435T>C) rs1045642 belonged to type CC (Fig. 2C), and it means that the efficiency of lopinavir and ritonavir tablets in this patient would be significantly decreased. The flow cytometry results of lymphocyte subsets showed PD‐1+ CD95+ cells was accounting for 94.8% in CD3+ CD8+ T subset, and for 94.8% in CD3+ TCRγδ+ T subset (Fig. 3).
Fig. 3.
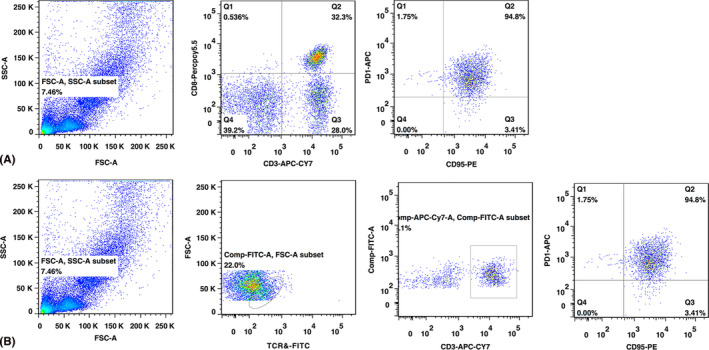
Flow cytometry results of lymphocyte subsets: (A) PD‐1+ CD95+ cells were accounting for 94.8% in CD3+ CD8+ T subset and (B) for 94.8% in CD3+ TCRγδ+ T subset.
To sum up, his nasopharyngeal swabs were positive for 22 days, but the eye swabs were still continuously positive for 2 weeks. The low level of lymphocyte and the high level IL‐6 lasted for almost 4 weeks and then became near normal.
Discussion
According to recent news reports, several ophthalmologists in Wuhan (China) were sacrificed due to COVID‐19. Although COVID‐19 indeed transmitted mainly through respiratory droplets and contact with infected patients, some studies suspected that it might be related with the contaction of body fluid of patients, including tear. Xia and colleagues suggested that SARS‐Cov2 transmission through the ocular surface should not be ignored (Lu et al. 2020). In this case, we collected the eye swab from a clinical cured COVID‐19 patient and detected the positive expression of SARS‐CoV‐2.
Respiratory‐related public health events, such as SARS, were reported to be associated with ophthalmology (Loon et al. 2004; Vegh et al. 2015). No matter SARS or Ebola, the patients’ tears were detected the virus nucleic of coronavirus or Ebola virus (Bausch et al. 2007). The novel virus SARS‐CoV‐2, which caused COVID‐19, is similar to SARS virus, which it shares more than 79% of its sequence. Our case positively detected SARS‐CoV‐2 virus in tears and conjunctival secretions. Similarly, some published paper has the same results of SARS‐CoV‐2 virus in tears (Xia et al. 2020). However, our case has many features differently with other reported cases. There were 5 points:
Virus was detected for continuously until turned negative, not only one time‐point.
The case was not conjunctivitis patient and positively detection when recovered.
Different results between two eyes of the patient were related with obstruction of common lacrimal duct on left eye.
Positive eye sample virus lasted for 2 weeks more after nasopharyngeal swab became negative.
System immune states were evaluated including blood routine, other virus co‐infection and Lymphocyte subsets.
Continuously detection may comprehensive analysis the dynamic change of this case. The occurrence of asymptomatic patients with SARS‐CoV‐2 may pose a significant public health issue and infection control challenge. China's National Centre for Disease Control analysed 72 314 COVID‐19 cases and found out 889 (1.2%) cases were asymptomatic (Novel Coronavirus Pneumonia Emergency Response Epidemiology 2020). One report indicated that an asymptomatic person was able to transmit SARS‐CoV‐2 to another patient in Germany (Rothe et al. 2020). These results reminded us that asymptomatic cases might play a role in the transmission. Similarly, the patient in our case did not suffer conjunctival congestion or conjunctivitis, but positive virus in eye sample may cause infection through eye tissues/tears or when doing ophthalmic examination. It reminded ophthalmologists should take more carefully protection for themselves and ophthalmic patients.
Coincidentally, we positively detected SARS‐CoV‐2 in his left eye sample repeatedly, of which common lacrimal duct was obstructed. Some study revealed that lacrimal duct obstruction and acute dacryocystitis were associated with Epstein–Barr virus infection (Steele & Meyer 1993). Therefore, we speculated that this case might concern with obstruction of common lacrimal duct. To our knowledge, obstruction of common lacrimal duct may decrease the virus clear through lacrimal drainage system. This point may be the reason why the different results between two eyes. Further works are needed to demonstrate this speculation.
In this case, the man entered into convalescence as his body temperature returned to normal and the clinical symptoms like cough were all disappeared. Positive eye sample virus lasted for 2 weeks more after nasopharyngeal swab became negative. However, the SARS‐CoV‐2 could still be detected, which mean that the virus was shedding persistently. In 2005, a retrospective study depicted the dynamic profiles of viral persistence in SARS patients. Cultivable SARS‐CoV‐2 was detected in stool or urine specimens for longer than 4 weeks in three convalescent patients (Xu et al. 2005). In 2018, a study revealed that the prolonged shedding of Avian Influenza A (H7N9) RNA in the respiratory tract was associated with delayed initiation of neuraminidase inhibitor (NAI) treatment and with use of corticosteroids (Wang et al. 2018). Recently, an article showed the median duration of viral shedding of SARS‐CoV‐2 was 20 days from illness onset. The shortest was 8 days, and the longest among survivors was 37 days (Zhou et al. 2020). In our case, the nasopharyngeal swabs kept positive for 22 days and the eye swabs kept positive for nearly 2 weeks more after the nasopharyngeal swabs became negative. After lacrimal duct irrigation and topical eye drops treatments, the SARS‐Cov‐2 in his eye samples turned undetectable. Thus, the duration of viral shedding was 36 days, might even be 38 days, as we did not collect eye swabs on Day 37 and 38.
The reasons of the prolonged shedding of SARS‐CoV‐2 in eye sample might be related with these factors as follows. (1) The patients had low efficiency for lopinavir and ritonavir because of type CC of ABCB1 (3435T>C) rs1045642. Although recognized specific drug for COVID is not found, lopinavir and ritonavir were recognized that they might inhibit SARS‐CoV‐2. Therefore, lopinavir and ritonavir are our routine treatment in our hospital. The patients’ gene determined his low effective for the two drugs and slowly virus shedding. Recently, a study revealed that in 199 hospitalized adult patients with severe COVID‐19, no benefit was observed with lopinavir–ritonavir treatment beyond standard care (Cao et al. 2020). Future trials are needed in the further. (2) Relative immunosuppressive state may also influence virus clear from his body. The low level of lymphocyte and the high level IL‐6 lasted for almost 4 weeks, high PD‐1+ CD95+ cells proportion in CD3+ CD8+ T subset and combined herpes simplex virus type 1 (HSV1) and HHV6B virus latent infection may be reflect that his immune was suppressed. These were no enough functional lymphocyte to kill the virus, and PD‐1+ CD95+ cells can inhibition of T‐cell activation and induction of immunocytes apoptosis (Brezar et al. 2017). In the NGS testing report, HSV1 and HHV6B nucleic acid were also detected. HSV1 and HHV‐6B are also responsible for lifelong chronic infections, most often asymptomatic, in the vast majority of the general adult population (Agut et al. 2017). In ophthalmology, HSV1 and HHV6 could remain latent in the human anterior segment and aqueous humour. When immune was suppressed, the two viruses can cause ocular diseases and be detected. Therefore, we speculated that the latent infection of HSV1 and HHV6B might be activated as immunocompromised during the course of COVID‐19.
Our case reminds that more eye swabs should be collected from confirmed and suspected COVID‐19 patients, especially from those immunocompromised, such elder patients or had a history of immunodeficiency diseases, those with eye symptoms, such as conjunctival congestion or conjunctivitis and those had a history of ocular diseases, such as obstruction of common lacrimal duct. Furthermore, when nasopharyngeal swab is negative and the eye swab is positive, it is a question that whether this man is a convalescent patient or asymptomatic infected patient. Deeply studies should be researched. It should be emphasized that PCR assay only detect the fragments of viral nucleic acid, so it represents differences from isolation and identification of the virus. And positive virus nucleic detection in tears does not absolutely mean that the virus definitely transmits from eyes.
Hu Yaguang and Chen Tianyan contributed equally to this work.
Contributor Information
Yawen Wang, Email: wangyw1269@xjtu.edu.cn.
Li Li, Email: eyelili2010@mail.xjtu.edu.cn.
References
- Agut H, Bonnafous P & Gautheret‐Dejean A (2017): Update on infections with human herpesviruses 6A, 6B, and 7. Med Mal Infect 47: 83–91. [DOI] [PubMed] [Google Scholar]
- Bausch DG, Towner JS, Dowell SF et al. (2007): Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 196(Suppl 2): S142–S147. [DOI] [PubMed] [Google Scholar]
- Brezar V, Hani L, Surenaud M, Hubert A, Lacabaratz C, Lelievre JD, Levy Y & Seddiki N (2017): Negative modulation of suppressive HIV‐specific regulatory T cells by IL‐2 adjuvanted therapeutic vaccine. PLoS Pathog 13: e1006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Wang Y, Wen D et al. (2020): A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loon SC, Teoh SC, Oon LL, Se‐Thoe SY, Ling AE, Leo YS & Leong HN (2004): The severe acute respiratory syndrome coronavirus in tears. Br J ophthalmol 88: 861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CW, Liu XF & Jia ZF (2020): 2019‐nCoV transmission through the ocular surface must not be ignored. Lancet 395: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel Coronavirus Pneumonia Emergency Response Epidemiology T (2020): [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 41: 145–151.32064853 [Google Scholar]
- Rothe C, Schunk M, Sothmann P et al. (2020): Transmission of 2019‐nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med 382: 970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele RJ & Meyer DR (1993): Nasolacrimal duct obstruction and acute dacryocystitis associated with infectious mononucleosis (Epstein‐Barr virus). Am J Ophthalmol 115: 265–266. [DOI] [PubMed] [Google Scholar]
- Vegh M, Roth HW, Hari‐Kovacs A & Facsko A (2015): Ocular symptoms and treatment of Ebola virus disease. Orv Hetil 156: 431–433. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo Q, Yan Z et al. (2018): Factors associated with prolonged viral shedding in patients with avian influenza A(H7N9) virus infection. J Infect Dis 217: 1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Tong J, Liu M & Shen Y & Guo D (2020): Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS‐CoV‐2 infection. J Med Virol. 10.1002/jmv.25725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Zhang Z, Jin L et al. (2005): Persistent shedding of viable SARS‐CoV in urine and stool of SARS patients during the convalescent phase. Eur J Clin Microbiol Infect Dis 24: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R et al. (2020): Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]


