1. INTRODUCTION
World Health Organization declared on 11 March, COVID‐19 as a pandemic infection that has spread rapidly across the globe. COVID‐19 currently has no known treatment or a vaccine. Oral health professionals are at risk of developing COVID‐19 infection as they come into close contact with patients and accompanying relatives who may be carrying the virus. Since January 2020, and by the end of April this year, more than 150 medical doctors in Italy and over 100 National Health Service workers in the UK have died in the COVID‐19 crisis. While the transmission of COVID‐19 via human exhaled droplets and direct contact is clear, the potential for aerosol transmission is a significant risk particularly for dental practices. The objective of this brief review is to highlight ways how dental manpower could protect from the spread of disease.
2. RECOGNISING COVID‐19 SYMPTOMATOLOGY TO SPOT THE DISEASE
Dentists will be called to see patients with emergency oral and dental problems of unknown COVID status. Attending on a COVID‐19 infected patient has the potential for direct disease transmission and for contamination in the dental practice environment. For oral health professionals and all in the dental team, it is important to recognise the full list of symptoms of COVID‐19, as it presents now: fever, cough, shortness of breath or difficulty breathing, chills, repeated shaking with chills, muscle pain, headache, sore throat, conjunctival hyperaemia, and new loss of taste or smell (Vetter et al., 2020). Any of these symptoms may appear anytime from 2 to 14 days after exposure to the virus. The most common symptoms of COVID‐19 are fever, dry cough and tiredness. According to the WHO, some patients may have aches and pains, nasal congestion or diarrhoea. Knowledge of these symptoms would help the dental surgery staff to triage patients when it comes to spotting COVID‐19‐infected persons. A history of international travel was important but following lockdown of airports is now irrelevant.
Patient recognition is complicated as these symptoms are initially mild and begin gradually. Some infected people may only have very mild symptoms, and some might not even show symptoms at all. As a result, asymptomatic transmission is likely.
Though infection in children from COVID‐19 is less common, with only a “handful of deaths” reported so far, a mysterious new coronavirus‐related rare condition among young children is also reported. The presentation of a sick child with a persistent red rash covering arms and legs, dry, cracked lips, lumps on tongue or a red, erythematous tongue has suggested a link between the Kawasaki‐like disease and coronavirus. Also referred to as Paediatric multisystem inflammatory syndrome in children doesn't show the hallmarks of coronavirus; it presents with symptoms similar to Kawasaki disease and toxic shock syndrome.
Of significance is that some adult patients may present in a dental practice reporting of loss of taste. In a case series of patients hospitalised in Italy, the authors reported taste disorders in 10% of cases (dysgeusia 8.5% and ageusia 1.7%) and mixed taste and olfactory disorders in another 20% of patients (Giacomelli et al., 2020). A young child may be brought in with a red tongue as a primary symptom of COVID‐19.
In most cases, individuals are usually considered infectious while they have symptoms; how infectious individuals are, depends on the severity of their symptoms and stage of their illness. However, it is now widely recognised of possible infectivity prior to the onset of symptoms. Virus shedding, as detected in the mouth or nose, could be present prior to onset of symptoms. A PCR test can determine whether the tested person is infected with SARS‐CoV‐2 and considered to be able to transmit the disease (a positive test) or is negative for the virus.
3. MANAGEMENT OF EMERGENCIES
Patients seeking emergency care could be assessed by telephone and/or video in order to triage the patients’ initial complaint. Many patients with oral problems can be managed with advice and treatment with analgesia and antibiotics. A follow‐up telephone/video review within 3–7 days should be arranged for reassurance. However, a patient may need a dental visit for an emergency extraction, incision and drainage of a dental abscess, or for urgent visual oral examination for symptoms suspicious of oral cancer (e.g. a non‐healing oral ulcer). When planning emergency treatment, the dentists should avoid or minimise operations that can produce droplets or aerosols (Meng, Hua, & Bian, 2020). Management of dental emergencies during COVID‐19 lockdown is beyond the scope of this publication and dentists would find guidelines circulated by NHS Education for Scotland useful (www.sdcep.org.uk). Oral diseases have a significant interplay with systemic health especially among the elderly (Jin et al., 2015). When managing the elderly, who may be in self‐isolation during the COVID outbreak a consultation with the patient's GP would be desirable before prescribing over the phone.
To minimise spread and protect staff, initial risk assessment for COVID status where possible should take place by phone, before making an appointment to visit the surgery. When booking emergency dental appointments, having a checklist of COVID‐19 symptoms at the reception may help to inform the dentist about symptomatic patients, to postpone non‐urgent therapies and to direct such patients to hospital centres equipped for handling infected subjects and their relatives, living together.
Patients may be reluctant to discuss symptoms related to oral cancer and may postpone visiting a dentist during the coronavirus outbreak. Arduino, Conrotto, and Broccoletti, (2020) in this journal have recently highlighted the issue of missed diagnoses of oral cancer that may later present in advanced stages. Care should be taken not to miss oral malignancies due to surgery closures.
4. SOURCES OF INFECTION
According to current evidence, COVID‐19 virus is primarily transmitted between people through respiratory droplets and contact routes. Dental surgery environments and procedures convey higher risks of transmission. An oral examination can generate an aerosol. Aerosol generating procedures (AGP) present risk of aerosolised transmission and high‐speed drilling and ultrasonic scalers are particularly considered AGPs. Other AGPs include extractions, incision and drainage of a dental abscess.
Coronaviruses can survive on surfaces (inanimate objects) and can remain viable for 3–5 days, dependent on the surface type, worst being plastics. Fortunately, the infectivity decreases with time.
Dental patients could have a strong urge to spit after a procedure. COVID‐19 has been found in infected saliva (To et al., 2020); patient spitting could enhance the spread of the COVID‐19 within the dental premises.
5. PROTECTION: HAND WASHING AND PERSONAL PROTECTIVE EQUIPMENT (PPE)
The same precautions should apply for all patients regardless of case status (positive, carrier or negative) during the period of sustained COVID‐19 transmission. Hand hygiene should be practised and extended to exposed forearms. It is important to carry out hand hygiene after each patient contact. The appropriate use of personal protective equipment (PPE) will protect the dentist and staff from contamination in most circumstances. The PPE to be used in dental practices should be in line with the national recommendations given by the government Chief Dental Officer or the professional organisation to which the dentist may belong to. For undertaking any direct patient care, dentists and dental surgery assistants and other oral health professionals are advised to wear, disposable gloves, aprons, eye protection and face shields where there is a risk of saliva, blood, other body fluids, secretions or excretions splashing into the face and eyes. Sessional use of some PPE, other than hand gloves, may be rational.
6. DISINFECTION AND DECONTAMINATION
Challacombe, Kirk‐Bayley, Sunkaraneni, and Combes (2020) propose the use of 10 ml of povidone iodine (PVP‐I) 0.5% solution ‐ an effective virucide‐ applied as a mouth rinse for all patients (in those without contraindications to its use eg. history of allergy to PVP, thyroid disease etc) requiring dental treatment during the current COVID‐19 pandemic, just prior to treatment. Decontamination of equipment and the care environment must be performed after each patient and should be carried out as per practice protocols. Decontamination of all areas of the practice including the toilets can effectively limit the concentration of SARS‐CoV‐2 RNA in aerosols (Liu et al., 2020). It is generally advisable to avoid the use of fans that re‐circulate the air. All non‐essential items including toys, books and magazines should be removed from reception and waiting areas.
Careful and thorough decontamination practice of laboratory items (e.g. impressions, prostheses) remains the responsibility of dental practices before any such items are dispatched to dental laboratories, in order to prevent all types of cross‐infection.
7. BCG VACCINATION AS A MEANS OF PROTECTION
Demographic data on the current COVID‐19 pandemic suggest that the disease incidence and observed fatality proportion of cases vary widely in different parts of the world. Bacillus Calmette–Guérin (BCG) vaccination status has been implicated as providing some immune protection to vaccinated communities. BCG utilises a weakened strain of Mycobacterium bovis to vaccinate against tuberculosis. Many countries began BCG vaccination programmes in the 1940s, and in particular, the Commonwealth countries have continued this public health practice to vaccinate babies at birth or at school entry. Between 1980 and 2007 several countries have ceased their BCG vaccination campaigns notably in Europe, Spain, Denmark Austria, Germany, the Isle of Man, Slovenia, UK, Finland and France, and some countries have not had a universal BCG vaccination policy, for example Italy, USA, Lebanon, the Netherland and Belgium. A global map (Figure 1) displaying BCG vaccination policy by country has been published (Zwerling et al., 2011; Zwerling & Pai, 2011). Using European data, Hegarty, Sfakianos, Giannarini, DiNardo, and Kamat (2020) and global data Miller et al. (2020) have shown considerable overlap with the map of countries with and without a national programme of BCG vaccination and the incidence of COVID‐19 infection. They speculate that countries with BCG vaccination programmes have far fewer coronavirus cases (by a factor 10), compared to where BCG programmes are no longer deployed. For example, Italy, where the COVID‐19 mortality is very high, never implemented universal BCG vaccination. Portugal which had an effective BCG vaccination policy demonstrated a low incidence of COVID‐19 and fewer deaths, than neighbouring Spain.
Figure 1.
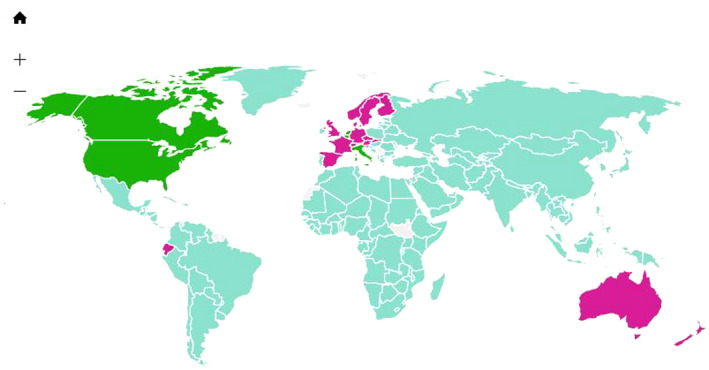
Map displaying BCG vaccination policy by country. Blue: Countries currently has universal BCG vaccination program. Purple: Countries used to recommend BCG vaccination for everyone, but currently does not. Green: Countries never had universal BCG vaccination programs. [ Reproduced from: PLoS Med 8(3): e1001012, with permission; Dr Madhukar Pai]
BCG vaccination has shown to stimulate the innate immune system to develop “memory,” termed trained immunity, which helps to eliminate various non‐mycobacterium infections including influenza (Covián et al., 2019).
In view of BCG vaccine's heterologous beneficial effect against non‐tuberculosis infections (Miller et al., 2020), the question has been raised “whether BCG may enhance one's immunity against COVID‐19.” There are two trials under way studying the effect of BCG vaccination to increase resistance to infections in the elderly population and to try and to help prevent severe COVID‐19 infection in healthcare workers: BRACE in Australia (https://clinicaltrials.gov/ct2/show/NCT04327206] and BCG‐CORONA in the Netherlands [https://clinicaltrials.gov/ct2/show/NCT04328441).
8. VACCINES FOR COVID‐19
A lot of pharma companies are working on vaccines and the development of those vaccines are at various stages. On 23rd April, WHO announced that seven vaccines have now been approved for human testing through clinical trials (World Health Organization, 2020). Of the seven, 3 are being tested in China, one in the UK, two in USA and one in Germany. The University of Oxford has taken the lead with a double‐blind RCT testing their ChAdOx1 nCoV‐19 vaccine, which uses an adenovirus vaccine vector and the SARS‐CoV‐2 spike protein. The trial vaccine is being tested on close to 1,100 healthy volunteers and using a vaccine against meningococcus as a control. A timeframe for vaccine development of 12 to 18 month is needed but it is believed that an effective candidate may be announced sooner. Equal distribution of the vaccine around the world would remain a challenge.
9. CONCLUSION
As means of protecting dental staff and patients, triaging patients over the telephone or video systems, room ventilation, appropriate PPE, sanitization of protective apparel is recommended. Proper use and disinfection of all areas of the practice including the toilets can effectively limit the concentration of SARS‐CoV‐2 RNA in aerosols (Liu et al., 2020). Relatives or friends accompanying patients to a dental practice should be restricted to essential visitors only, such as parents of paediatric patients. At patient reception areas, distancing measures should be carried out—ensuring a distance of two metres is kept. Dentists should keep up to date with and have regard to the latest advice from Government, the Health Service and the Chief Medical (Dental) Officer. The position may change daily and therefore it is important to check that advice regularly.
CONFLICT OF INTEREST
None.
Warnakulasuriya S. Protecting dental manpower from COVID‐19 infection. Oral Dis.2021;27(Suppl. 3):651–654. 10.1111/odi.13410
REFERENCES
- Arduino, P. G. , Conrotto, D. , & Broccoletti, R. (2020). The outbreak of Novel Coronavirus disease (COVID‐19) caused a worrying delay in the diagnosis of oral cancer in north‐west Italy: The Turin Metropolitan Area experience. Oral Diseases, 1–2. 10.1111/odi.13362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challacombe, S. J. , Kirk‐Bayley, J. , Sunkaraneni, V. S. , & Combes, J. (2020). Povidone iodine. British Dental Journal, 228(9), 656–657. 10.1038/s41415-020-1589-4 [DOI] [PubMed] [Google Scholar]
- Covián, C. , Fernández‐Fierro, A. , Retamal‐Díaz, A. , Díaz, F. E. , Vasquez, A. E. , Lay, M. K. , … Kalergis, A. M. (2019). BCG‐Induced cross‐protection and development of trained immunity: Implication for vaccine design. Frontiers in Immunology, 10, 2806. 10.3389/fimmu.2019.02806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli, A. , Pezzati, L. , Conti, F. , Bernacchia, D. , Siano, M. , Oreni, L. , … Gallii, M. (2020) Self‐reported olfactory and taste disorders in SARS‐CoV‐2 patients: A cross‐sectional study. Clinical Infectious Diseases. 10.1093/cid/ciaa330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty, P. K. , Sfakianos, J. P. , Giannarini, G. , DiNardo, A. R. , & Kamat, A. M. (2020). COVID‐19 and Bacillus Calmette‐Guérin: What is the Link? European Urology Oncology, 10.1016/j.euo.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, L. J. , Lamster, I. B. , Greenspan, J. S. , Pitts, N. B. , Scully, C. , & Warnakulasuriya, S. (2015). Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral Diseases, 22(7), 609–619. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Ning, Z. , Chen, Y. U. , Guo, M. , Liu, Y. , Gali, N. K. , … Lan, K. E. (2020). Aerodynamic analysis of SARS‐CoV‐2 in two Wuhan hospitals. Nature, 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- Meng, L. , Hua, F. , & Bian, Z. (2020). Coronavirus Disease 2019 (COVID‐19): Emerging and Future Challenges for Dental and Oral Medicine. Journal of Dental Research, 99(5), 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A. , Reandelar, M. J. , Fasciglione, K. , Roumenova, V. , Yan, L. I. , & Otazu, G. H. (2020). Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID‐19: an epidemiological study. Medicine, 10.1101/2020.03.24.20042937 [DOI] [Google Scholar]
- To, K.‐W. , Tsang, O.‐Y. , Yip, C.‐Y. , Chan, K.‐H. , Wu, T.‐C. , Chan, J.‐C. , … Yuen, K.‐Y. (2020). Consistent Detection of 2019 Novel Coronavirus in Saliva. Clinical Infectious Diseases, 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter, P. , Vu, D. L. , L’Huillier, A. G. , Schibler, M. , Kaiser, L. , & Jacquerioz, F. (2020). Clinical features of covid‐19. British Medical Journal, 369, m1470. 10.1136/bmj.m1470 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020). DRAFT landscape of COVID‐19 candidate vaccines – 23 April 2020. WHO. https://www.who.int/blueprint/priority‐diseases/key‐action/draft‐landscape‐COVID‐19‐candidatevaccines‐23‐April‐2020.pdf Accessed 1st May 2020. [Google Scholar]
- Zwerling, A. , Behr, M. A. , Verma, A. , Brewer, T. F. , Menzies, D. , & Pai, M. (2011). The BCG World Atlas: A database of global BCG vaccination policies and practices. PLoS Medicine, 8(3), e1001012. 10.1371/journal.pmed.1001012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerling, A. , & Pai, M. (2011). The BCG world atlas: a new, open‐access resource for clinicians and researchers. Expert Review of Anti‐infective Therapy, 9(8), 559–561. 10.1586/eri.11.71 [DOI] [PubMed] [Google Scholar]


