Abstract
Background
Different skin manifestations of COVID‐19 are being reported. Acral lesions on the hands and feet, closely resembling chilblains, have been recognized during the peak incidence of the COVID‐19 pandemic.
Material and methods
A retrospective review of 22 children and adolescents with chilblain‐like lesions seen over a short period of time in the Emergency Department of a children's hospital during the peak incidence of COVID‐19 in Madrid, Spain.
Results
All patients had lesions clinically consistent with chilblains of the toes or feet, with three also having lesions of the fingers. Pruritus and mild pain were the only skin symptoms elicited, and only 10 had mild respiratory and/or GI symptoms. None had fever. Coagulation tests, hemogram, serum chemistry, and lupus anticoagulant were normal in all patients tested. One out of 16 tested cases had elevated D‐dimer results, but without systemic symptoms or other laboratory anomalies. SARS‐CoV‐2 PCR tested in 19 cases was positive in just one case. Skin biopsies obtained in six patients were consistent with chilblains. On follow‐up, all cases showed spontaneous marked improvement or complete healing.
Conclusion
Acute chilblains were observed during COVID‐19 pandemic in children and teenagers. It is a mildly symptomatic condition with an excellent prognosis, usually requiring no therapy. Etiopathogenesis remains unknown.
Keywords: acral ischemia, chilblain, COVID‐19, dermoscopy, pernio, SARS‐CoV‐2, skin
1. INTRODUCTION
Since the beginning of the coronavirus disease 2019 (COVID‐19) pandemic, scattered reports have described different skin manifestations in infected patients, including urticarial, morbilliform, vesicular, and petechial exanthems, as well as vasospastic manifestations such as livedo reticularis and acral ischemic lesions. 1 , 2 , 3 , 4 , 5 Acral ischemia was initially described in adult patients with severe disease featuring extensive thrombotic events, usually in intensive care units with hypercoagulability and elevated D‐dimer levels. 6 Skin lesions on the toes, feet, fingers, and hands identical to chilblains were later reported in a few adolescents and young adults. 7 Since then, rapid spread through the dermatological and social networks in Spain, throughout Europe and the United States, has revealed hundreds of cases of chilblains occurring during the COVID‐19 pandemic.
We present a series of 22 cases of chilblains in children and adolescents in the setting of COVID‐19 seen in a very short period of time in the Emergency Department of a tertiary children's hospital in Madrid.
2. MATERIAL AND METHODS
A retrospective study was conducted in children and adolescents (up to 18 years of age) presenting to the Emergency Department in a 12‐day period (April 6‐17, 2020) with skin manifestations of chilblains, in the form of erythematous to purpuric macules and violaceous swellings located on the toes, feet, fingers, and hands.
Approval from the institutional Ethics Committee and Board was obtained. Standard informed consents were obtained for recording images in all patients and for skin biopsies when considered.
We recorded age, sex, personal history of previous diseases, contacts with potentially infected relatives, skin symptoms, type and location of lesions, dermoscopy, systemic symptoms, and therapies administered. Laboratory analyses performed were also recorded, as well SARS‐CoV‐2 PCR from oropharyngeal and nasopharyngeal swabs.
Skin biopsies obtained were processed for light microscopy and stained with hematoxylin and eosin, PAS stain, and colloidal iron.
3. RESULTS
Twenty‐two patients (13 male and 9 female), age range 6‐17 years (median 12 years), were seen in the Emergency and Dermatology Departments of the Children's University Hospital Niño Jesús in Madrid, Spain, with acrally located erythemato‐purpuric lesions consistent with chilblains (Table 1). Duration of lesions before consultation ranged from 1 to 28 days (median 7 days).
TABLE 1.
Summary of clinical data of 22 patients
| Number (%) | |
|---|---|
| Gender | |
| Male | 13 (59) |
| Female | 9 (41) |
| Age (y) | |
| 6‐9 | 1 (4) |
| 10‐13 | 13 (59) |
| 14‐17 | 8 (36) |
| History of Raynaud phenomenon | |
| Yes | 0 (0) |
| No | 22 (100) |
| Duration of cutaneous symptoms before coming to the ER (d) | |
| 1‐7 | 12 (55) |
| 8‐14 | 6 (27) |
| >14 | 4 (18) |
| Site of involvement | |
| Feet | 22 (100) |
| Hands | 3 (14) |
| Concomitant erythema multiforme | |
| Yes | 4 (18) |
| No | 18 (82) |
| Symptoms | |
| Cutaneous symptoms | |
| Local pruritus | 9 (41) |
| Local pain or tenderness | 7 (31) |
| Systemic symptoms | |
| Respiratory symptoms (cough or rhinorrhea) | 9 (41) |
| GI symptoms (abdominal pain or diarrhea) | 2 (9) |
| Shortness of breath | 0 (0) |
| Fever | 0 (0) |
| Time lapse from systemic symptoms to onset of chilblain (n = 10) | |
| 0‐7 d | 4 (40) |
| 7‐14 d | 2 (20) |
| 14‐28 d | 4 (40) |
| Epidemiologic background | |
| Household contact with a probable case of COVID‐19 | 12 (55) |
| Household contact with a confirmed case of COVID‐19 | 1 (4) |
| Laboratory tests | |
| Coagulation tests done (PT, aPTT, fibrinogen) | 18 (82) |
| Normal | 18 |
| Abnormal | 0 |
| D‐dimer levels done | 16 (73) |
| Normal | 15 |
| Abnormal | 1 |
| Hemogram done | 10 (45) |
| Normal | 10 |
| Abnormal | 0 |
| Serum chemistry done (LDH, ALT, AST) | 4 (18) |
| Normal | 4 |
| Abnormal | 0 |
| SARS‐CoV‐2 RT‐PCR test done | 19 (86) |
| Positive | 1 |
| Negative | 18 |
| Follow‐up | |
| Phone call | 22 (100) |
| Office visit | 21(95) |
| Skin biopsy obtained | 6 (27) |
| Outcome | |
| Improvement and/or healing | 22 (100) |
| Worsening | 0 (0) |
No patient had a history of rheumatic disease, lupus erythematosus, Raynaud's phenomenon, acrocyanosis, or previous history of chilblains. Five of them had a diagnosis of attention deficit hyperactivity disorder (ADHD) and were on treatment with methylphenidate hydrochloride (three cases), methylphenidate hydrochloride, aripiprazole, and intuniv (one case) and lisdexamfetamine (one case) for more than 1 year, with no change in dosage within the last 6 months.
Feet were affected in all 22 cases (Figure 1). The typical lesions consisted of acrally located, erythemato‐violaceous or purpuric macules on the toes and lateral aspects of the feet and heels. The tips and periungual or distal subungual areas of the toes were commonly involved. In other instances, patients showed swollen toes with dusky, violaceous discoloration. Less frequently, dark ischemic areas with superficial blisters were seen. As lesions evolved, skin hyperpigmentation was observed in follow‐up visits. Three patients also showed similar lesions on the fingers, located predominantly on the periungual areas. Dermoscopy was recorded in 10 patients, and the signs observed (Figure 2) included violaceous erythema, dilated capillaries, ischemic areas, purpuric dots, and hyperpigmentation.
FIGURE 1.
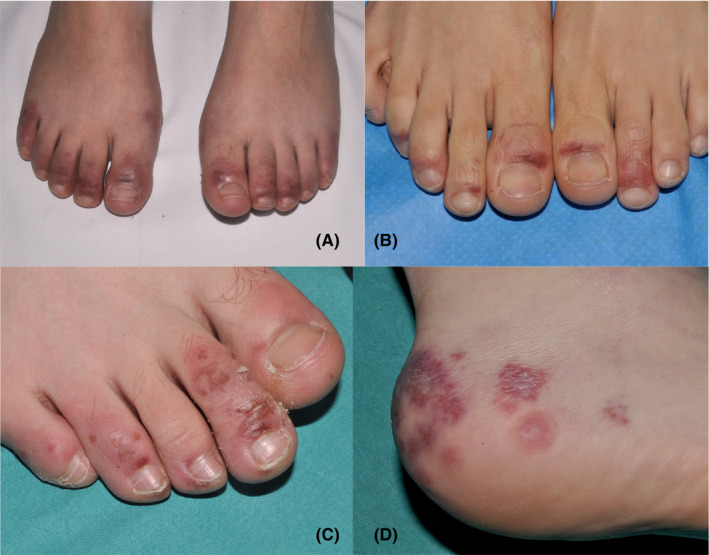
A‐D, Clinical spectrum of chilblains in four patients in the series
FIGURE 2.
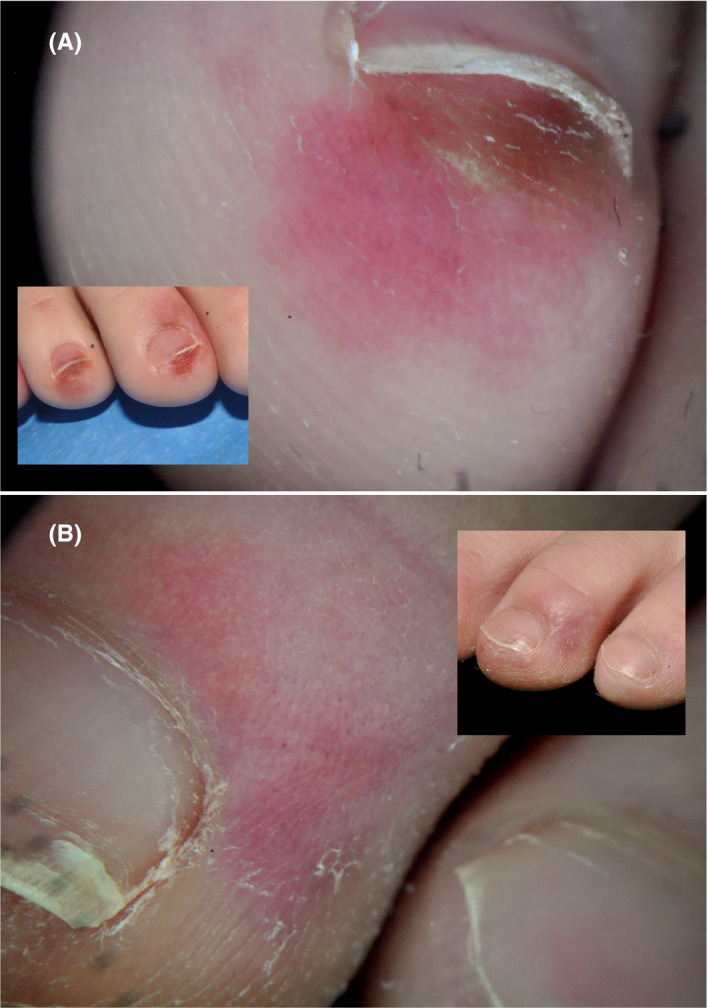
Dermoscopic features. A, Violaceous erythema, purpuric dots, and subungual hyperpigmentation. B, Erythema, vasodilatation, and purpuric dots
Pruritus (9, 41%) and mild pain (7, 32%) were present in some cases. Systemic symptoms appeared in 10 patients; they consisted of mild respiratory symptoms (cough and rhinorrhea) in nine patients (41%) and gastrointestinal complaints (abdominal pain and diarrhea) in two patients (9%); one patient presented with both respiratory and gastrointestinal symptoms. These symptoms had appeared 1‐28 days before the onset of chilblains (median 14 days). None presented with body temperature over 38.3°C during the course of the disease. Four patients had concomitant skin manifestations that were diagnosed as erythema multiforme.
Regarding epidemiologic data, one patient had household contact with a single confirmed case (positive PCR) of COVID‐19. Twelve cases recalled household contact with probable cases of COVID‐19 with respiratory symptoms (WHO definition for non‐PCR‐tested patients). No chilblains were reported in household contacts, but two of the patients in the series were brothers.
Coagulation studies were normal in 18 patients tested. D‐dimer levels were obtained in serum in 16 cases and were elevated only in one case (900 ng/mL; normal <500 ng/mL), but this abnormal result was believed to have no clinical significance. This patient was in good health and had no systemic symptoms; other coagulation tests were normal, and his lesions had similar evolution and outcome to others. Hemogram (10 cases), serum chemistry (four cases), and lupus anticoagulant (one case) were within normal limits in all patients tested. Oropharyngeal and nasopharyngeal PCR test for SARS‐CoV‐2 was performed in 19 cases and was positive in just one case. This patient did not recall any close contact with confirmed or possible cases of COVID‐19. Also, she had mild GI symptoms 2 days before chilblains, her other laboratory studies were normal, and she had a good outcome with almost complete resolution of her skin lesions in her last visit.
A skin biopsy was obtained from the acral lesions (four from the feet, two from the toes) in six patients (Figure 3). All showed similar results, with variable degree of intensity. Superficial and deep angiocentric and eccrinotropic lymphocytic infiltrate, papillary dermal edema, vacuolar degeneration of the basal layer and lymphocytic exocytosis to the epidermis and acrosyringia were found. Features of lymphocytic vasculopathy were seen in all of them. Some cases showed mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation, and focal thrombosis mostly confined to scattered papillary dermal capillaries, but also in vessels of the reticular dermis.
FIGURE 3.
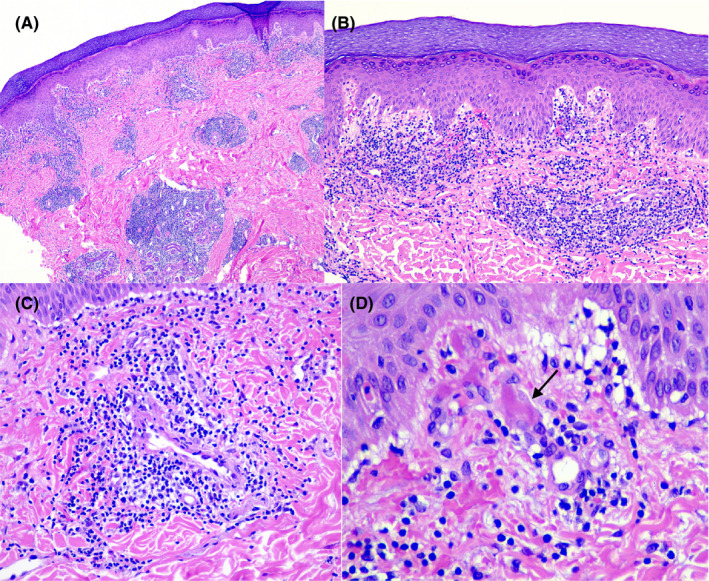
A, Dense, superficial, and deep angiocentric and eccrinotropic lymphocytic infiltrate (H&E stain, 10×). B, Papillary dermal edema, vacuolar degeneration of the basal layer and lymphocytic exocytosis. Endothelia of small vessels appear swollen (H&E stain, 20×). C, Intense lymphocytic vascular reaction in dermal vessels (H&E stain, 40×). D, Red cell extravasation and focal thrombosis (arrow) in papillary dermis capillaries (H&E stain, 100×)
Oral analgesics for pain relief and oral antihistamines for pruritus were the only treatments administered when needed. Topical corticosteroids were prescribed for one patient and a short course of oral steroids for another case, both for associated erythema multiforme.
All cases were first seen in the emergency department, and 21 of them were seen in the dermatology clinic 1‐10 days after their initial visit. Then, all 22 cases were contacted on the telephone, except for patients biopsied, who were seen again 7 days later. The lesions showed marked improvement or almost complete resolution 3‐5 weeks after their onset.
4. DISCUSSION
We present a series of 22 children and adolescents with lesions that are clinically and histologically characteristic of chilblains. Chilblains are uncommon in children, and these patients presented in a short period of time and under warm weather conditions (average maximum 16.13°C, average minimal 9.73°C). None of them had any predisposing condition (such as lupus erythematosus, Raynaud's phenomenon, or acrocyanosis) or previous history of chilblains. This accumulation of cases coincides with the peak of incidence of COVID‐19 in Madrid (cumulated incidence by April 6, 2020, of 422.43 confirmed cases per 100 000 habitants) and the occurrence of many similar cases seen by physicians in Spain and other heavily affected countries. It is thus most likely that these lesions are related to the COVID‐19 pandemic.
Epidemiologic features that link chilblains with COVID‐19 in our series should be interpreted with caution. In 59% of cases, a history of close contact with a symptomatic adult in the family was elicited, and all of these had a mild or moderate disease course. In 55%, chilblains were the only manifestation present, and 45% had mild symptoms that could be related to COVID‐19. In these, chilblains were noted after a mean of 16 days from the first symptoms. Only one of 19 tested patients was PCR positive for SARS‐CoV‐2. It is likely that test sensitivity is lower in mild cases and in children, possibly because of a low viral load. Considering that PCR is positive in only 11.2% of children requiring hospital admission for suspected COVID‐19, 8 it is not surprising that only one in 19 tests in our series gave a positive result. Alternatively, the appearance of chilblains could have been a late event in the disease course, and PCR would have turned negative by the time it was tested.
All patients had an excellent outcome, without complications or severe disease manifestations. The lesions started to fade after 7‐10 days, until they eventually disappeared or markedly improved. Mild symptoms such as pruritus or minimal pain were frequent (72% of cases), requiring only symptomatic treatment. No specific treatment for chilblains was indicated, and hence resolution of lesions was considered purely spontaneous.
The skin lesions in our patients were unequivocally categorized as chilblains, both clinically and histopathologically. 9 , 10 , 11 Dermoscopic features of chilblains have not been described to our knowledge; the most relevant dermoscopic features of our patients were violaceous erythema, ischemic areas, dilated capillaries, purpuric dots, and, in late phases, pigmented dots.
Chilblains, also called pernio, is a cutaneous localized inflammatory reaction resulting from a maladaptive vascular response to non‐freezing cold. 9 It is most common in women and middle‐aged adults and seems to be uncommon in children. 9 , 12 , 13 , 14 Chilblains may be classified as primary (idiopathic, cold‐related) or secondary to an underlying condition, including (a) connective tissue disease (lupus erythematosus, Behçet disease, antiphospholipid syndrome, rheumatoid arthritis, Sjögren syndrome); (b) cryopathies (cryoglobulinemia, cryofibrinogenemia, cold agglutinin disease); (c) hemoproliferative or neoplastic disease (leukemia, metastatic breast carcinoma); (d) blood hyperviscosity (macroglobulinemia); (e) genetic disease (genetic interferonopathies such as familial chilblain lupus, STING‐associated vasculopathy of infantile onset [SAVI], Aicardi‐Goutières syndrome, and IRAK4 deficiency); and (f) anorexia and diseases causing weight reduction. 10 , 15 , 16
None of these possible etiologies were present in our patients, not even cold exposure, and given the timing, SARS‐CoV‐2 is felt to be the most likely etiology of chilblains in these patients. Five of our patients were receiving medications for ADHD, and these have been linked to blue toe syndrome and cyanotic peripheral vasculopathy. 17 , 18 However, these drug‐related conditions run a chronic course and appear at onset or after dose increase of the medications. Our patients were taking these medications for more that 6 months at the same dosage, and all had an acute course of chilblains. A history of a viral illness may occasionally precede chilblains, 10 and cryoproteins, which may be produced after viral illnesses, were detected in four out of the eight cases in a pediatric series over 10 years in Colorado (three with cryoglobulins and one with cold agglutinins). 14 However, cryoproteins are very seldom detected in cases of chilblains. 10 Considering the very high incidence of viral illnesses in children, the incidence of virus‐induced chilblains has historically been very low, which is in sharp contrast to the large number of cases collected in our institution in such a short period of time.
Severe acral ischemic lesions due to thrombosis have been observed in COVID‐19 patients, usually admitted to intensive care units with very severe disease. 6 These patients have a hypercoagulable state and very elevated D‐dimer levels. This skin manifestation of COVID‐19 should not be confused with the mildly symptomatic chilblains seen in the children in this series, though it is possible that they may represent different points on the severity spectrum.
Chilblains may be a manifestation of lupus erythematosus (LE), a condition named chilblain LE. Perniotic lesions in chilblain LE are histopathologically identical to idiopathic chilblains, 19 although some features such as the presence of perieccrine infiltrate and the absence of dermal mucin are more indicative of idiopathic chilblains. However, most cases of idiopathic chilblains have an acute course, and perniotic lesions in LE are chronic, not related to temperature and are associated with clinical or laboratory anomalies of LE. 9 , 20 Familial chilblain LE is an autoinflammatory interferonopathy associated with a TREX1 mutation; other related interferonopahies such as Aicardi‐Goutières syndrome and SAVI may show skin features indistinguishable from chilblains LE. 21 , 22 Chronic chilblain‐like lesions in interferonopathies are most likely the result of chronic release of interferon. Because COVID‐19 is a potent trigger of the expression of type 1 interferon, 23 a link with chilblains has been hypothesized. 24 The acute and self‐healing nature of COVID‐19‐related chilblains, the rarity of chilblains in children and teenagers with other viral illnesses with similar interferon release, the absence of chilblains in patients treated with recombinant interferons, and the absence of other interferon‐related symptoms in most children with COVID‐19‐related chilblains argue against this hypothesis.
The pathophysiology of acute idiopathic chilblains is unknown. Persistent or prolonged cold‐induced vasospasm has been proposed to lead to hypoxemia and a subsequent secondary inflammatory response. 9 , 25 Also, neurovascular instability with inappropriate neural responses to temperature has been suggested for chilblains, 26 and neuropathy has been reported in patients with COVID‐19. 27 The pathophysiology of acute chilblain in COVID‐19 is unknown, and cold is not a trigger in our cases. A direct effect of the virus on the endothelia and the role of immune complexes or autoantibodies remain to be studied.
In conclusion, acute chilblains are a newly recognized manifestation of COVID‐19 in children and teenagers. It is a mildly symptomatic condition with an excellent prognosis. Most patients are negative on PCR testing, and all have an asymptomatic or minimally symptomatic COVID‐19 disease course as expected for this age group. Blood samples have been stored in order to perform reliable serologic tests, when available, to these patients.
Andina D, Noguera‐Morel L, Bascuas‐Arribas M, et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol. 2020;37:406–411. 10.1111/pde.14215
REFERENCES
- 1. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16387 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2. Mahé A, Birckel E, Krieger S, Merklen C, Bottlaender L. A distinctive skin rash associated with Coronavirus Disease 2019? J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16471 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;S0190‐9622(20)30657‐5. 10.1016/j.jaad.2020.04.044 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16472 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID‐19: transient livedo reticularis. J Am Acad Dermatol. 2020;S0190‐9622(20)30558‐2. 10.1016/j.jaad.2020.04.018 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. [DOI] [PubMed] [Google Scholar]
- 7. Mazzotta F, Troccoli T. Acute acro‐ischemia in the child at the time of COVID‐19. Eur J Pediatr Dermatol. 2020. https://www.ejpd.com/images/acroischemia‐ENG.pdf. Accessed April 22, 2020. Ahead of print. [Google Scholar]
- 8. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain. JAMA Pediatr. 2020;e201346. 10.1001/jamapediatrics.2020.1346. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nyssen A, Benhadou F, Magnée M, André J, Koopmansch C, Wautrecht JC. Chilblains. Vasa. 2020;49(2):133‐140. [DOI] [PubMed] [Google Scholar]
- 10. Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89(2):207‐215. [DOI] [PubMed] [Google Scholar]
- 11. Cribier B, Djeridi N, Peltre B, Grosshans E. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45(6):924‐929. [DOI] [PubMed] [Google Scholar]
- 12. Simon TD, Soep JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116(3):e472‐e475. [DOI] [PubMed] [Google Scholar]
- 13. Kearby R, Bowyer S, Sharrer J, Sharathkumar A. Case report: six‐year‐old girl with recurrent episodes of blue toes. Clin Pediatr (Phila). 2010;49(5):495‐498. [DOI] [PubMed] [Google Scholar]
- 14. Weston WL, Morelli JG. Childhood pernio and cryoproteins. Pediatr Dermatol. 2000;17(2):97‐99. [DOI] [PubMed] [Google Scholar]
- 15. Lutz V, Cribier B, Lipsker D. Chilblains and antiphospholipid antibodies: report of four cases and review of the literature. Br J Dermatol. 2010;163(6):645‐646. [DOI] [PubMed] [Google Scholar]
- 16. Gurung P, Lee ASW, Armon K, Millington GWM. Chilblains accompanying interleukin‐1 receptor‐associated kinase (IRAK)‐4 deficiency. Clin Exp Dermatol. 2018;43(5):596‐597. [DOI] [PubMed] [Google Scholar]
- 17. Al Aboud A, Abrams M, Mancini AJ. Blue toes after stimulant therapy for pediatric attention deficit hyperactivity disorder. J Am Acad Dermatol. 2011;64(6):1218‐1219. [DOI] [PubMed] [Google Scholar]
- 18. Syed RH, Moore TL. Methylphenidate and dextroamphetamine‐induced peripheral vasculopathy. J Clin Rheumatol. 2008;14(1):30‐33. [DOI] [PubMed] [Google Scholar]
- 19. Wang ML, Chan MP. Comparative analysis of chilblain lupus erythematosus and idiopathic perniosis: histopathologic features and immunohistochemistry for CD123 and CD30. Am J Dermatopathol. 2018;40(4):265‐271. [DOI] [PubMed] [Google Scholar]
- 20. Viguier M, Pinquier L, Cavelier‐Balloy B, et al. Clinical and histopathologic feature sand immunologic variables in patients with severe chilblains. A study of the relationship to lupus erythematosus. Medicine (Baltimore). 2001;80(3):180‐188. [DOI] [PubMed] [Google Scholar]
- 21. Kolivras A, Aeby A, Crow YJ, Rice GI, Sass U, André J. Cutaneous histopathological findings of Aicardi‐Goutières syndrome, overlap with chilblain lupus. J Cutan Pathol. 2008;35:774‐778. [DOI] [PubMed] [Google Scholar]
- 22. Kim H, Sanchez GA, Goldbach‐Mansky R. Insights from mendelian interferonopathies: comparison of CANDLE, SAVI with AGS, monogenic lupus. J Mol Med (Berl). 2016;94(10):1111‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou Z, Ren L, Zhang L, et al. Overly exuberant innate immune response to SARS‐CoV‐2 infection. SSRN Electron J. 2020. 10.2139/ssrn.3551623 Epub ahead of print. [DOI] [Google Scholar]
- 24. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020. 10.1016/j.jdcr.2020.04.011 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shahi V, Wetter DA, Cappel JA, Davis MD, Spittell PC. Vasospasm is a consistent finding in pernio (chilblains) and a possible clue to pathogenesis. Dermatology. 2015;231(3):274‐279. [DOI] [PubMed] [Google Scholar]
- 26. George R, Fulchiero GJ Jr, Marks JG Jr, Clarke JT. Neurovascular instability syndrome: a unifying term to describe the coexistence of temperature‐related vascular disorders in affected patients. Arch Dermatol. 2007;143(2):274‐275. [DOI] [PubMed] [Google Scholar]
- 27. Gutiérrez‐Ortiz C, Méndez A, Rodrigo‐Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID‐19. Neurology. 2020;10.1212/ WNL.0000000000009619. 10.1212/WNL.0000000000009619 Epub ahead of print. [DOI] [PubMed] [Google Scholar]


