Dear Editor,
Novel coronavirus 2019 (SARS‐CoV2) pandemic has particularly affected Italy, with a profound impact on the therapeutic strategy for complex disorder such as psoriasis, whose extensive skin damage might expose to an increased infective risk compared to the general population. 1 , 2 , 3 , 4 Psoriasis treatment relies on immunosuppression, and although most experts agree that the benefit‐to risk‐ratio is in favour of maintaining selective biological therapies, and small molecules such as apremilast, they recommend dismission if severe COVID‐19 symptoms occur. 5 , 6
We report a patient with erythrodermic psoriasis, with contraindication to most treatments because of a recurrent brain oligodendroglioma, who was under apremilast therapy while contracting SARS‐CoV‐2 pneumonia. The 45‐year‐old obese (BMI 36.33) white man, with a decennial history of severe psoriasis and arthritis, treated with all traditional and biological drugs had to start chemotherapy with temozolomide, for the brain oligodendroglioma not operable recurrence. Its psoriasis had worsened to erythroderma (Fig. 1), only partially controlled by prednisone 50 mg/day, and in agreement with the neuro‐oncologist, apremilast 30 mg orally twice a day was started. The patient gradually improved, allowing prednisone tapering to a minimal dose of 12.5 mg daily. On February 19, 2020, he was enough stabilized to travel to Milan, Northern Italy, to be evaluated for brain radiotherapy. On February 26, he developed a severe cough with high fever (TC 40°C) and a chest X‐Ray revealed bilateral interstitial pneumonia with positive swab to SARS CoV2. After referral to the Infective Disease Unit, the patient started treatment with Lopinavir/Ritonavir400/100 mg twice a day and intravenous Ceftriaxone 2 g/day. He was discharged on March 3, clinically healed after two consecutive negative SARS‐CoV‐2 swabs. Apremilast had never been stopped during the COVID‐19 hospitalization, with acceptable control of the psoriasis, limited to mild scaling and erythema, especially on the trunk (Fig. 2).
Figure 1.

Severe erythrodermic psoriasis (PASI 45) before apremilast treatment.
Figure 2.
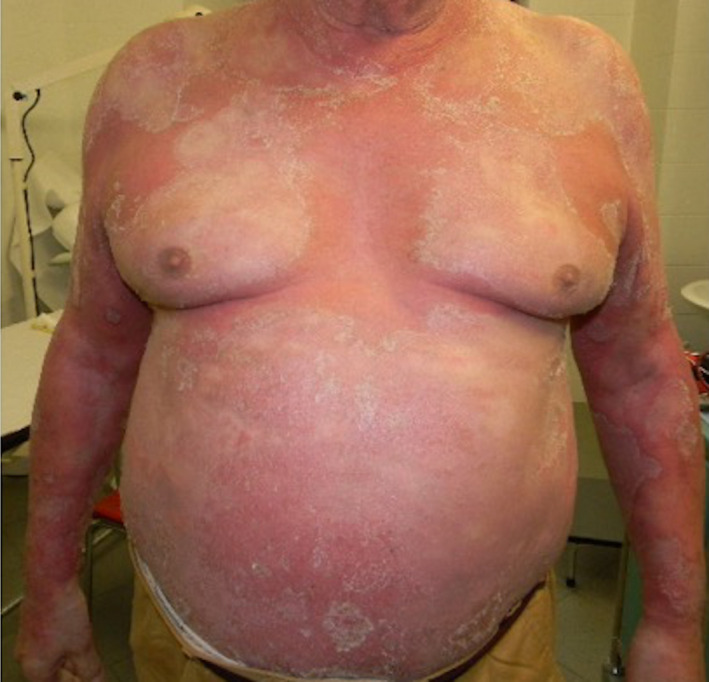
The patient improvement under apremilast treatment, after the COVID‐19 recovery.
The fact that patient with a severe form of psoriasis contracted the COVID‐19 pneumonia, while on treatment with apremilast is worth of some considerations. First of all, the information of apremilast safety, not interfering with the infection, as the drug was not interrupted during the whole course of the infection. Our patient had several risk factors for a worst outcome: obesity, recent chemotherapy, persistence of brain oligodendroglioma and viral contagion in a nosocomial setting. By converse, the infection recovered rapidly and the patient was discharged 6 days after the onset of symptoms. Apremilast has previously demonstrated a long‐term safety profile in the setting of serious infections such as HIV, HBV and HCV. 7 , 8 However, therapeutic strategy during severe COVID‐19 pneumonia are still in the process of definition, and it was quite surprising apremilast was maintained. We cannot rule out that apremilast anti‐inflammatory activity might have played a role in the rapid recovery. The selective inhibition of the enzyme phosphodiesterase 4 (PDE4) allows higher levels of cyclic AMP, which decreases the production of inflammatory cytokines such as tumour necrosis factor‐alpha (TNF‐α). 7 Interestingly, the efficacy of apremilast has been reported in acute lung injury caused by the anticancer proteasome inhibitor carfilzomib, characterized by an exaggerated inflammatory response. 9 Another experiment in mouse has documented an inhibitory effect of apremilast on the release of profibrotic cytokine from macrophages, including interleukin‐6. 10 During COVID19, pneumonia has been documented a ‘cytokines storm’, with markedly higher levels of IL‐6, and TNF‐α, suggesting the use of interleukin‐6 receptor blocker tocilizumab in severe cases. 11 Recently, another Italian psoriasis patient contracting COVID‐19 under IL‐23 inhibitor treatment (guselkumab) has been reported, and completely recovered from the infection. 12
From our experience, apremilast confirms its safety in very critical patients with severe infections, including COVID‐19. Its efficacy in our sub‐erythrodermic psoriasis was not completely satisfactory, but other treatments were contraindicated for the recurrent brain oligodendroglioma. Further studies are warrant to explore the intriguing immune modulating activities of this very manageable drug.
Acknowledgement
The patient in this manuscript has given written informed consent to the publication of his case details.
Funding source
none.
Copyright: The authors certify that the manuscript is original, never submitted to other journal for publication before. All authors contributed equally to the manuscript and had the opportunity to revise and approve the final text.
References
- 1. Gisondi P, Piaserico S, Conti A, Naldi L. Dermatologists and SARS‐CoV‐2: The impact of the pandemic on daily practice. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16515. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tartari F, Guglielmo A, Fuligni F, Pileri A. Changes in emergency service access after spread of COVID19 across Italy. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16553. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Radi G, Diotallevi F, Campanati A, Offidani A. Global coronavirus pandemic (2019‐nCOV): implication for an Italian medium size dermatological clinic of a II level hospital. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16386. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4. Atzori L, Mugheddu C, Addis G et al. Psoriasis health care in the time of the coronavirus pandemic: insights from dedicated centers in sardinia (Italy). J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16473. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Altobrando A, Patrizi A, Bardazzi F. Should SARS‐CoV‐2 influence immunosuppressive therapy for autoimmune blistering diseases? J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16491. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Price KN, Frew JW, Hsiao JL, Shi VY. COVID‐19 and Immunomodulator/ Immunosuppressant use in dermatology. J Am Acad Dermatol 82: e173–e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. Pharm Ther 2015; 40: 459–500. [PMC free article] [PubMed] [Google Scholar]
- 8. Sp R Shah VV, Wu Jj. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol 2017; 31: e481–e482. [DOI] [PubMed] [Google Scholar]
- 9. Imam F, Al‐Harbi NO, Al‐Harbi MM et al. Apremilast ameliorates carfilzomib‐induced pulmonary inflammation and vascular injuries. Int Immunopharmacol 2019; 66: 260–266. [DOI] [PubMed] [Google Scholar]
- 10. Maier C, Ramming A, Bergmann C et al. Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin‐6 from M2 macrophages. Ann Rheum Dis 2017; 76: 1133–1141. [DOI] [PubMed] [Google Scholar]
- 11. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and Interleukin‐6 receptor (IL‐6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents 2020: 105954. 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Messina F, Piaserico S. SARS‐CoV‐2 infection in a psoriatic patient treated with IL‐23 inhibitor. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16468. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


