To the Editor,
Since the beginning of the well‐known severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) pandemic, skin involvement both in adults and children has been signaled. 1 , 2 However, large‐scale studies describing skin manifestations and their etiopathogenic correlation with coronavirus disease 2019 (COVID‐19) in detail have not been reported yet. Through the analysis of emerging data from literature 3 and the direct observation of three patients with COVID‐19 (SARS‐CoV‐2 detection from nasopharyngeal swab samples through RNA extraction) and dermatological manifestations, we have hypothesized different mechanisms for their development.
CASE 1: A 55‐year‐old woman was admitted to the Infectious Diseases Department of the General Regional Hospital of Ancona for pyrexia, dry cough, and dyspnea. Upon admission, she had undergone nasopharyngeal swab for SARS‐CoV‐2 isolation, with positive laboratory report. The day before, she had performed a chest X‐ray showing a right parahilar pulmonary consolidation. At the admission, high‐resolution computed tomography scan of the chest revealed a diffuse bilateral ground‐glass opacity, then diagnosis of COVID‐19 interstitial pneumonia was made. Her comorbidities included obesity (BMI = 30.2) and hypertension, in treatment with Bisoprolol Fumarate 5 mg once a day.
Dermatological consultation was immediately requested for skin rash appeared 72 hours before hospital admission. It was therefore observed a generalized urticarial skin rash characterized by erythematous, smooth, slightly elevated papules and wheals, associated to severe pruritus. The patient did not report neither similar episodes in the past, nor allergies to drugs or foods. Furthermore, the patient had not taken any new medication before the rash appeared. Blood test revealed normal blood count (no lymphopenia or lymphocytosis or eosinophilia), slight increase of procalcitonin serum level (0.14 ng/mL), C‐reactive protein (CRP, 12.1 mg/dL), and liver enzymes (glutamic oxaloacetic transaminase [GOT], glutamate pyruvate transaminase [GPT], lactate dehydrogenase [LDH], gamma‐glutamyl ranspeptidase [GGT] fourfold levels). A systemic treatment with intravenous daily administration of betamethasone sodium phosphate 4 mg and chlorphenamine maleate 10 mg, in addition to antiviral therapy with lopinavir/ritonavir for pneumonia, was started. In the following days urticaria improved gradually (Figure 1). Twenty‐five days after entering into hospital, the patient was discharged for resolution of pneumonia and negative extraction of SARS‐CoV‐2 RNA from her nasopharyngeal swab in two consecutive times.
Figure 1.
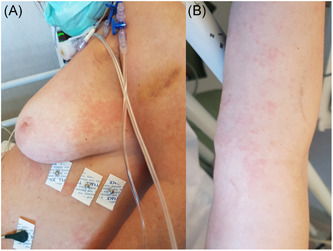
A and B, Urticarial rash in female patient on the way to recovery after bilateral interstitial pneumonia from COVID‐19. COVID‐19, coronavirus disease 2019
CASE 2: Dermatological consultation was requested from anesthesiology division because of the appearance of an urticarial rash in a 64‐year‐old patient with acute respiratory distress syndrome (PaO2/FiO2 ≤ 100 mm Hg) caused by COVID‐19 (Figure 2). Skin rash was already present at the time of hospital admission. As in previous clinical case, neither history of allergy to drugs or foods, nor recent intake of new drugs were reported into patient's medical record. Patient was, at that moment, in treatment with lopinavir/ritonavir and hydroxychloroquine from 1 week, and no new drug introduction had been made in the last 3 weeks before skin rash development.
Figure 2.
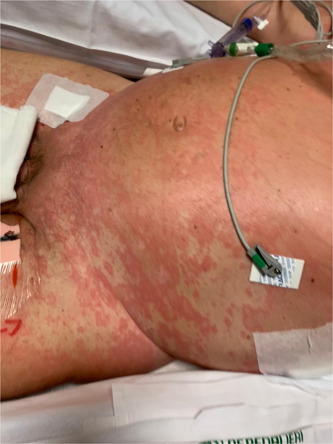
Urticarial rash in male patient with severe respiratory distress syndrome related to COVID‐19. COVID‐19, coronavirus disease 2019
Blood test revealed abnormal blood count with neutrophil leukocytosis (neutrophil granulocytes 8.600/mm3), and mild lymphopenia (lymphocites 700/mm3), moderate increase of pro‐calcitonin serum levels (0.87 ng/mL), marked increase of CRP (10.2 mg/dL), and liver enzymes (GOT, GPT, LDH, GGT fourfold levels) serum levels. Patient was receiving mechanical ventilation for respiratory failure.
As in previous case, diagnosis of urticarial skin rash was made, and treatment with intravenous administration of methylprednisolone 40 mg/die and bilastine 20 mg/die was started. Currently, patient is still hospitalized in Anesthesiology Department, in stable clinical conditions, skin rash is slightly improved after 48 hours from the beginning of the treatment.
CASE 3: A 12‐year‐old girl presented to our observation with erythematous‐oedematous purple lesions affecting the skin of the distal phalanges of all 10 toes with clear demarcation from the remaining skin of the feet (Figure 3A). Both patient and her mother reported that these chilblain‐like lesions had never occurred before, and were not accompanied by pain or itching, or other systemic symptoms. A family history of autoimmune diseases has not been reported and the patient's mother denied that her daughter suffered from other diseases or that she recently had exposure to the cold. The patient's mother reported 1 day fever occurred, 10 days before, with a single body temperature spike of 37.9°C. Patient's father had presented respiratory symptoms compatible with COVID‐19 infection about 20 days earlier, although he had not performed the diagnostic swab yet. Due to recent reports of similar skin lesions during COVID‐19, a diagnostic swab was requested, and unfortunately denied (in Italy swab is allowed only in patients with concomitant fever > 37.5°C).
Figure 3.
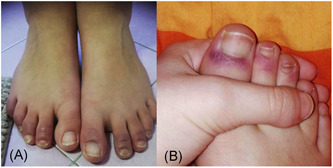
A, Chilblain‐like lesions in a SARS‐CoV‐2 positive 12 year‐old girl and (B) in 8 year‐old boy with a SARS‐CoV‐2 positive father. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
Seventy‐two hours after, the patient father's nasopharyngeal swab was positive for SARS‐CoV‐2. After that, nasopharyngeal swab performed to our patient resulted positive too.
Similar clinical presentations were observed few days later in another 8‐year‐old boy with father recently recovered from COVID‐19 (Figure 3B). Unfortunately, in this case nasopharyngeal swab was not performed.
From the observation of these four patients, together with data from literature, it can be inferred that the attention to skin involvement during COVID‐19 should be recommended.
A study carried out by Recalcati 4 on 88 patients affected by COVID‐19 showed presence of widespread urticaria (3 patients), erythematous rush (14 patients), and chickenpox‐like vesicles (1 patient). Other frequently described manifestation are chilblain‐like lesions, especially located on the feet of adolescent subjects without typical respiratory symptoms of COVID‐19, sometimes positive to the diagnostic swab. 5
There are still no studies to accurately identify which are the dermatological manifestations of COVID‐19, and why they occur. However, several hypotheses could be formulated from integration of our clinical observations and data from literature. In our opinion, skin involvement in course of SARS‐CoV‐2 may be more than a mere coincidence.
Skin lesions could be ascribed into two main clinical categories: early (urticarial rash, exanthemas, and chickenpox‐like vesicles) and late (chilblains‐like lesions) cutaneous manifestations.
Urticarial rash, exanthemas, chickenpox‐like vesicles occur in most cases at the beginning of respiratory symptoms (as reported in clinical cases 1 and 2), they may be related either to initial viral replication of SARS‐CoV‐2 (so‐called “viral sepsis”), or to cytokine storm characteristic of COVID‐19 disease. Not yet knowing how the above mentioned processes can occur, 6 , 7 further pathogenic hypothesis would be speculative.
Other, generally later reported, COVID‐19 related skin features are chilblains‐like lesions.
Many Dermatologists, around the world have reported a recent increase in chilblains‐like lesions, unrelated to cold exposure, located at the feet in adolescents. In many cases, as shown by a recent Italian study, 5 many patients have positive swab for SARS‐CoV‐2. Very few information on the histology of these skin injuries are available. One described case including skin histology in chilblain‐like lesions 8 showed an interesting superficial and deep lichenoid, perivascular and peri‐eccrine infiltrate of lymphocytes with occasional plasma cells without detection of fibrin or intra luminal thrombi. These findings, combined with evidence of their onset several days after respiratory symptoms appearance (or in the absence of symptoms) might indicate that these lesions have other origin than those of the urticarial rashes, exanthemas, and chickenpox‐like vesicles. They might be related to a secondary cell‐mediated immune response, following the initial viral infection. Further studies are needed to verify these hypotheses, however, in our opinion it is essential to focus clinical attention on all dermatological manifestations previous described, especially if they occur for the first time in healthy subjects.
A careful personal and family history should be collected for each patients, even if paucisymptomatic, in this subset of patients (with a careful epidemiological investigation of possible COVID‐19 infection), claiming for SARS‐CoV‐2 nasofaryngeal swab.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Federico Diotallevi and Anna Campanati contributed equally to the manuscript.
REFERENCES
- 1. Lu S, Lin J, Zhang Z, et al. Alert for non‐respiratory symptoms of coronavirus disease 2019 (COVID‐19) patients in epidemic period: a case report of familial cluster with three asymptomatic COVID‐19 patients [published online ahead of print March 19, 2020]. J Med Virol. 10.1002/jmv.25776 [DOI] [PubMed] [Google Scholar]
- 2. Campanati A, Brisigotti V, Diotallevi F, et al. Active implications for dermatologists in “SARS‐CoV‐2 era”: personal experience and review of literature [published online ahead of print May 19, 2020]. J Eur Acad Dermatol Venereol. 10.1111/jdv.16646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Radi G, Diotallevi F, Campanati A, Offidani A. Global coronavirus pandemic (2019‐nCOV): implication for an Italian medium size dermatological clinic of a II level hospital [published online ahead of print March 22, 2020]. J Eur Acad Dermatol Venereol. 10.1111/jdv.16386 [DOI] [PubMed] [Google Scholar]
- 4. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective [published online ahead of print March 26, 2020]. J Eur Acad Dermatol Venereol. 10.1111/jdv.16387 [DOI] [PubMed] [Google Scholar]
- 5. Piccolo V, Neri I, Filippeschi C, et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients [published online ahead of print April 24, 2020]. J Eur Acad Dermatol Venereol. 10.1111/jdv.16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li H, Liu L, Zhang D, et al. SARS‐CoV‐2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517‐1520. 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skroza N, Bernardini N, Balduzzi V, et al. A late onset widespread skin rash in a previous COVID‐19 infected patient: viral or multidrug effect? [published online ahead of print May 18, 2020] J Eur Acad Dermatol Venereol. 10.1111/jdv.16633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection–induced chilblains: a case report with histopathologic findings [published online ahead of print April 18, 2020]. JAAD Case Rep. 10.1016/j.jdcr.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]


