Abstract
Immunocompromised patients may be at increased risk for complications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, comprehensive data of SARS-CoV-2 infection in solid organ transplant (SOT) recipients are still lacking. We performed a multicenter nationwide observational study within the Swiss Transplant Cohort Study (STCS) to describe the epidemiology, clinical presentation, treatment and outcomes of the first microbiologically documented SARS-CoV-2 infection among SOT recipients. Overall, 21 patients were included with a median age of 56 years (10 kidney, 5 liver, 1 pancreas, 1 lung, 1 heart and 3 combined transplantations). The most common presenting symptoms were fever (76%), dry cough (57%), nausea (33%), and diarrhea (33%). Ninety-five percent and 24% of patients required hospital and ICU admission, respectively, and 19% were intubated. After a median of 33 days of follow-up, 16 patients were discharged, 3 were still hospitalized and 2 patients died. These data suggest that clinical manifestations of SARS-CoV-2 infection in middle-aged SOT recipients appear to be similar to the general population without an apparent higher rate of complications. These results need to be confirmed in larger cohorts.
KEYWORDS: clinical research/ practice, complication: infectious, infection and infectious agents, infection and infectious agents - viral, infectious disease
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel respiratory virus from the Betacoronavirus family, which was first described in December 2019 during an outbreak of coronavirus disease (COVID-19) including severe pneumonia in Wuhan, China.1 Since then, it has spread in China and abroad, rapidly becoming the most important pandemic of the 21st century, causing more than three million infections and more than two-hundred thousands of deaths worldwide.2
The clinical course of this infection is mild in 80% of cases, typically presenting as an influenza-like illness. However, it tends to be more severe in persons aged ≥ 65 or with significant comorbid diseases such as cardiac, respiratory, kidney or liver disease, diabetes mellitus and immunosuppression, causing severe viral pneumonia and acute respiratory distress syndrome (ARDS).3 Solid organ transplant (SOT) recipients are generally at higher risk for complications of respiratory viral infections (in particular influenza), due to their chronic immunosuppressive regimen,4, 5, 6, 7 and this may hold true also for SARS-CoV-2 infection. On the other hand, impaired outcomes of patients with COVID-19 have been linked to the development of a profound pro-inflammatory state leading to ARDS. Whether chronic immunosuppressive therapy may prevent a worse prognosis in SOT recipients is still debated, as only a few cohorts of adult SOT recipients with COVID-19 have been reported, most data coming from single centers.8, 9, 10, 11, 12 Clinical trials and observational data of larger populations of SARS-CoV-2 infected SOT recipients are therefore needed.
Taking advantage of the Swiss Transplant Cohort Study (STCS), a nationwide cohort of SOT recipients,13 we performed a multicenter nationwide observational study to comprehensively describe the epidemiology, clinical presentation, treatment and outcomes of the first microbiologically documented SARS-CoV-2 infections among adult SOT recipients.
2. MATERIALS AND METHODS
2.1. Swiss Transplant Cohort Study (STCS)
The STCS is a prospective multicenter cohort study enrolling > 90% of all patients undergoing SOT (heart, lung, kidney, liver, kidney-pancreas) in Switzerland since 2008.13 Clinical and laboratory data are collected at the time of transplantation, 6 and 12 months after transplantation, and yearly afterwards. It provides patients’ demographics, underlying organ disease, baseline organ function, as well as detailed information regarding patient- and graft-specific complications, notably infectious diseases.
Specific information regarding SARS-CoV-2 infection were collected through an electronic Case Report Form (eCRF) on secure Redcap electronic data capturing software, which served as an addendum to the STCS database.
Ethics approval was obtained in all participating centers; all enrolled patients gave written informed consent. The study was approved by the Scientific Committee of the STCS, which granted permission to the investigators to use the data from the STCS.
2.2. Patients and study design
All adult recipients of SOT who have been enrolled in the STCS since May 2008, and who were diagnosed with a microbiologically proven SARS-CoV-2 infection by real-time PCR between March 9 and April 6, 2020, were included in the analysis. Clinical presentation, risk factors, evolution, incidence of complications and outcomes, as well as treatments directed against SARS-CoV-2 and adaptation of the immunosuppressive regimen were described during this period.
2.3. Statistical analysis
Descriptive statistics were used to characterize patients’ demographics, transplantation characteristics, clinical and biological data.
3. RESULTS
3.1. Study participants
At the beginning of the COVID-19 epidemic in Switzerland on February the 25th 2020, more than 5000 SOT recipients were currently followed within the STCS. No systematic screening was performed; screening was performed in the presence of symptoms and/or signs suggestive of SARS-CoV-2 infection, or exposure to infected individuals. As of April 6th 2020, SARS-CoV-2 infection was diagnosed in 21 SOT recipients (10 kidney, 5 liver, 2 kidney-pancreas, 1 kidney-lung, 1 pancreas, 1 lung and 1 heart) actively followed within the STCS. Median time from transplantation to infection was 47 months (IQR 23-97). There were 6 females (33%) and median age was 56 years (IQR 49-65). Additional medical comorbidities were present in 20/21 of the participants. All detailed patients’ characteristics are further described in Table 1.
TABLE 1.
Patients’ characteristics
| All patients (n = 21) | |
|---|---|
| Age, median (IQR) | 56 (49-65) |
| Male sex, n (%) | 15 (71%) |
| Transplanted Organ, n (%) | |
| Kidney | 10 (47.6%) |
| Liver | 5 (23.8%) |
| Kidney-pancreas | 2 (9.5%) |
| Kidney-lung | 1 (4.8%) |
| Lungs | 1 (4.8%) |
| Heart | 1 (4.8%) |
| Pancreas | 1 (4.8%) |
| Months from transplantation, median (IQR) | 47 (23-97) |
| Induction therapy, n (%) | |
| Basiliximab | 5 (23.8%) |
| Anti-lymphocyte serum | 4 (19%) |
| N/A | 12 (57.1%) |
| Immunosuppressive agent, n (%) | |
| Tacrolimus | 18 (85.7%) |
| Mycophenolate | 17 (80.1%) |
| Prednisone | 9 (42.9%) |
| Ciclosporin | 2 (9.5%) |
| Azathioprine | 2 (9.5%) |
| mTOR inhibitor | 1 (4.8%) |
| Immunosuppressive regimen, n (%) | |
| Calcineurin inhibitor + antimetabolite | 10 (47.6%) |
| Calcineurin inhibitor + antimetabolite + prednisone | 8 (38.1%) |
| Calcineurin inhibitor + prednisone | 1 (4.8%) |
| mTOR inhibitor + antimetabolite | 1 (4.8%) |
| Calcineurin inhibitor alone | 1 (4.8%) |
| Underlying conditions, n (%) | |
| Hypertension | 14 (66.7%) |
| Diabetes mellitus | 9 (42.9%) |
| Obesity | 5 (23.8%) |
| Ischemic heart disease | 5 (23.8%) |
| Atrial fibrillation | 4 (19%) |
| Chronic Obstructive Pulmonary Disease | 4 (19%) |
| Cirrhosis | 4 (19%) |
| Solid tumor (localized) | 3 (14.3%) |
| Connective tissue disease | 2 (9.5%) |
| Peripheral arterial disease | 2 (9.5%) |
| Stroke / TIA | 1 (4.8%) |
| Chronic heart failure | 1 (4.8%) |
| Active smoker | 1 (4.8%) |
3.2. Characteristics of SARS-CoV-2 infection in SOT
All the SOT recipients infected with SARS-CoV-2 developed at least one symptom or sign compatible with SARS-CoV-2 infection ( Table 2). Mean time from first symptoms until diagnosis was 3 days (IQR 2-6 days). Most frequent SARS-CoV-2 symptoms at presentation were fever (76%), dry cough (57%), nausea (33%), diarrhea (33%) and dyspnea (30%). Hypoxemia with the need for oxygen therapy was present in 9 (43%) patients at admission. Anosmia was reported by one patient. Two patients (10%) developed neither fever nor respiratory symptoms. One kidney transplant recipient, admitted because of febrile allograft pyelonephritis with Escherichia coli bacteremia, underwent testing for SARS-CoV-2 as a screening procedure. One patient was diagnosed before the onset of symptoms during screening because of household exposure to a SARS-CoV-2 infected family member. Extra-respiratory symptoms were present in 33% of patients.
TABLE 2.
Patients’ clinical, biological, and imaging presentations and management
| Days of symptoms at SARS-CoV-2 diagnosis, median (IQR) | 3 (2-6) |
|---|---|
| Number of follow-up days, median (IQR) | 33 (27-42) |
| SARS-CoV-2 symptoms at admission, n (%) | |
| Fever | 16 (76%) |
| Dry cough | 12 (57.1%) |
| Diarrhea | 7 (33.3%) |
| Nausea/vomiting | 7 (33.3%) |
| Dyspnea | 6 (30%) |
| Myalgia/arthralgia | 6 (28.6%) |
| Rhinorrhea | 5 (23.8%) |
| Headache | 4 (19%) |
| Fatigue | 3 (14.3%) |
| Rigors | 3 (14.3%) |
| Thoracic pain | 1 (4.8%) |
| Laboratory values at admission, median (IQR | |
| Leucocyte counts (G/L), N = 21 | 4.8 (3.6-7.8) |
| Lymphocyte counts (G/L), N = 20 | 0.63 (0.45-0.97) |
| C-reactive protein (mg/L), N = 21 | 33 (9-95) |
| Procalcitonin (μg/L), N = 10 | 0.16 (0.07-0.70) |
| D-dimers (μg/mL), N = 9 | 0.39 (0.28-0.66) |
| Radiographic findings at admission, n (%) | |
| Not done | 2 (9.5%) |
| Normal | 8 (39.1%) |
| Interstitial infiltrates / ground-glass | 11 (52%) |
| ICU admission, n (%) | 5 (23.8%) |
| Mechanical ventilation, n (%) | 4 (19%) |
| SARS-CoV-2 directed treatment, n (%) | |
| None | 14 (66.7%) |
| Lopinavir/ritonavir | 3 (14.3%) |
| Hydroxychloroquine | 4 (19%) |
| Received antibiotics, n (%) | 11 (52%) |
| Complications, n (%) | |
| Acute kidney injury | 9 (42.9%) |
| AKIN 1 | 5 (24%) |
| AKIN 2 | 2 (9.5%) |
| AKIN 3 | 2 (9.5%) |
| ARDS | 4 (19%) |
| Community-acquired pneumonia | 3 (14.3%) |
| Hospital-acquired pneumonia | 2 (9.5%) |
| Other bacterial infection | 2 (9.5%) |
| Septic shock | 2 (9.5%) |
| Acute thromboembolic event | 1 (4.8%) |
| Outcomes, n (%) | |
| Ambulatory care | 1 (4.8%) |
| Discharged from hospital | 15 (71.4%) |
| Still in hospital, medical ward | 1 (4.8%) |
| Still in the ICU | 2 (9.5%) |
| Death | 2 (9.5%) |
All 21 patients had a laboratory workup, with median leucocytes count of 4.8 (IQR 3.6-7.8; 21 patients), median lymphocytes counts of 0.63 G/L (IQR 0.45-0.97; 20 patients), median CRP values of 33 mg/L (IQR 9-95; 21 patients), median PCT values of 0.16 μg/L (IQR 0.07-0.70; 10 patients) and median D-dimers values of 0.39 μg/mL (IQR 0.28-0.66; 9 patients).
A chest X-ray made on the day of admission in 14 (67%) patients showed interstitial infiltrates in 6 (43%) cases and was normal in 8 (57%) patients. Three out of 8 patients with a normal chest X-ray at admission had a second chest imaging, all of which showed new infiltrates. Thoracic CT-scans were done on the day of admission in 5 patients and showed ground-glass and interstitial infiltrates in 5 (100%) patients. Two patients (10%) had no imaging.
3.3. Management and outcomes of SARS-CoV-2 infection in SOT recipients
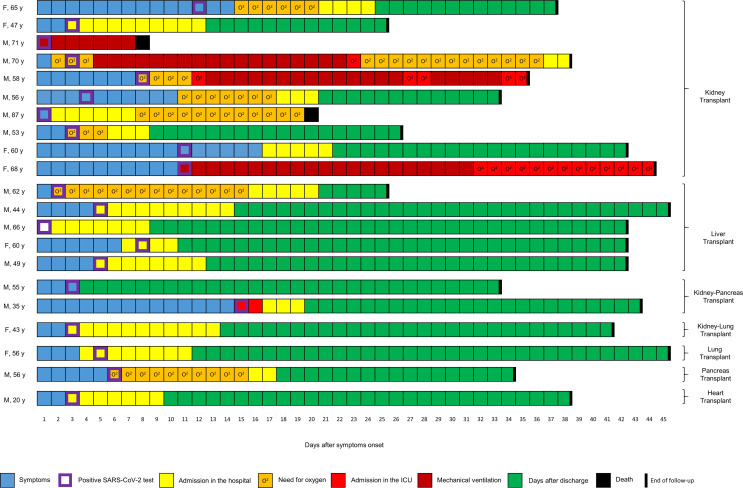
All but one patient were admitted to the hospital following SARS-CoV-2 infection. In patients requiring hospitalization, 80% (15/20) were admitted at time of diagnosis or in the following two to three days. Three patients were admitted 2 to 3 days and one patient 6 days after diagnosis because of clinical deterioration ( Figure 1).
FIGURE 1.
Clinical course of 21 SOT recipients with SARS-CoV-2 infection. Patients are grouped according to type of transplant. Blue squares represent days with symptoms, purple squares day of SARS-CoV-2 positive test, yellow squares days in hospital, orange squares days with oxygen, light-red squares days in ICU, dark red squares mechanical ventilation, black squares death, and green squares days after discharge. O2, oxygen therapy
Five patients (24%) needed intensive care unit (ICU) admission (4 kidney and one kidney-pancreas recipients), and 4 of them received mechanical ventilation. Compared to patients not admitted to the ICU, they tended to be older (median age 56 vs 68 years) and to suffer more underlying cardiovascular diseases (hypertension; 10/16 vs 4/5, ischemic heart disease; 2/16 vs 3/5, and atrial fibrillation; 2/16 vs 2/5), but without apparent differences in diabetes (7/16 vs 2/5) and obesity (4/16 vs 1/5) rates. One patient (35 years old) was admitted to the ICU because of acute kidney injury with severe metabolic impairment, but without hypoxia, and was discharged from ICU after 2 days.
Drugs with possible anti-SARS-CoV-2 activity were administered to 7 (33.3%) patients. Hydroxychloroquine was administered to 3 kidney transplant recipients admitted to the ICU, and azithromycin was co-administered to one of them. One combined kidney-lung and 2 kidney transplants received the lopinavir/ritonavir combination. All 3 had immunosuppressive drug monitoring; 2 required no modification in their immunosuppressive regimen, 1 patient had a transient interruption of azathioprine. Globally immunosuppressive regimen was modified in 14 (66.7%) patients. The most frequent intervention was withdrawal of anti-proliferative drugs (10 patients; mycophenolate in 8 and azathioprine in 3 patients, respectively), with concomitant reduction in calcineurin inhibitors in 3 patients. In 3 additional patients the dosage of mycophenolate was reduced by 50%, in one patient isolated tacrolimus dosage reduction was performed. Steroids dosages were not modified.
The most frequent medical complications were acute kidney injury (9/21, 43%), ARDS (4/21, 19%), community-acquired pneumonia (3/21,14%), hospital-acquired pneumonia (2/21, 10%), and septic shock (2/21, 10%). Despite extensive workup, no microbiological documentation was obtained neither for the pneumonia, nor for the septic shocks. Two patients presented microbiological proven infections: one had renal graft E. coli pyelonephritis, and one had Campylobacter infection. A total of 11 patients received antibiotics. One patient had newly diagnosed Norovirus infection. One patient in the ICU presented central pulmonary embolism and multiples deep venous thrombosis.
After a median of 33 days of follow-up, 16 patients had been discharged (among them 1 patient needed further care in a rehabilitation hospital), 3 patients were still hospitalized (among whom 2 were in the ICU but were extubated) and 2 patients had died.
The first patient who died was a 71-year-old kidney recipient, transplanted 7 years ago, with a history of hypertension and ischemic heart disease. At admission, he presented with hypoxemic respiratory failure, required ICU admission and an oro-tracheal intubation shortly thereafter. The diagnostic work-up showed a marked elevation of inflammatory parameters (CRP 230 mg/L, PCT 33 mcg/L, IL-6 3919 ng/L) and a CT-scan showed bilateral interstitial infiltrates with ground-glass and multiple consolidations in the right lung. The clinical course was complicated by a septic shock with mesenteric ischemia, which lead to fatal outcome 8 days after admission.
The second patient who died was a 87-year-old kidney recipient, who developed nosocomial SARS-CoV-2 infection while admitted for other medical issues. The clinical presentation was initially mild and the initial chest x-ray normal. Seven days after diagnosis, the patient developed progressive hypoxemia. Because of major comorbidities, he was not eligible for ICU admission and died in a setting of worsening hypoxemia 18 days after diagnosis.
4. DISCUSSION
To our knowledge, this is to date one of the few multicenter nationwide reports of microbiologically confirmed SARS-CoV-2 infection in SOT recipients, which brings important information about presentation, clinical course, and outcomes in this particular population.
Given the relatively low number of patients presented in this series, it is difficult to estimate whether SOT recipients are at higher risk for the development of SARS-CoV-2 infection. In Switzerland (up to April 6th) SARS-CoV-2 incidence in the general population has been estimated to be 284 cases per 100 000 inhabitants. Taking into consideration the total number of patients included in the STCS (more than 5000) and the number of patients included in this series, the rate of SARS-CoV-2 infection in SOT recipients appears to be similar to that of the general population. These numbers are, however, limited by a potential diagnostic and hospitalization bias in SOT recipients, or, on the other hand, by missing diagnosis in patients not consulting the transplant center. Whether the actual incidence of SARS-CoV-2 is influenced by a higher susceptibility to infection or, on the other hand, by better adherence to confinement rules, will need to be determined by large epidemiologic surveys.
The most frequently reported symptoms in SARS-CoV-2 infection in the general population were fever and dry cough, with headache, myalgia, nausea and diarrhea being also frequent. In our case series, the clinical presentation in SOT recipients did not differ significantly from the general population, contrasting with the recently reported experience with influenza in SOT recipients, which presents more frequently without fever, and with more severe respiratory and extra-respiratory symptoms.4
So far, no treatment has been proven effective in the therapy of SARS-CoV-2 infection. Lopinavir/ritonavir did not show a significant clinical effect in an open-label randomized study in China,14 and its administration in SOT recipients is challenging because of significant drug-drug interactions with immunosuppressive regimens. Hydroxychloroquine was shown to have in vitro activity against SARS-CoV-215 and is currently being evaluated in several clinical trials to assess its clinical effect. Given the low number of patients receiving these drugs in an uncontrolled manner, we cannot extract any conclusion about their use in SOT recipients. As it occurs with other respiratory viral infections in SOT recipients, the most common therapeutic intervention was transient reduction or discontinuation of antimetabolites, while maintaining the same doses of calcineurin inhibitors and steroids.
After a median follow-up of 33 days, 5 (24%) SOT recipients presented a severe clinical course and required ICU admission, and 2 (9.5%) patients eventually died. Approximately half of the patients had relatively mild infections with short hospital stays and no need for oxygen therapy,16 despite the fact that most of these patients had several underlying diseases that were potentially risk factors for severe complications. Compared to the general population, these data are similar to the preliminary results of the first 200 patients admitted with COVID-19 at the Lausanne University Hospital (Regina et al personal communication). In this cohort, 19% of patients required ICU admission and mechanical ventilation and 12.5% of patients died during hospitalization. The median age of our SOT recipient cohort was lower (56 years vs 70 years), which can partially explain the relatively good outcomes observed in our series.
Other recent studies have reported worse outcomes in cohorts of SOT recipients. In New York, two different cohorts showed mortality rates of 18% among 90 SOT recipients17 and 28% among 36 kidney transplant recipients.12 In Madrid, case fatality rate was 27% (5/18).11 It is not yet known whether these higher mortality rates are due to a higher representation of severe cases due to limited testing access, overload of the health resources including availability of ICU beds, or demographic differences with inclusion of more vulnerable elderly patients.
Early reports of pathological findings caused by SARS-CoV-2-induced ARDS showed overactivation of T cells in lung tissue,18 which function could be impaired in patients receiving immunosuppressive drugs such as tacrolimus, ciclosporin, mycophenolate or corticosteroids. Moreover, calcineurin inhibitors have shown some in vitro activity against different human coronavirus including SARS-CoV-1, and could therefore exert a protective effect.19 , 20 The immunosuppressive regimens of SOT recipients could therefore alter the clinical presentation of SARS-CoV-2 infection, and potentially act as a protecting factor against severe, uncontrolled, inflammatory response contributing to ARDS development.21 Related to that, we observed lower inflammatory parameters in our SOT recipient cohort as compared to the hospitalized general population (Regina et al personal communication), with a median CRP of 33 mg/L vs. 48 mg/L and a median D-dimers of 0.39 µg/mL vs 0.96 µg/mL, respectively. However, the significance of these observations needs to be analyzed in a larger cohort of patients with appropriate controls.
Pneumonia and sepsis were treated with antibiotics on clinical suspicion in several patients without microbiological documentation despite extensive workup. We observed similar clinical presentations in the general population at the University Hospitals of Geneva (van Delden C et al personal communication). Whether these are part of the viral disease and/or its triggered cytokine storm syndrome mimicking bacterial infection needs further investigation.22 , 23
There are evident limitations to our study. First, this is an observational study in a relatively small number of heterogenous SOT recipients regarding their transplanted organ, age, and comorbid diseases. This precluded us to perform any correlations between symptoms, laboratory values or imaging, as well as treatments and severity of infection. Second, the follow-up is also relatively short for assessing all outcomes. Third, there is a selection bias towards symptomatic patients, as pauci- or asymptomatic SOT recipients might not have sought medical attention and missed diagnosis. However, this would even further support our overall observation concerning the absence of severely symptomatic SARS-CoV-2 infections in SOT recipients. Larger ongoing cohorts with prolonged follow-up, as well as serologic surveys are needed. Nevertheless, our experience within the STCS suggests that, in the context of low threshold for testing, the clinical course of SARS-CoV-2 infection in SOT recipients appears to be similar to that observed in the general population, without a significant increased rate of severe complications.
ACKNOWLEDGMENTS
We thank all patients who participate in the Swiss Transplant Cohort Study, the study nurses, the central and local data managers and all the investigators involved in the STCS.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The dataset used for this study is stored at the STCS data center and it is available upon request.
Funding information This study has been conducted in the framework of the Swiss Transplant Cohort Study, supported by the Swiss National Science Foundation (grant 33CS30_177522) and the Swiss University Hospitals (G15) and transplant centers.
Footnotes
Jonathan Tschopp and Arnaud G. L’Huillier contributed equally.
Appendix 1. List of active members of the STCS
Patrizia Amico, John-David Aubert, Vanessa Banz, Guido Beldi, Christian Benden, Christoph Berger, Isabelle Binet, Pierre-Yves Bochud, Sanda Branca, Heiner Bucher, Thierry Carell, Emmanuelle Catana, Yves Chalandon, Sabina de Geest, Olivier de Rougemont, Michael Dickenmann, Michel Duchosal, Laure Elkrief, Thomas Fehr, Sylvie Ferrari-Lacraz, Christian Garzoni, Paola Gasche Soccal, Christophe Gaudet, Emiliano Giostra, Déla Golshayan, Karine Hadaya, Jörg Halter, Dimitri Hauri, Dominik Heim, Christoph Hess, Sven Hillinger, Hans H. Hirsch, Günther Hofbauer, Uyen Huynh-Do, Franz Immer, Richard Klaghofer, Michael Koller (Head of the data center), Bettina Laesser, Guido Laube, Roger Lehmann, Christian Lovis, Pietro Majno; Oriol Manuel, Hans-Peter Marti, Pierre Yves Martin, Michele Martinelli, Pascal Meylan, (Head, Biological samples management group), Nicolas J Mueller (Chairman Scientific Committee), Antonia Müller, Thomas Müller, Beat Müllhaupt, Manuel Pascual (Executive office), Jakob Passweg, Klara Posfay-Barbe, Juliane Rick, Eddy Roosnek, Anne Rosselet, Silvia Rothlin, Frank Ruschitzka, Urs Schanz, Stefan Schaub, Aurelia Schnyder, Macé Schuurmans, Christian Seiler, Jan Sprachta; Susanne Stampf, Carolin Steinack, Jürg Steiger (Head, Executive Office), Guido Stirnimann, Christian Toso, Christian Van Delden (Executive office), Jean-Pierre Venetz, Jean Villard, Madeleine Wick (STCS coordinator), Markus Wilhelm, Patrick Yerly.
REFERENCES
- 1.Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease (COVID-2019) situation report-99. April 28, 2020 [Accessed April 29, 2020].
- 3.Guan W-J, Ni Z-Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar D, Ferreira VH, Blumberg E, et al. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis. 2018;67(9):1322–1329. doi: 10.1093/cid/ciy294. [DOI] [PubMed] [Google Scholar]
- 5.Ison MG, Hirsch HH. Community-acquired respiratory viruses in transplant patients: diversity, impact, unmet clinical needs. Clin Microbiol Rev. 2019;32(4) doi: 10.1128/CMR.00042-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manuel O, Estabrook M. American Society of Transplantation Infectious Diseases Community of Practice. RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13511. doi: 10.1111/ctr.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mombelli M, Kampouri E, Manuel O. Influenza in solid organ transplant recipients: epidemiology, management, and outcomes. Expert Rev Anti Infect Ther. 2020;18(2):103–112. doi: 10.1080/14787210.2020.1713098. [DOI] [PubMed] [Google Scholar]
- 8.Zhu L, Xu X, Ma KE, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020; 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed]
- 9.Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020; 10.1111/ajt.15874 [DOI] [PMC free article] [PubMed]
- 10.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020; 10.1016/j.healun.2020.03.006 [DOI] [PMC free article] [PubMed]
- 11.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020; 10.1111/ajt.15929 [DOI] [PMC free article] [PubMed]
- 12.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020; 10.1056/NEJMc2011117 [DOI] [PMC free article] [PubMed]
- 13.Koller MT, van Delden C, Müller NJ, et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol. 2013;28(4):347–355. doi: 10.1007/s10654-012-9754-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020; 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed]
- 15.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention Novel Coronavirus Pneumonia Emergency Response Epidemiology T. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020. 10.1111/ajt.1594 [DOI] [PMC free article] [PubMed]
- 18.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carbajo-Lozoya J, Ma-Lauer Y, Malešević M, et al. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including Alisporivir. Virus Res. 2014;184:44–53. doi: 10.1016/j.virusres.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbajo-Lozoya J, Muller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165(1):112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonio R, Silvia M. Immunosuppression drug-related and clinical manifestation of Coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. 2019;2020 doi: 10.1111/ajt.15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020. 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed]
- 23.Mehta P, McAuleay DF, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020. 10.1016/S0140-6736(20)30630-9 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used for this study is stored at the STCS data center and it is available upon request.