1.
To the Editor:Novel coronavirus disease (COVID‐19) is spreading around the world and although clinical data are limited, immunomodulatory agents such as chloroquine and hydroxychloroquine are used as off‐label treatment. 1 While these medications have an established clinical safety profile for their common use, (eg, malaria) their efficacy and safety in COVID‐19 pneumonia remains unclear. 1 This is as most of the evidence to support use of chloroquine, or the less toxic hydroxychloroquine against the disease, comes from a small single arm trial. 2 As we demonstrate in this correspondence, the use of chloroquine for treatment of patients with COVID‐19 infection is not without risks.
A 56‐year‐old man, with a medical history of diabetes mellitus type 2, presented to the emergency department with a 6‐day history of myalgia and a dry cough. Oxygen saturation was 94% with room air and respiratory rate 24/min. There was no fever and his pulse and blood pressure were normal. So, COVID‐19 was suspected which was confirmed by a real‐time‐PCR assay. A chest CT scan showed bilateral ground glass opacities. The patient was admitted for observation on a COVID‐19 ward. During the following 2 days, his condition deteriorated with increasing need for oxygen administration. On the third day of admission his peripheral oxygen saturation dropped to 83% despite the use of a non‐rebreathing mask with 15 L/min of oxygen. His respiratory rate was 30/minute. He was admitted to the intensive care unit (ICU) for initiation of mechanical ventilation. Treatment with chloroquine was started consisting of a first dose of 600 mg, followed by 300 mg twice a day (for 5 days). 1 Initial ICU laboratory results demonstrated a hemoglobin level of 11.4 g/dL (reference 13.7‐17.7 g/dL), 12 hours later his hemoglobin level dropped to 8.9 g/dL and additional laboratory investigations demonstrated signs of severe hemolysis. A peripheral blood smear revealed findings consistent with hemolysis (Figure 1). Arterial blood gas results demonstrated increased levels of methemoglobin (9.1%; reference <1.5%). Given his ethnic background (African‐Caribbean), glucose‐6‐phospate dehydrogenase (G6PD) deficiency was suspected and chloroquine was stopped. 3 He received 3 units of packed red blood cells in the following 48 hours. Although his methemoglobin level was relatively low, 1000 mg ascorbic acid (vitamin C) was administered intravenously four times a day for 2 days, to help optimize his oxygenation. His methemoglobin normalized within 6 days and laboratory testing for G6PD deficiency confirmed very low G6PD activity in the patientʼs red blood cells (Figure 1A,B). Genetic analysis demonstrated variant G6PD A‐ (the African variant).
FIGURE 1.
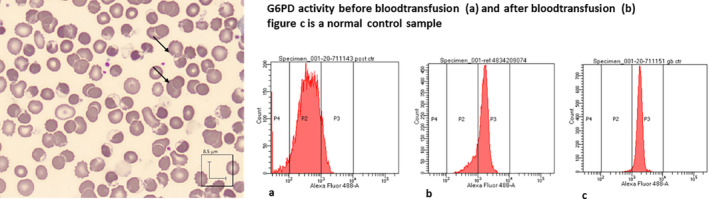
Signs of hemolysis and low G6PD activity. Glucose‐6‐phosphate dehydrogenase (G6PD) deficiency in red blood cells was suspected by the presence of blister cells on peripheral blood smear (left) and was confirmed by a low G6PD enzyme activity assay (right, x‐axis G6PD activity; fluorescence intensity 490/525 nm in arbitrary units ( ferryl Hb as measure for G6PD activity), y‐axis number of red blood cells). 5 A, demonstrates the lack of G6PD activity in red blood cells 12 hours after chloroquine. Mean G6PD activity of 0.1 IE/gHb (reference 3.8‐5.9). B, demonstrates that after ongoing hemolysis and a blood transfusion the main fraction of red blood cells in the circulation had normal G6PD activity (mean 4.0 IE/gHb) with only a minor fraction deficient cells. C, demonstrates G6PD activity in a healthy control (mean 5.0 IE/gHb)
Note, G6PD deficiency is an X‐linked disease that affects 400 million people worldwide. 3 During a period of oxidative stress, intracellular levels of the reduced form of the nicotinamide adenine dinucleotide phosphate (NADPH) in these patients are depleted. This leads to the accumulation of oxidative damaged proteins and lipids in their red blood cells, resulting in hemolysis of deficient red blood cells. Chloroquine is on the list of oxidative drugs known to cause hemolysis in patients with G6PD deficiency. 3 The G6PD A‐ variant results in a moderate enzyme deficiency and clinically insignificant hemolysis. A short duration of chloroquine treatment at the above mentioned dose usually does not result in severe hemolysis, unless the antioxidant reserves are depleted by another (pre‐existing) trigger, such as intensive systemic inflammation. In our patient, the ongoing inflammation due to the COVID‐19 pneumonia had probably resulted in excessive consumption of intracellular antioxidants and thus NADPH. Under these circumstances, chloroquine possibly triggered a complete depletion of NADPH resulting in severe hemolysis. Our patient also suffered from a functional anemia due to methemoglobinemia. Hemoglobin is transformed to methemoglobin once ferrous iron (Fe2+) of the heme group is oxidized to ferric iron (Fe3+). Methemoglobin has such a high oxygen affinity that it virtually cannot release its oxygen in the tissues and this usually becomes clinically apparent at a level of 15% or more. 4 However, patients with severe anemia may have symptoms at lower levels. The most frequent cause of methemoglobinemia is exposure to oxidative drugs such as chloroquine. 5 , 6 Under normal circumstances methemoglobin is rapidly converted back to hemoglobin by the NADH‐dependent cytochrome‐b5 methemoglobin reductase (CYB5R) enzyme. Oxidizing agents may overwhelm this reducing system and cause methemoglobin levels to rise. Mutations affecting the activity of CYB5R enzyme can lead to congenital methemoglobinemia. 5 Molecular analysis of the CYB5R enzyme in our patient, however, did not show any pathogenic variants. Based on this result together with the normalization of the methemoglobin level after chloroquine discontinuation and no medical history of methemoglobinemia, the reducing capacity of the deficient G6PD in our patient was probably overwhelmed by the antioxidant consumption during COVID‐19 in combination with chloroquine use.
Widespread off‐label use of chloroquine harbors potential benefit, but also a risk of harm. Monitoring of well‐known side effects such as QT prolongation, bone marrow suppression, and mental disturbances is recommended. 1 This case‐report illustrates that hemolytic anemia due to G6PD deficiency is another complication that can occur and exacerbate an already compromised oxygenation in the setting of COVID‐19 pneumonia.
Pending results from well‐designed clinical trials, we recommend caution with using chloroquine as treatment of COVID‐19. If feasible, G6PD deficiency should be ruled out before administering chloroquine in patients with COVID‐19.
2. CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
REFERENCES
- 1. Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID‐19. J Crit Care. 2020;57:279‐283. 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;105949. 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Roper D, Layton M, Rees D, et al. Laboratory diagnosis for G6PD deficiency. A British Society for Haematology Guideline. Br J Haematol. 2020;189(1):24‐38. [DOI] [PubMed] [Google Scholar]
- 4. Schuurman M, van Waardenburg D, Da Costa J, Niemarkt H, Leroy P. Severe hemolysis and methemoglobinemia following fava beans ingestion in glucose‐6‐phosphatase dehydrogenase deficiency: case report and literature review. Eur J Pediatr. 2009;168(7):779‐782. [DOI] [PubMed] [Google Scholar]
- 5. Peters AL, Veldthuis M, Leeuwen van K, et al. Comparison of Spectrophotometry, Chromate Inhibition, and Cytofluorometry Versus Gene Sequencing for Detection of Heterozygously Glucose‐6‐Phosphate Dehydrogenase‐Deficient Females. J Histochem Cytochem. 2017;65(11):627‐636. 10.1369/002215541773002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sonbol MB, Yadav H, Vaidya R, Rana V, Witzig TE. Methemoglobinemia and hemolysis in a patient with G6PD deficiency treated with rasburicase. Am J Hematol. 2013;88(2):152‐154. [DOI] [PubMed] [Google Scholar]


