Abstract
The coronavirus disease 2019 pandemic has led to the manufacturing of novel devices to protect clinicians from the risk of transmission, including the aerosol box for use during tracheal intubation. We evaluated the impact of two aerosol boxes (an early‐generation box and a latest‐generation box) on intubations in patients with severe coronavirus disease 2019 with an in‐situ simulation crossover study. The simulated process complied with the Safe Airway Society coronavirus disease 2019 airway management guidelines. The primary outcome was intubation time; secondary outcomes included first‐pass success and breaches to personal protective equipment. All intubations were performed by specialist (consultant) anaesthetists and video recorded. Twelve anaesthetists performed 36 intubations. Intubation time with no aerosol box was significantly shorter than with the early‐generation box (median (IQR [range]) 42.9 (32.9–46.9 [30.9–57.6])s vs. 82.1 (45.1–98.3 [30.8–180.0])s p = 0.002) and the latest‐generation box (52.4 (43.1–70.3 [35.7–169.2])s, p = 0.008). No intubations without a box took more than 1 min, whereas 14 (58%) intubations with a box took over 1 min and 4 (17%) took over 2 min (including one failure). Without an aerosol box, all anaesthetists obtained first‐pass success. With the early‐generation and latest‐generation boxes, 9 (75%) and 10 (83%) participants obtained first‐pass success, respectively. One breach of personal protective equipment occurred using the early‐generation box and seven breaches occurred using the latest‐generation box. Aerosol boxes may increase intubation times and therefore expose patients to the risk of hypoxia. They may cause damage to conventional personal protective equipment and therefore place clinicians at risk of infection. Further research is required before these devices can be considered safe for clinical use.
Keywords: aerosol box, barrier device, COVID‐19, intubation, personal protective equipment, PPE, rapid sequence induction, RSI
Introduction
The severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) pandemic has put the lives of healthcare workers at great risk, as evidenced by the growing numbers of healthcare worker deaths in many nations [1, 2]. The threat to healthcare workers from the virus, and the associated fear, has been further exacerbated by a worldwide shortage of personal protective equipment (PPE) [3, 4]. SARS‐CoV‐2, which causes coronavirus disease 2019 (COVID‐19), is predominantly spread by droplets but has the potential for aerosol transmission [5]. This has seen a significant focus on the utility and application of PPE during aerosol‐generating procedures including intubation [6]. Data from the severe acute respiratory syndrome (SARS) epidemic of 2002–2003 suggest that healthcare workers involved in intubation were at six times the risk of acquiring that coronavirus [7]. The correct application of PPE and infection control techniques were shown to significantly reduce this risk [8, 9]. Recently, international guidelines have been developed to assist healthcare workers involved in the airway management (including intubation) of COVID‐19 patients [10, 11].
Ongoing clinician concerns and anxiety about safety, especially in light of PPE shortages, has driven solutions including increased manufacturing of PPE, suggestions regarding the reuse of PPE and in some cases the creation of novel devices to protect healthcare workers [12, 13]. Such innovation has virtue, especially in times of supply shortages, but the inherent risks of this approach are also described. For example, while regulated equipment is usually subjected to testing in a range of circumstances by various users, improvised equipment is not and is therefore likely to have a higher failure rate in clinical use [14].
One such novel device is the aerosol box. First described by Dr Lai Hsien‐yung [15], these devices typically consist of a transparent plastic cube covering a patient’s head and shoulders, with access holes for the intubating proceduralist’s arms and sometimes additional holes for an assistant. These devices have been discussed in both the traditional medical literature [16, 17, 18] but more commonly on social media and medical education websites, often being praised for their ingenuity [19, 20, 21, 22]. Despite there being no published research on the safety or efficacy of the aerosol boxes, these devices are being used in clinical practice and manufacturers have already distributed hundreds of units to hospitals in the USA, the UK and Australia [12, 17, 23, 24, 25, 26, 27].
There is no doubt that genuine fear is motivating the distribution and use of these devices, especially in systems with critical PPE shortages. Additional drivers of this rapid implementation may also include ‘Gizmo Idolatry’ (the implicit conviction that a more technological approach is intrinsically better than one that is less technological [28]), and ‘MacGyver bias’ (the inherent attraction of our own personal improvised devices [14]). We contend that these devices require further evaluation before implementation. We sought to investigate the use of airway boxes and the time to successful intubation of a COVID‐19 patient in a simulated environment. We aimed to also explore their effects on pre‐oxygenation, laryngoscopy and conventional PPE.
Methods
We evaluated the impact of two aerosol boxes on tracheal intubation of a simulated patient with severe COVID‐19 in the intensive care unit (ICU) in an in‐situ simulation crossover study. The simulated process of intubation was designed to comply with the Safe Airway Society COVID‐19 airway management guidelines [10], hereafter referred to as the ‘SAS guidelines’. The SAS guidelines are endorsed by 13 specialist colleges, societies and associations in Australia and New Zealand and thus inform the practice of most frontline workers involved in COVID‐19 airway management in Australia. They are largely consistent with other international guidelines including the Difficult Airway Society guidelines [11].
The study received ethics approval from the Cabrini Institute Ethics Committee. Written consent was obtained from research participants and additional consent was obtained for the publication of images.
Local designers provided the aerosol boxes. Designs for a variety of aerosol boxes have been published online and are being mass produced. We studied two devices; one which closely resembled the original box described by Canelli et al. [16] (the ‘early‐generation’ box) and another which was the most modified and advanced version available to us (the ‘latest‐generation’ box) (Fig. 1). The early‐generation box resembles that originally designed by Dr Lai Hsien‐yung of Taiwan [15], but with modifications similar to those suggested by an American manufacturer in order to make it suitable for larger body habitus patients [23]. The latest‐generation box represents a local designer’s most recent adaption and includes holes for the assistant’s hands, a hole on top for insertion of a bougie to aid intubation, and ports for applying suction to generate a negative pressure (not used in this study). On this model, we followed the designer’s recommendation that occlusive dressings (Tegaderm Film, 3M, St Paul, MN, USA) cover the holes to create a tighter seal, and a plastic drape be placed over the patient’s chest in place of the plastic wall which had been removed in this model.
Figure 1.

The early‐generation aerosol box (left) and the latest‐generation aerosol box (right) which were studied. Dimensions of both boxes were the same: 65‐cm wide, 50‐cm tall, and 40‐cm deep. The primary arm holes are 12.5 cm in diameter and positioned identically in both boxes
A total of 35 specialist (consultant) anaesthetists formed a new roster to support the expanded ICU in preparation for the COVID‐19 pandemic in our hospital. All were invited via electronic message to participate; the first 12 volunteers were accepted as participants. Three intubations were performed by each participant; one with no aerosol box and one with each of the aerosol boxes (12 participants; 36 intubations in total). The order of intubations for each participant was block randomised.
The study was performed in a negative‐pressure room in the ICU at Cabrini Hospital, a large tertiary metropolitan hospital in Melbourne, Australia, that has experience in the care of severe COVID‐19 patients. An ICU bed capable of being lowered to a height of 56 cm from the floor to the top of the mattress was used. Consistency of the head‐up bed positions was ensured by using a plumb‐string, the markings for which were made using a protractor. A simulated vital‐sign monitor (SimMon version 1.8.6, Castle + Andersen ApS, Hillerød, Denmark) was displayed. The airway assistant for all intubations was a single experienced intensive care nurse not involved in the project design or in data collection.
An Airsim Advance Crico (Trucorp, Lurgan, Ireland) was used as the airway manikin. The tongue was inflated to generate a modified Cormack‐Lehane grade 2A on the videolaryngoscope monitor, which was verified by an independent specialist anaesthetist not involved in the study. The manikin’s base (but not the head or neck itself) was secured to the bed using a bandage in order to prevent an unrealistic degree of movement; the independent anaesthetist confirmed the fixation was realistic in representing the degree of movement that might be expected when intubating a normal adult.
All participants were familiar with the SAS guidelines. Participants were oriented to the simulation environment and the aerosol boxes and training was provided in both boxes by the same doctor who directed the local hospital’s COVID‐19 airway training. Each participant was offered at least 5 min of instruction and the opportunity to perform two simulated intubations in each box before the study simulations.
For the study, the anaesthetist wore PPE consistent with the local guidelines for intubation of a patient with COVID‐19, consisting of a face‐shield, goggles or glasses, mask, gown and gloves. Due to concerns about PPE stocks, a surgical mask was worn instead of a P2/N95 mask. Pre‐oxygenation was performed with the manikin in a 45° head‐up position for 5 min, as required by the SAS guidelines. At any time, the anaesthetist could request the bed height be adjusted and a step was available if requested. After 5 min, administration of the induction agent and neuromuscular blocking drug was simulated and the head of the bed was lowered to 20°. The assistant provided gentle mask ventilation during this time, as may be required for a critically ill patient with severe COVID‐19 [10]. Intubation commenced 60s after induction. An example of a simulation is shown in the supporting information (Figure S1).
A tracheal tube (internal diameter 8.5 mm), bougie and malleable stylet were provided. A videolaryngoscope (Karl Storz C‐MAC, Karl Storz SE and Co.,Tuttlingen, Germany) with a disposable blade (Mac‐4) was used for all intubations. Having practiced on the aerosol boxes, anaesthetists were free to choose their preferred primary and rescue techniques with the equipment provided but their approach had to remain consistent for all three intubations. Re‐oxygenation of the patient between intubations attempts was not permitted as this would interfere with the primary outcome measure. Intubation was considered to have failed completely if it took more than 3 min from removal of the facemask, in which case the time to intubation was censored at 3 min.
The primary outcome measure was intubation time, defined as the time from removing the facemask until the first breath delivered by a correctly‐placed tracheal tube with an inflated cuff.
Secondary outcomes included: first‐pass intubation success; intubation grade (modified Cormack‐Lehane grade, on the video screen); breaks in pre‐oxygenation mask‐seal; and breaches or damage to PPE. At the end of each simulation, participants were asked for qualitative comments on their experience. Data was collected contemporaneously; video recordings were available for review if required.
Power was set at 0.95. A‐priori assumptions used to calculate minimum sample size were a mean (SD) time difference of 10 (7.5)s with a normal parent distribution, using a one‐tailed paired Wilcoxon test for an alpha of 0.025. A sample of 10 was required, with the protocol allowing for additional participants on the same day (interim statistical analysis was prohibited). The primary outcome was analysed using a one‐tailed paired Wilcoxon (signed‐rank) test using SPSS version 1.0.0.1347 (IBM, NY, USA). An alpha of 0.025 was used as a Bonferroni correction for two comparisons. Power was calculated using G*Power version 3.1.9.6 (Heinrich Heine University, Düsseldorf, Germany). Only descriptive statistics were used for secondary outcomes due to the small sample size.
Results
Twelve participants (2 women, 10 men) performed 36 intubations. Participants had a median (IQR [range]) age of 45 (42–48 [40–56]) years. Participants reported a median (IQR [range]) of 10.5 (9–13.5 [7.0–23.0]) years experience as a specialist anaesthetist (i.e. experience since qualifying as a consultant). All participants were currently practicing full‐time in anaesthesia and were fellows of the Australia and New Zealand College of Anaesthesia (ANZCA). Quantitative results are shown in Table 1.
Table 1.
Primary and secondary outcomes for simulated patients intubated with no aerosol box, early‐generation box or latest‐generation box. Values are median (IQR [range]) or number (proportion)
| No aerosol box | Early‐generation aerosol box | Latest‐generation aerosol box | |
|---|---|---|---|
| n = 12 | n = 12 | n = 12 | |
| Time to intubation; s | 42.9 (32.9–46.9 [30.9–57.6]) | 82.1 (45.1–98.3 [30.8–180.0]) | 52.4 (43.1–70.3 [35.7–169.2]) |
| First‐pass success | 12 (100%) | 9 (75%) | 10 (83%) |
| Breaks in pre‐oxygenation | 0 | 1 (8%) | 1 (8%) |
| Laryngoscopy grade a |
2A: 9 (75%) 2B: 3 (25%) |
2A: 8 (67%) 2B: 4 (33%) |
2A: 10 (83%) 2B: 2 (17%) |
| PPE breaches | 0 | 1 (8%) | 7 (58%) |
PPE, personal protective equipment.
All laryngoscopy grades were either 2A or 2B.
After familiarisation training with both aerosol boxes, all anaesthetists elected to intubate with a bougie on the first attempt. Intubation time with no aerosol box was significantly shorter than with the early‐generation box (median (IQR [range]) 42.9 (32.9–46.9 [30.9–57.6]) s vs. 82.1 (45.1–98.3 [30.8–180.0]) s, p = 0.002) and the latest‐generation box (52.4 ( 43.1–70.3 [35.7–169.2]) s, p = 0.008). There was one failed intubation (> 180 s) in the early‐generation box group. Intubation times without an aerosol box were consistent with a normal distribution on formal testing. However, intubation times for both boxes were positively skewed with some anaesthetists experiencing intubation times significantly more prolonged than others (Fig. 2).
Figure 2.
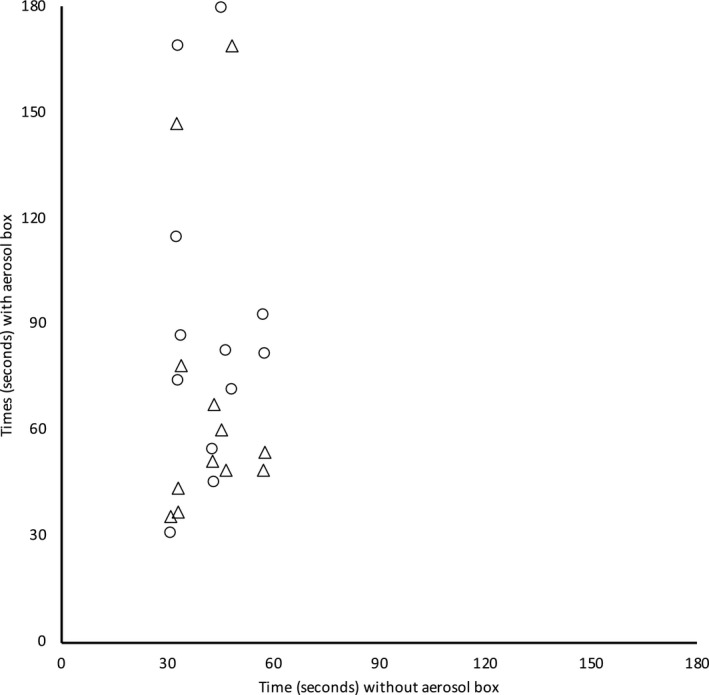
Comparison of paired intubation times with no aerosol box vs. with the early‐generation aerosol‐box (○) or the latest‐generation aerosol box (△). Unsuccessful intubation was censored and is shown at 180 s. Intubation times with both aerosol boxes were significantly longer than with no aerosol box.
Compared with using no aerosol box, the increase in intubation time was a mean (SD) of 48.4 (46.4; 95%CI 18.9–77.9) s with the early‐generation box and 28.2 (44.1; 95%CI 0.1–56.2) s with the latest‐generation box. No intubations without an aerosol box took more than 1 min, whereas 14 out of 24 (58%) intubations with a box took over 1 min and 4 out of 24 (17%) took over 2 min (including the failure). Only 2 out of 12 anaesthetists (17%) achieved intubations of under 60 s using both boxes. There was no relationship between intubation times with the early‐generation box and the latest‐generation box (Fig. 3); some participants who had relatively quick intubations with one box experienced very long times with the other. Post‐hoc analysis found no difference in intubation times between the two boxes (p = 0.209). Without an aerosol box, all anaesthetists obtained first‐pass success; with the early‐generation and latest‐generation boxes, 9 out of 12 (75%) and 10 out of 12 (83%) participants obtained first‐pass success, respectively. Breaks in pre‐oxygenation and laryngoscopy grade were similar between intubations (Table 1).
Figure 3.
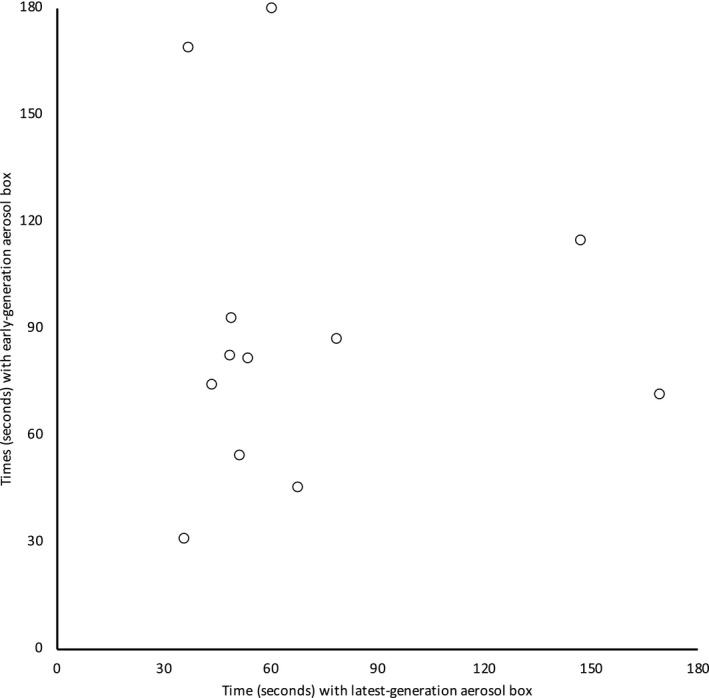
Comparison of paired intubation times with the latest generation box (abscissa) and the early generation box (ordinate). Unsuccessful intubation was censored and is shown at 180 s. There was no association between the intubation times of the two aerosol boxes.
There were eight breaches of PPE (see also supporting information, Figure S2). One was a tear in a gown sleeve and the remainder were gowns pulled back from the glove exposing skin. One breach occurred using the early‐generation box and the remainder (including the gown tear) occurred using the latest‐generation box. All PPE breaches appeared to be due to the gown becoming stuck or held‐up at the arm hole, in particular becoming caught on the occlusive dressings used on the latest‐generation box.
Free‐form commentary was sought from participants immediately after each simulated intubation (Table 2). The most frequently mentioned factors relating to the aerosol boxes were discomfort of the arms, back or knees (6 out of 12 participants; 50%) and an increased cognitive load (4 out of 12 participants; 33%).
Table 2.
Factors reported by anaesthetists performing simulated intubations with aerosol boxes. Values are number (proportion) with either box
| Factor | Participants |
|---|---|
| n = 12 | |
| Discomfort using box | 6 (50%) |
| Increased cognitive load from use of box | 4 (33%) |
| Use of airway device restricted by box | 3 (25%) |
| Issue with laryngoscope contacting box | 3 (25%) |
| Migration of box off bed | 3 (25%) |
| Concerns that contact with the box may cause circuit components to become disconnected | 2 (17%) |
| Required assistance to hold box | 2 (17%) |
Discussion
Before a novel medical device is considered for clinical use, it must be assessed for efficacy and safety. In the case of aerosol boxes, efficacy is the ability of the device to provide protection to healthcare workers from infection. Safety of both the patient and staff must also be considered, which includes allowing efficient intubation (to avoid patient harm from hypoxia) as well as the device not causing injury to either the patient or healthcare worker during its use. To our knowledge this is the first formal study of these devices that has been conducted.
This study found that use of either of the two different aerosol boxes significantly slowed intubation times when used by experienced airway specialists. First‐pass success rates were lower with both boxes, although the study size was small. Contributing factors to procedural difficulty include reduced ability to manipulate devices within the box, reduced arm movement and increased cognitive load. Cognitive overload is recognised to impair decision making and lead to potential patient harm during airway management [29, 30]. The observed delays in intubation are important; the desaturation of patients with COVID‐19 on induction of anaesthesia may be rapid and profound [10, 31]. With both pulmonary disease and in a hypermetabolic state, it would be expected that the observed delays would result in increased rates of critical desaturation and put the patient at significant risk of harm [32]. The measured intubation time in this study only included the actual attempt(s) at intubation and not the 60 s period between induction and laryngoscopy; the times reported in this study are therefore actually 60 s less than the potential apnoeic time of the patient. In practice, these patients with severe COVID‐19 requiring intubation will likely need to be re‐oxygenated after a short attempt at laryngoscopy and this could exaggerate the differences found in intubation times further.
An alternative approach to our primary outcome would have been to measure intubation success as defined by an arbitrary time cut‐off, such as 60 s. We used intubation time as the primary outcome because continuous data allow for more comprehensive interpretation of results. Additionally, we felt that setting an arbitrary cut‐off time for failed intubation might leave us open to the accusation of deliberately setting a cut‐off time to achieve a statistically significant result which may or may not be clinically significant.
The frequency and severity of PPE damage observed in this study was concerning. Seven events occurred with the latest‐generation box which may be contributed to by the occlusive dressings used on the arm holes (intended to create a tight seal around the arms), but one event also occurred on the early‐generation box without these dressings. Some practitioners use long‐cuffed surgical gloves for COVID‐19 intubations to minimise the risk of wrist exposure; however, we used standard clinical gloves in our simulation so as not to deplete precious PPE supplies. While the use of surgical gloves may have reduced the risk of wrist exposure, many events exposed more skin than these would have covered, nor would gloves have conceivably prevented the gown tear which occurred. While these devices are intended to protect healthcare workers from viral exposure, it is possible that by damaging their PPE they may have the opposite effect. PPE breaches often seemed to go unrecognised by participants, potentially increasing their risk further.
The primary limitation of this study was the small sample size. Although strongly statistically significant, the sample size limits inferences that can be drawn on secondary outcomes. Neither participants nor researchers were able to be blinded. We decided against a non‐inferiority study but we did set a‐priori power at 0.95 so that if the mean difference in intubation times was less than 10 s, one would be able to draw a meaningful conclusion.
The external validity of this study may be limited by the variety of aerosol box designs available; however, the study tested two different designs including an early‐generation and a late‐generation box, and obtained similar results for both. Any similar devices should have significant design modifications to mitigate against the problems identified in this study and should be subjected to similar safety testing before being considered for clinical use. All participants elected to use a bougie for intubation. It is possible that bougies are particularly difficult to use with these boxes, although the decision to use a bougie was made by each experienced anaesthetist independently after practicing with both boxes. Additionally, one of the devices studied had been optimised for bougie use, as bougies are a fundamental tool for tracheal intubation in the critically ill patient.
This study examined pre‐oxygenation and simple intubation; however, airway management is a process that involves much more than these two procedures. Oropharyngeal suctioning; hyper‐angulated videolaryngoscopy; supraglottic airway insertion; patient repositioning; front‐of‐neck access (cricothyrotomy); and fibreoptic intubation were not examined. These procedures remain untested in these novel devices and should be studied before aerosol boxes can be used safely. Similarly, the implications for management of the morbidly obese patient, the agitated patient or the patient requiring erect pre‐oxygenation also need to be studied for safety.
This study did not examine the efficacy of aerosol boxes in reducing the viral exposure risk to clinicians. There are no published studies examining whether aerosol boxes reduce healthcare worker viral exposure. This cannot be assumed and research is required. It has been suggested that the box should be removed if difficulty is encountered [16, 17]. We did not allow this in our study but it is likely that a number of boxes would have been removed. We did not examine the process of the emergency removal of the boxes; further investigation would be needed to examine if this was achievable in a timely and safe manner. Concerns with an emergency removal of the box during airway management include patient or healthcare worker injury, dispersal of aerosols and droplets from within the box, and the contamination of healthcare workers from the box surfaces.
Finally, we examined the use of these devices by the most experienced airway specialists, being specialist (consultant) anaesthetists. The volume of practice for intubations among intensive care specialists is significantly less [33]. It is possible that a non‐anaesthetist group of specialists would experience more difficulty with these techniques.
The SARS‐CoV‐2 pandemic has caused healthcare workers to fear for their personal safety at work and the genuine motivation to protect oneself from harm is easily understood. Nevertheless, this study demonstrates significant patient safety concerns in the use of aerosol boxes for intubation of COVID‐19 patients. Furthermore, aerosol boxes may paradoxically increase the risk to clinicians involved in COVID‐19 airway management by causing breaches of PPE.
Supporting information
Figure S1. Example of the simulated intubations used in this study, in this case using the early‐generation aerosol box.
Figure S2. Examples of damage to PPE associated with the use of aerosol boxes for intubation. Left: A gown caught on an occlusive dressing at the box’s arm‐hole. Middle: A gown strains and pulls loose from the glove during laryngoscopy. Right: A torn gown sleeve which was damaged during pre‐oxygenation (white cloth has been inserted for visual contrast).
Acknowledgements
No external funding or competing interests declared.
References
- 1. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID‐19 pandemic. Journal of the American Medical Association 2020; 323: 2133–34. [DOI] [PubMed] [Google Scholar]
- 2. Medscape . In Memoriam: healthcare workers who have died of COVID‐19. 2020. https://www.medscape.com/viewarticle/927976 (accessed 28/04/2020).
- 3. Artenstein AW. In pursuit of PPE. New England Journal of Medicine 2020; 382(18): e46. Epub 17 April. doi.org/10.1056/NEJMc2010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jayawardena A. Waiting for something positive. New England Journal of Medicine 2020; 382: e89. [DOI] [PubMed] [Google Scholar]
- 5. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. New England Journal of Medicine 2020; 382: 1564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheung JC, Ho LT, Cheng JV, Cham EYK, Lam KN. Staff safety during emergency airway management for COVID‐19 in Hong Kong. Lancet Respiratory Medicine 2020; 8: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7: e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. British Journal of Anaesthesia 2003; 90: 715–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One 2010; 5: e10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brewster D, Chrimes N, Do T, et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID‐19 adult patient group. Medical Journal of Australia 2020; 212: 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cook TM, El‐Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID‐ 19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia 2020; 75: 785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colasimone D. Australian doctors design and make life‐saving equipment needed for coronavirus pandemic. 2020. https://www.abc.net.au/news/2020-04-06/doctors-designing-medical-equipment-to-face-coronavirus-covid-19/12120588 (accessed 28/04/2020).
- 13. Singer AJ, Morley EJ, Henry MC. Staying ahead of the wave. New England Journal of Medicine 2020. Epub 13 April. doi.org/10.1056/NEJMc2009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duggan LV, Marshall SD, Scott J, Brindley PG, Grocott HP. The MacGyver bias and attraction of homemade devices in healthcare. Canadian Journal of Anesthesia 2019; 66: 757–61. [DOI] [PubMed] [Google Scholar]
- 15. Everington K. Taiwanese doctor invents device to protect US doctors against coronavirus. 2020. https://www.taiwannews.com.tw/en/news/3902435 (accessed 28/04/2020).
- 16. Canelli R, Connor CW, Gonzalez M, Nozari A, Ortega R. Barrier enclosure during endotracheal Intubation. New England Journal of Medicine 2020. Epub 3 April. doi.org/10.1056/NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leyva Moraga FA, Leyva Moraga E, Leyva Moraga F, et al. Aerosol box, an operating room security measure in COVID‐19 pandemic. World Journal of Surgery 2020. Epub 26 April. doi.org/10.1007/s00268‐020‐05542‐x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kearsley R. Intubation boxes for managing the airway in patients with COVID‐19. Anaesthesia 2020; 75: 969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. #GetUsPPE . Aerosol Boxes (Intubation Boxes). 2020. https://getusppe.org/makers/aerosol-boxes (accessed 28/04/2020).
- 20. Chan A. Should we use an “aerosol box” for intubation? 2020. https://litfl.com/should-we-use-an-aerosol-box-for-intubation (accessed 28/04/2020).
- 21. Learning From Excellence . LFE in COVID19. 2020. https://learningfromexcellence.com/covid19 (accessed 28/04/2020).
- 22. Yu C. Anesthesiologist trials taiwanese intubating box. 2020. https://www.youtube.com/watch?v=Hj0L_Dw5zU4 (accessed 28/04/2020).
- 23. The RB. Intubation Box is an inexpensive and reusable personal protective device that functions as a barrier between Covid‐19 patients and healthcare providers. 2020. https://intubationbox.com (accessed 30/04/2020).
- 24. Babcock S. Here’s how Shore Plastics spearheaded local production of doc‐protecting Aerosol Boxes. 2020. https://technical.ly/baltimore/2020/04/07/aerosol-boxes-protect-doctors-during-intubation-covid-19-shore-plastics-spearheaded-local-production-quake-scientific (accessed 28/04/2020).
- 25. Buja M, Russom P. Waltham company makes aerosol boxes to help prevent spread of COVID‐19 among health care workers. 2020. https://www.nbcboston.com/news/coronavirus/waltham-company-makes-aerosol-boxes-to-help-prevent-spread-of-covid-19-among-health-care-workers/2104103 (accessed 28/04/2020).
- 26. Canning M. Limavady firm's aerosol box protects medics from Covid‐19 in NHS 'hour of need'. 2020. https://www.belfasttelegraph.co.uk/business/northern-ireland/limavady-firms-aerosol-box-protects-medics-from-covid-19-in-nhs-hour-of-need-39135617.html (accessed 28/04/2020).
- 27. Konica KC. Minolta looks to help Aus healthcare workers with 3D printing. 2020. https://itbrief.com.au/story/konica-minolta-looks-to-help-aus-healthcare-workers-with-3d-printing (accessed 28/04/2020).
- 28. Leff B, Finucane TE. Gizmo idolatry. Journal of the American Medical Association 2008; 299: 1830–2. [DOI] [PubMed] [Google Scholar]
- 29. Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. British Journal of Anaesthesia 2015; 115: 827–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stiegler MP, Neelankavil JP, Canales C, Dhillon A. Cognitive errors detected in anaesthesiology: a literature review and pilot study. British Journal of Anaesthesia 2012; 108: 229–35. [DOI] [PubMed] [Google Scholar]
- 31. Sorbello M, El‐Boghdadly K, Di Giacinto I, et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia 2020; 75: 724–32. [DOI] [PubMed] [Google Scholar]
- 32. Farmery AD. Simulating hypoxia and modelling the airway. Anaesthesia 2011; 66(Suppl. 2): 11–8. [DOI] [PubMed] [Google Scholar]
- 33. Brewster DJ, Nickson CP, Gatward JJ, Staples M, Hawker F. Should ongoing airway education be a mandatory component of continuing professional development for College of Intensive Care Medicine Fellows? Anaesthesia and Intensive Care 2018; 46: 190–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Example of the simulated intubations used in this study, in this case using the early‐generation aerosol box.
Figure S2. Examples of damage to PPE associated with the use of aerosol boxes for intubation. Left: A gown caught on an occlusive dressing at the box’s arm‐hole. Middle: A gown strains and pulls loose from the glove during laryngoscopy. Right: A torn gown sleeve which was damaged during pre‐oxygenation (white cloth has been inserted for visual contrast).


