Abstract
Lung ultrasound (LUS) plays a critical role in the SARS‐CoV‐2 pandemic. Evidence is mounting on its utility to diagnose, assess the severity and as a triage tool in the ED. Sonographic features correlate well to computed tomography (CT) chest findings and a bedside LUS performed by a trained clinician along with clinical examination, could be an alternative to chest X‐ray and CT chest in these highly infectious patients. In this article, we have described a step‐by‐step approach to LUS in COVID patients and the CLUE (COVID‐19 LUS in the ED) protocol, which involves an anatomical parameter, the severity of lung changes, objectively scored using the validated LUS scoring system and a physiological parameter, oxygen requirement. We believe this CLUE protocol can help risk‐stratify patients presenting to ED with suspected COVID‐19 and aid clinicians in making appropriate disposition decisions.
Keywords: CLUE, COVID‐19, emergency, lung ultrasound, POCUS
Lung ultrasound in COVID‐19: current evidence
Lung ultrasound (LUS) is a vital part of critical care evaluation of multiple lung pathologies, like pneumothorax, acute respiratory distress syndrome, pulmonary oedema, interstitial lung disease and pneumonia. 1 As SARS‐CoV‐2 infection causes interstitial pneumonitis, there is an extensive use of LUS in COVID‐19 patients in China 2 and Italy. 3 The detection of COVID‐19 by reverse transcription polymerase chain reaction testing of nasopharyngeal swabs, considered as the gold‐standard test, lacks sensitivity compared to computed tomography (CT) chest, 59% vs 88%, respectively. 4 Ultrasound has an excellent correlation to CT chest findings 2 and could be an alternative to ionising radiation imaging. 3 Poor sensitivity of 59% for chest X‐ray (CXR) to detect COVID‐19 changes 5 and superiority of ultrasound in similar interstitial lung disease, 6 makes it an attractive imaging option. Performance of LUS at bedside also allows concurrent execution of clinical examination and lung imaging by the same clinician, expedites clinical decision making. 7
Technical aspects of LUS in COVID‐19
A step‐by‐step approach to safely performing LUS is given in Table 1. We recommend chest be scanned systematically as 12 zones, six zones for the right lung (R1–R6) and six zones for the left lung (L1–L6, Fig. 1). Scanning the posterior lung zones (R5, R6, L5, L6) will improve the sensitivity of LUS, as most changes are in the posterior lung. 8 For safe scanning, the patient to sit facing away from the clinician and posterior, lateral (R3, R4, L3, L4) and even anterior (R1, R2, L1, L2) zones scanned by the clinician positioned behind the patient. If the patient is in the supine position (unwell to move or sedated), the posterior lung zones replaced by scanning areas slightly posterior to the posterior axillary line. In our limited experience with COVID‐19 patients, it takes less than 10 min to perform LUS, excluding cleaning time.
Table 1.
Step‐by‐step approach on scanning COVID‐19
| Don personal protective equipment (PPE) and double gloves |
| Perform ultrasound (US) only if needed and preferably along with clinical examination |
| Handheld US device (cover entire device) or Cartwheel US device (transparent plastic drape and transducer cover) |
| Use small disposable packets of gel |
| Position patient facing away from the sonographer (if possible) |
| Scan posterior lung zones (R5, R6, L5, L6), then lateral zones (R3, R4, L3, L4) and finally anterior zones (R1, R2, L1, L2) |
| Acquire video clips and label presets to minimise keyboard handling |
| After scanning, remove transducer cover, plastic drape and outer pair of gloves |
| Wearing the inner pair of gloves, wipe‐clean entire machine |
| Doff PPE, wear new gloves and wipe‐clean entire machine again |
Figure 1.
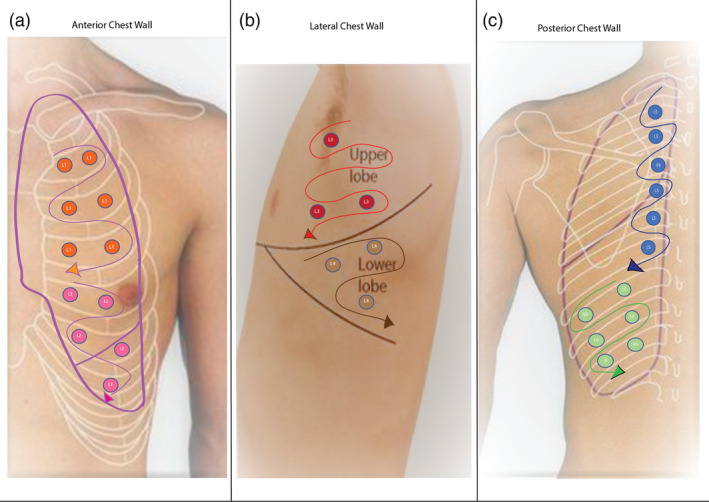
Left lung zones. L1, left upper anterior; L2, left lower anterior; L3, left upper lateral; L4, left lower lateral; L5, left upper posterior; L6, left lower posterior.
Coronavirus being a lipid‐based enveloped virus is susceptible to low‐level alcohol‐based disinfectant wipes 9 but strongly recommend involvement of the infection‐control department and the ultrasound manufacturer in disinfection planning and guideline development.
Sonographic features in COVID‐19
An appropriately optimised image of a normal LUS will feature A‐lines and few B‐lines (<3 B‐lines per intercostal space) and smooth thin pleural line. 1 Sonographic features of COVID‐19 pneumonitis are: 2
Increased number of B‐lines (discrete or confluent, multifocal and usually bilateral).
Thickening of pleura with pleural line irregularities.
Subpleural small consolidations (<1 cm height), which progress to large poorly vascularised or avascular consolidations 8 (>1 cm height), with occasional air bronchograms.
Pleural effusions are uncommon.
CLUE protocol: COVID‐19 LUS in ED protocol
CLUE protocol (Fig. 2) involves an anatomical parameter, LUS scoring system (LUSS) and a physiological parameter, oxygen requirement at the time of examination, to aid emergency clinician make disposition decision.
Figure 2.
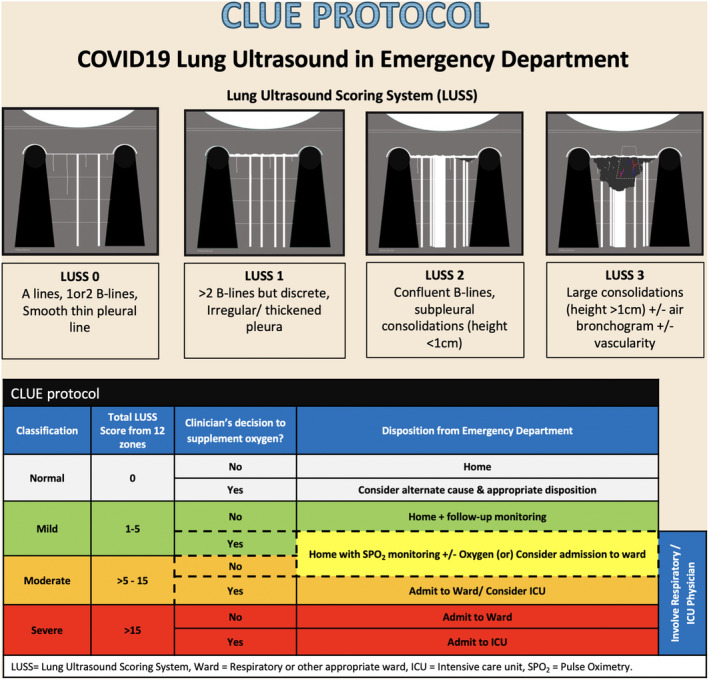
CLUE protocol. ICU, intensive care unit; LUSS, lung ultrasound scoring system; SPO2, pulse oximetry; ward, respiratory or other appropriate ward.
LUSS is a valid tool to assess regional and global lung aeration in acute respiratory distress syndrome 10 , 11 and can be used in COVID‐19 pneumonitis with several similar sonographic features. 2 At each zone, LUSS points range from 0 to 3, with higher points allocated to severe lung changes (Fig. 2). Based on the total score from 12 lung zones, the severity classified as mild (score 1–5), moderate (>5–15) and severe (>15). A normal lung will have a total score of 0.
A clinician's decision on the need for supplemental oxygen is a complex process, involving factors like oxygen saturation, work of breathing, respiratory rate and pre‐existing medical conditions (i.e. chronic obstructive pulmonary disease, heart disease). A single parameter like oxygen saturation or respiratory rate may not represent real‐time clinical practice.
CLUE protocol only provides a foundation, which is easy to use and flexible to accommodate complex clinical presentations. Some of the patients in the mild and moderate severity groups could safely go home from the ED, provided a proper self‐isolation facility, and adequate community follow‐up ensured. In patients, who are depicted in cells with dotted borders in the table ‘CLUE protocol’ in Figure 2, consider in‐hospital management if no pulse‐oximetry monitoring or home‐oxygen support provided.
Why CLUE protocol?
While Australia and New Zealand prepare for a figurative tsunami of highly infectious patients, we anticipate that a protocolised use of bedside LUS by emergency clinicians in COVID‐19 patients could alleviate some of the radiological resource burden expected.
Existing evidence supports LUS in COVID‐19, but none has a clear objective scoring system or incorporates clinician's assessment in decision making. CLUE protocol aims to address this gap and provide the emergency clinician with an appropriate disposition plan. CLUE protocol will provide instant, objective information of the severity of the disease and may avoid further imaging like CXR and CT chest. Absence of ionising radiation with ultrasound makes it an ideal imaging modality for serial assessments, providing an objective measure of disease progression. Ultrasound performed by the treating clinician during the clinical examination may minimise the number of staff encounters, potentially minimise healthcare worker infection rate and cross‐contamination among patients.
We anticipate several limitations. Firstly, LUSS and CLUE protocol have never been tested for use in COVID‐19 viral pneumonitis and currently a multicentre trial in Australia and New Zealand EDs in progress, to evaluate this scoring system. Secondly, LUS findings are not specific to COVID‐19 and may not correlate to clinical outcome. Thirdly, using ultrasound in COVID‐19 involves meticulous infection control practice. Finally, LUS requires an operator with a certain degree of training, and we strongly emphasise that beginners to LUS are not to train on these highly infectious patients.
Conclusion
CLUE protocol which incorporates LUSS and supplemental oxygen requirement at the time of examination, when performed by a trained emergency clinician, can help risk‐stratify suspected COVID‐19 patients. This protocol will aid the clinician to make rapid and appropriate bedside clinical decisions, potentially decrease reliance on CXRs or CT chest and aid disposition planning from the ED.
Acknowledgements
The authors thank Dr Elissa Kennedy‐Smith and Dr Cris Zollo for their valuable suggestions and review of manuscript.
Author contributions
All authors contributed to protocol development, manuscript writing, approval of the final version and agree to be accountable for all aspects of the work. VM is the project supervisor, researched and drafted the work. SS drafted Figure 1.
Competing interests
None declared.
Vijay Manivel, MBBS, FACEM, FEM, DDU, Senior Emergency Physician, Director Emergency Ultrasound Training, Clinical Senior Lecturer; Andrew Lesnewski, MBBS, Advanced Trainee; Simin Shamim, MBBS, Advanced Trainee; Genevieve Carbonatto, MBBS, FACEM, CCPU, Senior Emergency Physician; Thiru Govindan, MBBS, FACEM, CCPU, Senior Emergency Physician.
References
- 1. Mayo PH, Copetti R, Feller‐Kopman D et al. Thoracic ultrasonography: a narrative review. Intensive Care Med. 2019; 45: 1200–11. [DOI] [PubMed] [Google Scholar]
- 2. Peng QY, Wang XT, Zhang LN. Findings of lung ultrasonography of novel corona virus pneumonia during 2019‐2020 epidemic. Intensive Care Med. 2020; 46: 849–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poggiali E, Dacrema A, Bastoni D et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID‐19) pneumonia? Radiology 2020; 295: E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ai T, Yang Z, Hou H et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology 2020; 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vizioli L, Ciccarese F, Forti P et al. Integrated use of lung ultrasound and chest X‐ray in the detection of interstitial lung disease. Respiration 2017; 93: 15–22. [DOI] [PubMed] [Google Scholar]
- 7. Buonsenso D, Pata D, Chiaretti A. COVID‐19 outbreak: less stethoscope, more ultrasound. Lancet Respir. Med. 2020; 8: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang Y, Wang S, Liu Y et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non‐critical novel coronavirus pneumonia (COVID‐19). SSRN 2020; 10.2139/ssrn.3544750. [DOI] [Google Scholar]
- 9. Abramowicz JS, Basseal JM. World Federation for Ultrasound in Medicine and Biology position statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID‐19. Ultrasound Med. Biol. 2020; 10.1016/j.ultrasmedbio.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiumello D, Mongodi S, Algieri I et al. Assessment of lung aeration and recruitment by CT scan and ultrasound in acute respiratory distress syndrome patients. Crit. Care Med. 2018; 46: 1761–8. [DOI] [PubMed] [Google Scholar]
- 11. Mongodi S, Bouhemad B, Orlando A et al. Modified lung ultrasound score for assessing and monitoring pulmonary aeration. Ultraschall Med. 2017; 38: 530–7. [DOI] [PubMed] [Google Scholar]


