ABSTRACT
The SARS‐CoV‐2 pandemic is unprecedented in our professional lives and much effort and resources will be devoted to care of patients (and HCW) affected by this illness. We must also continue to aim for the same standard of care for our non‐COVID respiratory patients, while minimizing risks of infection transmission to our colleagues. This commentary addresses the key paired issues of minimizing performance of diagnostic/staging bronchoscopy in patients with suspected/known lung cancer while maximizing the safety of the procedure with respect to HCW transmission of COVID‐19.
Keywords: bronchoscopy and interventional techniques, coronavirus, COVID‐19, endobronchial ultrasound, infectious disease transmission, lung cancer
INTRODUCTION
Since late December 2019, an outbreak of infection caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has spread from Wuhan, China, to over 60 countries worldwide, with (at the time of writing) over 1 500 000 confirmed cases and more than 80 000 deaths. 1 Transmission is predominantly respiratory, 2 raising concerns that bronchoscopy may increase the risk of infection of healthcare workers (HCW). Evidence regarding this risk is unclear, with one study suggesting bronchoscopy does not generate aerosols, 3 and another study reporting only a strong trend towards increased risk of transmission to HCW during the H1N1 2009 influenza epidemic. 4 Nevertheless, bronchoscopy remains universally regarded as an aerosol‐generating procedure (AGP). 5 This, as well as prioritization of preservation of limited personal protective equipment (PPE) stock, had resulted in uniform agreement that non‐urgent bronchoscopy be postponed until the coronavirus disease 2019 (COVID‐19) pandemic has passed.
Guidelines developed to date have defined the role of bronchoscopy in patients with suspected or confirmed COVID‐19 infection. 6 Throughout the pandemic, however, patients will continue to present with symptoms and findings not related to COVID‐19 infection, but as a result of suspected lung cancer. In these patients, bronchoscopy remains a potentially important diagnostic (and therapeutic) tool. 7 In this manuscript, we review the diagnostic work‐up of patients with suspected/known lung cancer. We address how a reduction in the overall number of bronchoscopic procedures may be achieved, and how bronchoscopy, when performed, may be done as safely as possible. Considerations apply both to suspected non‐small cell lung cancer (NSCLC) and small cell lung cancer.
INDICATIONS
Non‐invasive imaging should still be completed in a routine manner, with positron emission tomography (PET) potentially even more important in guiding clinical decision‐making in the current climate. The following consideration regarding stage classification is based on PET findings, with recommendations summarized in Table 1.
Table 1.
Summary of recommendations based on clinical stage of suspected/confirmed lesion
| Clinical stage† | Recommended management strategy |
|---|---|
| Stage I | Percutaneous biopsy where technically possible |
| Consider resectional biopsy or empiric SABR | |
| Stage II | Percutaneous biopsy where technically possible |
| Consider surgical resection without tissue diagnosis | |
| Stage III | Percutaneous biopsy of primary lesion for bulky/multi‐station mediastinal involvement of PET/CT |
| EBUS‐TBNA for sampling of single‐station cN2/3 disease | |
| EBUS‐TBNA for tissue diagnosis ± ancillary molecular testing where no option for percutaneous sampling is available | |
| Stage IV | Percutaneous biopsy/(drain) where possible from extrathoracic site |
| EBUS‐TBNA for patients with extrathoracic sites unsuitable for minimally invasive biopsy or bony metastases as sole M1 site 26 |
On the basis of PET/CT.
CT, computed tomography; EBUS‐TBNA, endobronchial ultrasound‐guided transbronchial needle aspiration; PET, positron emission tomography; SABR, stereotactic ablative body radiotherapy.
Stage I
While preoperative diagnosis is preferred in many centres to minimize rates of benign resection, and to limit reliance of intraoperative frozen section for surgical decision‐making, primary diagnostic surgical resection is recommended in some guidelines for lesions whose radiological features indicate a high probability of malignancy.8, 9 We expect that a higher proportion of patients will undergo resectional biopsy ± frozen section assessment during COVID‐19.
Equally, where lesions have a sufficiently high likelihood of malignancy, even in high‐risk surgical patients, empiric stereotactic ablative body radiotherapy (SABR) may be delivered safely on the basis of radiological predictors of malignancy risk.10, 11 SABR is now well established as a safe and effective treatment modality for localized (N0) NSCLC and should be considered in suitable patients. Treatment of centrally positioned tumours by SABR is associated with a higher risk of major complications, and it is predominantly this subset of patients that will still require bronchoscopic diagnosis.
Radial endobronchial ultrasound (EBUS) or navigation bronchoscopy is frequently undertaken for diagnosis of peripheral pulmonary lesions. 12 Radial EBUS is the preferred approach 13 due to a high sensitivity and favourable complication profile. 14 Percutaneous lung biopsy with either computed tomography (CT) or ultrasound guidance demonstrates high diagnostic accuracy and is suggested as a diagnostic option. The procedure is generally performed with only local anaesthesia, under aseptic conditions, and presents a lower risk of transmission of infection than bronchoscopic procedures (see Section Anaesthesia). We recommend percutaneous biopsy where technically possible after review by an interventional radiologist.
We expect that a small number of patients will still require radial EBUS for diagnosis of centrally positioned lesions (or linear EBUS/EUS‐B15, 16) unsuitable for SABR (prior to conventionally fractionated radiotherapy), predominantly within patients at higher surgical risk due to either cardiorespiratory disease or radiological characteristics of the tumour.
Stage II
Many centres would undertake minimally invasive mediastinal LN staging patients with cN1 findings on PET due to the elevated risk of post‐operative upstaging to N2 in this group. 17 However, given the current risks and potentially reduced sensitivity of EBUS/endoscopic staging in this patient subset,17, 18 we suggest that such patients undergo percutaneous biopsy of the primary tumour prior to surgical resection, or may even be appropriate for surgical resection without a tissue diagnosis, given the high likelihood of malignancy.
Stage III (cN2/3)
In this staging group, we (simplistically) identify two separate scenarios based on PET findings: (i) single‐station N2 disease in otherwise operative candidates and (ii) multilevel/bulky mediastinal LN involvement.
For patients with single‐station LN involvement, we recommend performance of LN staging with linear EBUS due to the major impact on therapeutic decision‐making according to pathological status of this LN. Sampling via oesophageal endoscopic route (EUS‐B) is associated with shorter procedure times and may reduce cough, 19 and may be considered where expertise allows.
Systematic mediastinal LN staging by EBUS improves staging in patients with Stage III NSCLC 20 and significantly improves coverage of subclinical disease through detection of PET‐occult metastases. 21 Nevertheless, the proportion of patients in whom PET‐occult disease is detected20, 22 may not justify the risk to HCW resulting from performance of bronchoscopy. Patients with bulky/multi‐station mediastinal involvement on PET may be appropriately cared for by percutaneous biopsy, without need for (minimally invasive) mediastinal sampling. Sensitivity of PET in such instances is high and radiation fields can safely be constructed on the basis of PET‐identified disease extent. 23
Stage IV
Minimally invasive tissue diagnosis is perhaps most critical in this group due to the importance of NSCLC sub‐typing for treatment planning as well as the need to complete molecular characterization of the tumour, including detection of driver mutations 24 and assessment of PD‐L1 (Programmed Death Ligand ‐ 1) status, which can be achieved with high accuracy by EBUS. 25
Tissue confirmation of suspected metastatic site is recommended, and should provide sufficient tissue to allow molecular testing to be completed. Thus, percutaneous biopsy should be sufficient for most patients. We expect there will remain a minority of patients with clinical Stage IV NSCLC who require bronchoscopic tissue diagnosis, likely via linear EBUS. These include patients with suspected nodules in ipsilateral lobes (T4) or contralateral lung (M1a), as well as patients with extrathoracic sites identified on PET unsuitable for minimally invasive biopsy. This includes patients with only bony metastases, which are recommended to be avoided due to low yield of tumour cells and poor DNA quality consequent to decalcification acids. 26
PRE‐PROCEDURE SCREENING
The intent of pre‐procedure screening is to identify patients with possible/likely COVID‐19 infection in order to instigate isolation practices to minimize transmission risks, including to HCW. Patients should be screened (either prior by phone or at entry to the health facility) for clinical or epidemiological markers of risk for active COVID‐19 infection. Recent overseas travel or close contact with a known case of COVID‐19 should prompt a delay of the procedure by a fortnight (provided they do not subsequently develop symptoms of COVID‐19 infection). Presence of fever/respiratory illness, depending on local community prevalence rates/practices, may prompt testing for COVID and delay of bronchoscopy until confirmatory negative results are known.
Where resources allow, consideration should be given to pre‐bronchoscopic testing for asymptomatic COVID‐19 infection via nasopharyngeal swab. Negative results would allow patients to proceed to bronchoscopy/EBUS, while positive testing would suggest a delay in undertaking the procedure—while lung cancer diagnosis cannot wait several months, it certainly can wait 2 weeks, which is longer than the time to viral clearance following mild COVID‐19 illness. 27 If patients develop symptoms subsequent to a positive test, then a longer delay before bronchoscopy is needed.28, 29
Critically, we note that sensitivity of nasopharyngeal swab for COVID‐19 infection has been reported at 71–83% among symptomatic patients,30, 31 and may be even lower among asymptomatic patients. Given the numerous reports regarding false‐negative results for nasopharyngeal swab reverse transcriptase (RT)‐PCR testing,32, 33 we recommend universal precautions, including PPE (below), be applied even in the event of a negative nasopharyngeal swab result.
ANAESTHESIA
Bronchoscopy is considered an AGP; however, the highest risk of infection transmission has been reported with tracheal intubation and extubation. 34 Use of muscle relaxant may reduce cough and aerosol generation; however, it is not clear that this reduces aerosol generation associated with endotracheal intubation, and will not lessen the risk at the time of extubation. Therefore, given the large risk associated with intubation/extubation, we feel the preferred approach is for the procedure to be performed with moderate/deep intravenous sedation.
Aerosol generation is highest at the time of bronchoscopic intubation. 35 Use of a laryngeal mask airway (LMA) may allow deeper sedation and reduced cough, although the impact on potential for transmission of infection remains unknown.
Transmission of SARS‐CoV‐2 is predominantly via droplet spread. 2 Use of PPE (below) is essential to minimize the risk of HCW infection. Dispersal of droplets following respiratory expulsion may significantly exceed 1–2 m, 36 and viruses may survive in the air for hours and on surfaces for days. 37 Prevention of droplet dispersal may significantly mitigate HCW exposure, resulting in greater levels of protection than achieved through wearing of masks by HCW. Masks worn at the droplet ‘source’ (by the patient) significantly reduce aerosol dispersal 38 and viral content in dispersed respiratory droplets. 39 This can potentially be achieved during bronchoscopy through use of a ‘slotted mask’ during the procedure to minimize droplet dispersal following cough (Fig. 1).
Figure 1.
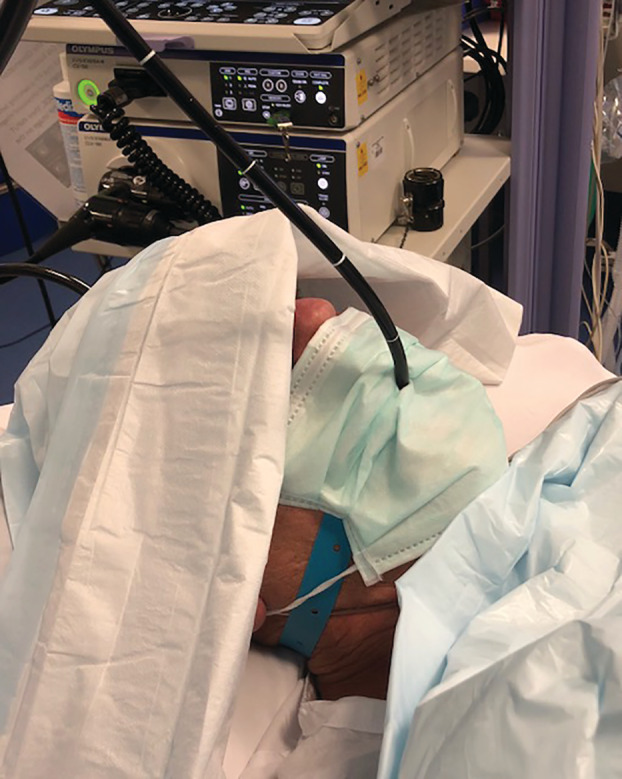
Oral intubation by the linear videobronchoscope through a small incision in a standard surgical mask. This ‘slotted mask’ allows airway access during a moderate sedation anaesthetic while minimizing droplet spread especially during episodes of cough.
Use of a surgical mask, or even FFP2/N95 masks, to the patient following LMA extubation may also be an important risk mitigation intervention.
No nebulizers including lignocaine should be used. Nebulization itself is an AGP, 40 and nebulization may convert bronchoscopy to a higher risk procedure also. 3
PROCEDURE
Where possible, procedures should be performed in a negative pressure room. Consider re‐application of lignocaine to minimize cough on/following extubation of the bronchoscope.
Both for the purposes of preservation of PPE, as well as minimization of HCW exposure, the procedure should be performed with the minimum number of staff in the procedure room. Guidelines will be available in individual institutions/jurisdictions, but should include use of P2/N95 high‐particulate respiratory masks and level 3–4 barrier protection (disposable gloves, impervious gown and eye protection). 41
It is self‐evident that the procedure should be performed in the shortest time possible and with the fewest number of sampling procedures required to achieve the clinical goal. Accordingly, procedures should be performed only by consultant interventional pulmonologists.
While use of rapid on‐site cytological evaluation (ROSE) may increase the number of HCW exposed, we believe use of ROSE is overall highly beneficial due to the impact on reduced number of needle passes as well as reduced requirement for additional bronchoscopy procedures to make a final diagnosis, 42 and the consequent reduced procedure times achieved by their utilization. 43 Consequently, our preference is to use ROSE during the COVID‐19 event. This should only be done in discussion with local experts/authorities.
FURTHER COMMENTS
It may be prudent to routinely submit bronchial washing specimens collect at bronchoscopy for COVID testing. Exact rates of asymptomatic infection in COVID are unknown but may be significant.44, 45 Asymptomatic cases likely contribute significantly to community transmission of COVID.46, 47, 48 Pandemic influenza is more readily detected in lower respiratory tract specimens,49, 50 with limited reports suggesting the same is true for SARS‐CoV‐2.33, 51 The most sensitive detection of coronavirus requires testing of both upper and lower respiratory samples.52, 53
Beyond the public health advantage of accurately determining infection numbers, significant benefits may follow detection of asymptomatic or mild/early cases. These patients are individually at risk of adverse outcomes of COVID‐19 infection, but equally in their subsequent care are likely to be in close contact with HCW, as well as a vulnerable group of fellow patients. It is possible they will be receiving immunosuppressive therapies as part of their treatment.
Routine testing is suggested by at least one international practice statement, 7 although it should be based on local resources and addressed in discussion with local health authorities. Practice may differ depending on the phase of the pandemic community infection prevalence.
CONCLUSIONS
The SARS‐CoV‐2 pandemic is unprecedented in our professional lives and much effort and resources will be devoted to care of patients (and HCW) affected by this illness. Nevertheless, we must also continue to aim for the same standard of care for our non‐COVID respiratory patients, while minimizing risks of infection transmission to our colleagues. This commentary has addressed the key paired issues of minimizing performance of bronchoscopy to only patients with no alternate option while maximizing the safety of the procedure with respect to HCW transmission.
Abbreviations
- AGP
aerosol‐generating procedure
- COVID‐19
coronavirus disease 2019
- CT
computed tomography
- EBUS
endobronchial ultrasound
- EBUS‐TBNA
EBUS‐transbronchial needle aspiration
- EUS‐B
endoscopic ultrasound using (video) bronchoscope
- HCW
healthcare worker
- LMA
laryngeal mask airway
- LN
lymph node
- NSCLC
non‐small cell lung cancer
- PCR
polymerase chain reaction
- PET
positron emission tomography
- PPE
personal protective equipment
- ROSE
rapid on‐site cytological evaluation
- SABR
stereotactic ablative body radiotherapy
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Steinfort DP, Herth FJF, Irving LB, Nguyen PT. Safe performance of diagnostic bronchoscopy/EBUS during the SARS‐CoV‐2 pandemic. Respirology. 2020;25:703–708. 10.1111/resp.13843
Handling Editors: Philip Bardin and Paul Reynolds
REFERENCES
- 1. John Hopkins University Coronavirus Resource Center . 2020. [Accessed 22 Apr 2020.] Available from URL: https://coronavirus.jhu.edu
- 2. World Health Organization . Modes of transmission of virus causing COVID‐19: implications for IPC precaution recommendations. Scietific brief. WHO reference number: WHO/2019‐nCoV/Sci_Brief/Transmission_modes/20202.
- 3. O'Neil CA, Li J, Leavey A, Wang Y, Hink M, Wallace M, Biswas P, Burnham CD, Babcock HM. Characterization of aerosols generated during patient care activities. Clin. Infect. Dis. 2017; 65: 1335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson KA, Pappachan JV, Bennett AM, Mittal H, Macken S, Dove BK, Nguyen‐Van‐Tam JS, Copley VR, O'Brien S, Hoffman P et al. Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic – the risk of aerosol generation during medical procedures. PLoS One 2013; 8: e56278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Centre for Disease Prevention and Control (ECDC) . Infection prevention and control for the careof patients with 2019‐nCoV in healthcare settings. 2020. [Updated Feb 2020; Accessed 1 Apr 2020.] Available from URL: https://www.ecdc.europa.eu/sites/default/files/documents/nove-coronavirus-infectionprevention-control-patients-healthcare-settings.pdf
- 6. Lentz RJ, Colt H. Summarizing societal guidelines regarding bronchoscopy during the COVID‐19 pandemic. Respirology 2020; 25: 547–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo F, Luo F, Darwiche K, Singh S, Torrego A, Steinfort DP, Gasparini S, Liu D, Zhang W, Fernandez‐Bussy S et al. Performing bronchoscopy in times of the COVID‐19 pandemic – practice statement from an international expert panel. Respiration 2020: 1–6. 10.1159/000507898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baldwin DR, Callister ME; Guideline Development Group . The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015; 70: 794–8. [DOI] [PubMed] [Google Scholar]
- 9. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non‐small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143(5 Suppl.): e278S–313S. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez Maldonado S, Delorme S, Husing A, Motsch E, Kauczor HU, Heussel CP, Kaaks R. Evaluation of prediction models for identifying malignancy in pulmonary nodules detected via low‐dose computed tomography. JAMA Netw. Open 2020; 3: e1921221. [DOI] [PubMed] [Google Scholar]
- 11. Herder GJ, van Tinteren H, Golding RP, Kostense PJ, Comans EF, Smit EF, Hoekstra OS. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F‐fluorodeoxyglucose positron emission tomography. Chest 2005; 128: 2490–6. [DOI] [PubMed] [Google Scholar]
- 12. Steinfort DP, Bonney A, See K, Irving LB. Sequential multimodality bronchoscopic investigation of peripheral pulmonary lesions. Eur. Respir. J. 2016; 47: 607–14. [DOI] [PubMed] [Google Scholar]
- 13. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143(5 Suppl.): e142S–65S. [DOI] [PubMed] [Google Scholar]
- 14. Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta‐analysis. Eur. Respir. J. 2011; 37: 902–10. [DOI] [PubMed] [Google Scholar]
- 15. Nakajima T, Yasufuku K, Fujiwara T, Chiyo M, Sekine Y, Shibuya K, Shibuya K, Hiroshima K, Yoshino I. Endobronchial ultrasound‐guided transbronchial needle aspiration for the diagnosis of intrapulmonary lesions. J. Thorac. Oncol. 2008; 3: 985–8. [DOI] [PubMed] [Google Scholar]
- 16. Steinfort DP, Farmer MW, Irving LB, Jennings BR. Pulmonologist‐performed per‐esophageal needle aspiration of parenchymal lung lesions using an EBUS bronchoscope: diagnostic utility and safety. J. Bronchology Interv. Pulmonol. 2017; 24: 117–24. [DOI] [PubMed] [Google Scholar]
- 17. Leong TL, Loveland PM, Gorelik A, Irving L, Steinfort DP. Preoperative staging by EBUS in cN0/N1 lung cancer: systematic review and meta‐analysis. J. Bronchology Interv. Pulmonol. 2019; 26: 155–65. [DOI] [PubMed] [Google Scholar]
- 18. Dooms C, Tournoy KG, Schuurbiers O, Decaluwe H, De Ryck F, Verhagen A, Beelen R, van der Heijden E, De Leyn P. Endosonography for mediastinal nodal staging of clinical N1 non‐small cell lung cancer: a prospective multicenter study. Chest 2015; 147: 209–15. [DOI] [PubMed] [Google Scholar]
- 19. Oki M, Saka H, Ando M, Tsuboi R, Nakahata M, Oka S, Kogure Y, Kitagawa C. Transbronchial vs transesophageal needle aspiration using an ultrasound bronchoscope for the diagnosis of mediastinal lesions: a randomized study. Chest 2015; 147: 1259–66. [DOI] [PubMed] [Google Scholar]
- 20. Steinfort DP, Siva S, Leong TL, Rose M, Herath D, Antippa P, Ball DL, Irving LB. Systematic endobronchial ultrasound‐guided mediastinal staging versus positron emission tomography for comprehensive mediastinal staging in NSCLC before radical radiotherapy of non‐small cell lung cancer: a pilot study. Medicine (Baltimore) 2016; 95: e2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cole AJ, Hardcastle N, Turgeon GA, Thomas R, Irving LB, Jennings BR, Ball D, Kron T, Steinfort DP, Siva S. Systematic endobronchial ultrasound‐guided transbronchial needle aspiration improves radiotherapy planning in non‐small cell lung cancer. ERJ Open Res. 2019; 5: pii: 00004‐2019. 10.1183/23120541.00004-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peeters ST, Dooms C, Van Baardwijk A, Dingemans AM, Martinussen H, Vansteenkiste J, Decaluwé H, De Leyn P, Yserbyt J, Nackaerts K et al. Selective mediastinal node irradiation in non‐small cell lung cancer in the IMRT/VMAT era: how to use E(B)US‐NA information in addition to PET‐CT for delineation? Radiother. Oncol. 2016; 120: 273–8. [DOI] [PubMed] [Google Scholar]
- 23. Lehman M. Clinical question: what are the principles of radiation therapy in the definitive management of stage III inoperable NSCLC. [Accessed 16 Apr 2015.] Available from URL: http://wiki.cancer.org.au/australia/Guidelines:Lung_cancer2015 and http://wiki.cancer.org.au/australia/Clinical_question:What_are_the_principles_of_radiation_therapy_in_the_definitive_management_of_stage_III_inoperable_NSCLC%3F
- 24. van der Heijden EH, Casal RF, Trisolini R, Steinfort DP, Hwangbo B, Nakajima T, Guldhammer‐Skov B, Rossi G, Ferretti M, Herth FF et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound‐guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014; 88: 500–17. [DOI] [PubMed] [Google Scholar]
- 25. Hendry S, Byrne DJ, Christie M, Steinfort DP, Irving LB, Wagner CA, Ellwood T, Cooper WA, Fox SB. Adequate tumour cellularity is essential for accurate PD‐L1 immunohistochemistry assessment on cytology cell‐block specimens. Cytopathology 2020; 31: 90–5. [DOI] [PubMed] [Google Scholar]
- 26. John T, Bowden JJ, Clarke S, Fox SB, Garrett K, Horwood K, Karapetis CS. Australian recommendations for EGFR T790M testing in advanced non‐small cell lung cancer. Asia Pac. J. Clin. Oncol. 2017; 13: 296–303. [DOI] [PubMed] [Google Scholar]
- 27. Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, Peiris M, Poon LLM, Zhang W. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect. Dis. 2020; pii: S1473‐3099(20)30232‐2. 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, Wu F, Song ZG, Huang W, Chen J et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. (Engl.) 2020; 133: 1039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang MG, Yuan X, Tao Y, Peng X, Wang F, Xie L, Sharma L, Dela Cruz CS, Qin E. Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection. Am. J. Respir. Crit. Care Med. 2020; 201: 1150–52. 10.1164/rccm.202003-0524LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, Zeng B, Li Z, Li X, Li H. Diagnosis of the coronavirus disease (COVID‐19): rRT‐PCR or CT? Eur. J. Radiol. 2020; 126: 108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of chest CT for COVID‐19: comparison to RT‐PCR. Radiology 2020: 200432. 10.1148/radiol.2020200432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology 2020: 200642. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li D, Wang D, Dong J, Wang N, Huang H, Xu H, Xia C. False‐negative results of real‐time reverse‐transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2: role of deep‐learning‐based CT diagnosis and insights from two cases. Korean J. Radiol. 2020; 21: 505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7: e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lavoie J, Marchand G, Cloutier Y, Halle S, Nadeau S, Duchaine C, Pichette G. Evaluation of bioaerosol exposures during hospital bronchoscopy examinations. Environ. Sci. Process Impacts 2015; 17: 288–99. [DOI] [PubMed] [Google Scholar]
- 36. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID‐19. JAMA 2020. 10.1001/jama.2020.4756. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N. Engl. J. Med. 2020; 382: 1564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diaz KT, Smaldone GC. Quantifying exposure risk: surgical masks and respirators. Am. J. Infect. Control 2010; 38: 501–8. [DOI] [PubMed] [Google Scholar]
- 39. Leung NHL, Chu DKW, Shiu E, Chan K, McDeviutt J, Hau B, Yen H, Li Y, Ip D, Peiris J et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020. 10.1038/s41591-020-0843-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y, Li J, Leavey A, O'Neil C, Babcock HM, Biswas P. Comparative study on the size distributions, respiratory deposition, and transport of particles generated from commonly used medical nebulizers. J. Aerosol Med. Pulm. Drug Deliv. 2017; 30: 132–40. [DOI] [PubMed] [Google Scholar]
- 41. Clinical Excellence Commission, NSW Government . Application of PPE in response to COVID‐19 pandemic. Version 1.4. 2020. [Accessed 8 Apr 2020]. Available from URL: http://cec.health.nsw.gov.au/__data/assets/pdf_file/0006/572883/Application-of-PPE-in-Responseto-COVID-19-19-March-2020-V1.4.pdf
- 42. Sehgal IS, Dhooria S, Aggarwal AN, Agarwal R. Impact of rapid on‐site cytological evaluation (ROSE) on the diagnostic yield of transbronchial needle aspiration during mediastinal lymph node sampling: systematic review and meta‐analysis. Chest 2018; 153: 929–38. [DOI] [PubMed] [Google Scholar]
- 43. Steinfort DP, Leong TL, Laska IF, Beaty A, Tsui A, Irving LB. Diagnostic utility and accuracy of rapid on‐site evaluation of bronchoscopic brushings. Eur. Respir. J. 2015; 45: 1653–60. [DOI] [PubMed] [Google Scholar]
- 44. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID‐19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro. Surveill. 2020; 25: 2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS‐CoV‐2 in women admitted for delivery. N. Engl. J. Med. 2020. 10.1056/NEJMc2009316. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, Wang M. Presumed asymptomatic carrier transmission of COVID‐19. JAMA 2020; 323: 1406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W et al. Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020; 382: 970–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang J, Tian S, Lou J, Chen Y. Familial cluster of COVID‐19 infection from an asymptomatic. Crit. Care 2020; 24: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bogoch II, Andrews JR, Zachary KC, Hohmann EL. Diagnosis of influenza from lower respiratory tract sampling after negative upper respiratory tract sampling. Virulence 2013; 4: 82–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blyth CC, Iredell JR, Dwyer DE. Rapid‐test sensitivity for novel swine‐origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009; 361: 2493. [DOI] [PubMed] [Google Scholar]
- 51. Winichakoon P, Chaiwarith R, Liwsrisakun C, Salee P, Goonna A, Limsukon A, Kaewpoowat Q. Negative nasopharyngeal and oropharyngeal swab does not rule out COVID‐19. J. Clin. Microbiol. 2020; 58: pii: e00297‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020. 10.1001/jama.2020.3786. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cheng PK, Wong DA, Tong LK, Ip SM, Lo AC, Lau CS, Yeung EY, Lim WW. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet 2004; 363: 1699–700. [DOI] [PMC free article] [PubMed] [Google Scholar]


