Abstract
Coronavirus disease (COVID‐19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) primarily affects the epithelium of the airways. With the increasing involvement of dermatologist in management of this crisis, cutaneous symptoms gained more and more attention. In this review, we will describe cutaneous symptoms of patients of all ages in association with COVID‐19. We will focus on such disorders that are caused by direct action of SARS‐CoV‐2 on tissues, complement, and coagulation system and on nonspecific eruption of the systemic viral infection. Drug‐induced reactions are only mentioned in the differential diagnoses. Although more systematic investigations are warranted, it becomes clear that some symptoms are clinical signs of a milder COVID‐19 course, while others are a red flag for a more severe course. Knowledge of the cutaneous manifestations of COVID‐19 may help in early diagnosis, triage of patients, and risk stratification.
Keywords: acro‐ischemia, androgenetic alopecia, chilblain‐like eruptions, COVID‐19, maculopapular rash, purpuric rash, SARS‐CoV‐2, urticaria
1. INTRODUCTION
Since the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) induced infectious disorder named coronavirus disease (COVID‐19) pandemic started in Wuhan city in Central China at the end of 2019, a total of 3.5 million patients have been tested positive worldwide with >240 000 related death recorded. 1
The virus is mainly spread by droplets, but direct contact and fecal excretions are other possible sources of infection. Vertical transmission may be possible. The primary target of SARS‐CoV‐2 is the upper respiratory mucosa, and angiotensin‐converting enzyme 2 (ACE2) acts as a functional receptor for the viral spikes and eventually viral entry into host cells. 2 Expression of the SARS‐CoV‐2 cell receptor gene ACE2 has been demonstrated in a number of human tissues including skin and adipose tissue. 3 One of the main reasons of pulmonary consolidation during the active disease is the development of extensive pulmonary fibrosis. SARS‐CoV‐2 induces pulmonary fibrosis in a tumor growth factor‐beta (TGF‐beta)/Smad‐dependent pathway. In both cutaneous and pulmonary fibrosis, transdifferentiation of adipocytes or lipofibroblasts into myofibroblasts is involved. Adipocytes can serve as a viral reservoir. 4
Incubation time of COVID‐19 is up to 14 days. Typical clinical symptoms include fever, dry cough, sore throat, fatigue, diarrhea, conjunctivitis, hyposmia, and hypogeusia. Diagnosis is based on medical and travel history, contact to COVID‐19 patients, and clinical symptoms. 2 Confirmation is done by detection of viral RNA by reverse‐transcriptase polymerase chain reaction (RT‐PCR) for nasopharyngeal swabs or bronchoalveolar fluid. About 50% of nasopharyngeal swabs may be false negative. 5 Independent factors for severe disease and poor outcome are age > 65 years of life, male gender, cardiovascular disorders, and diabetes mellitus. 6 , 7 So far, no specific cutaneous pathological findings were reported in autopsied COVID‐19 patients. 8
2. SKIN DISEASE IN COVID‐19
The initial studies from Central China reported low frequencies of skin disease in COVID‐19 patients. Among 1099 confirmed cases in Wuhan, only 0.2% presented with cutaneous symptoms. 9 With a closer involvement of dermatologist in the battle against the latest pandemic, the interest on cutaneous signs of SARS‐CoV‐2 infection increased.
The first report from Northern Italy on 88 COVID patients observed cutaneous symptoms in 18 patients (20.4%) of whom 8 patients developed cutaneous signs at the onset, 10 patients after the hospitalization. 10 Unfortunately, neither photographs nor histology was available.
In a recent letter from Thailand, it was stated that almost all COVID‐19 patients had cutaneous signs. 11
There were no cutaneous signs reported from Tibetan patients living in the high‐attitude plateau area, where the course of the disease was generally mild. 12
We do not know the reason for these different pictures. One explanation might be the involvement of dermatologists in the triage, which will result in a higher rate of skin diseases diagnosed. Another explanation is the setting. Patients with severe disease in intensive care unit (ICU) will get more attention for all possible clinical findings compared to those with mild disease and outpatient care. Genetic factors might also contribute, but further studies are needed.
An important initiative to gain valid data has recently been created by the American Academy of Dermatology (AAD) COVID‐19 Task Force, who has launched an online COVID‐19 dermatology registry: www.aad.org/covidregistry. The primary purpose is to rapidly collect the various cutaneous manifestations. 13
3. ACRO‐ISCHEMIA
Severe COVID‐19 can lead to a state of hypercoagulation and disseminated intravascular coagulation with laboratory findings such as increased levels of D‐dimer, fibrinogen and fibrinogen degradation products, and prolonged prothrombin time. These critical ill patients present acro‐ischemia with finger and toe cyanosis, cutaneous bullae, and dry gangrene. 14 Livedo‐like features and necrosis were noted in 6% of Spanish COVID‐19 patients, mostly elderly people. These findings were associated with a mortality rate of 10%. 15 Two more temporary cases were reported in a 47‐year‐old woman and a 67‐year‐old male from Atlanta, GA, USA. 16
Histopathological investigations of various tissues in three patients who died from COVID‐19 disease revealed hyaline thrombi in microvessels of skin. 17
4. CHILBLAIN‐LIKE ERUPTIONS ON FINGERS AND TOES
Chilblain‐like edematous and erythematous eruptions have been observed in milder cases of COVID‐19 and in particular in youngsters and young adults, which disappear after the infection without leaving scars. 18 Chilblain like eruptions are mostly asymmetrically distributed. Among 375 COVID‐19 patients of Spain, 19% presented with “pseudo‐chilblains.” They may be associated with itch or pain and disappear on average after 2 weeks. 15 In a WhatsApp group of French dermatologists, 295 cases with cutaneous manifestations were collected. The most common cutaneous finding was chilblain‐like eruption with 146 posts. 19 Most of the contributors were dermatologists in private practice with milder cases of possible COVID‐19 disease. Chilblain‐like lesions in pediatric dermatological outpatients (mean age 14 years) have been noted in 25 children in Spain. None of these children had the typical symptoms of COVID 19, except one who suffered from diarrhea. Two‐thirds of them were males, often the lesions were asymptomatic. Mild pain (22%) or pruritus (11%) were the associated symptoms. The lesions disappeared within 2 weeks without treatment. 20 From Lombardy, 14 cases including 11 children (average age 14 years) and 3 young adults (average age 29 years) with chilblain‐like eruptions were reported. There were slightly more females involved. The authors described erythemato‐violaceous papules and macules—some with bullae—and digital swelling. Mild itching was reported in three cases. 21 In a telemedicine attempt from Northern Italy, 63 cases of chilblain‐like acral eruption were clinically analyzed. There was no gender preference. The median age was 14 years. Toes and feet were more often affected than the fingers or hands. Erythematous‐edematous lesions were the predominant feature, while blistering was observed in about half of the cases (Figure 1 ). Pain and itch were reported in 27% each, pain with itch occurred in 20.6% of patients. The lesions were completely asymptomatic 25.4%. Median time from the onset to clinical diagnosis was 10 days. COVID‐19 disease was mild with pyrexia in less than 5%. 22 In a series of six patients with chilblain‐like acral eruptions, those who were young (15‐44 years) were either asymptomatic or had only mild symptoms of COVID‐19. A 91‐year‐old male patient was hospitalized but recovered after 3 weeks. 23
FIGURE 1.
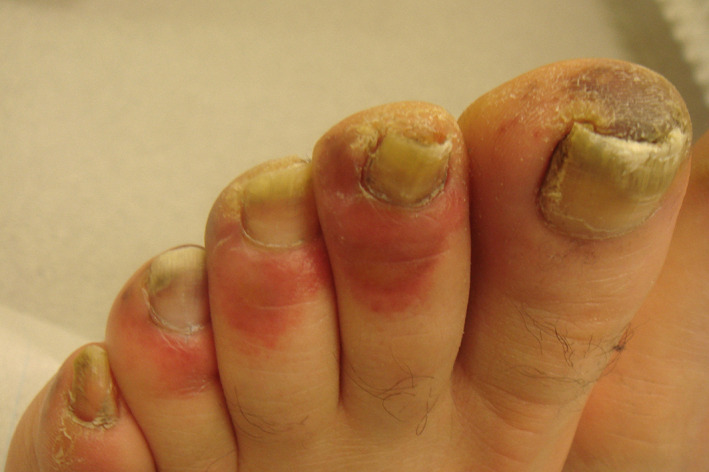
Chilblain‐like acral eruptions
In conclusion, chilblain‐like eruptions (COVID finger and toes) are signs of milder COVID‐19 disease in younger patients. The most important differential diagnoses are perniones and chilblain lupus.
5. RASH WITH PETECHIAE/PURPURIC RASH
A skin rash with petechiae resembling dengue fever has been observed in a COVID‐19 patient from Thailand. 24 A morbilliform rash sparing palmoplantar skin and mucosa with purpuric features was observed in a 32‐year‐old female occurring 6 days after the onset of COVID‐19 symptoms. Five days later, it started healing without leaving scars. 25 A 48‐year‐old symptomatic man from Spain presented with confluent erythematous macules, papules, and petechiae in a symmetric periflexural distribution. The affected parts were buttocks, popliteal fossae, proximal anterior thighs, and lower abdomen, but crural folds, face, palmoplantar skin, and mucosa remained uninvolved. A skin biopsy disclosed superficial perivascular lymphocytic infiltrate with abundant red cell extravasation and focal papillary edema. The epidermis showed focal parakeratosis and dyskeratotic cells. There were no signs of thrombotic vasculopathy. 26
Other investigators of purpuric skin lesions reported a pauci‐inflammatory thrombogenic vasculopathy, with deposition of complement components C5b‐9 and C4d in involved and normal‐appearing skin, sometimes colocalized with COVID‐19 spike glycoproteins. 27
In conclusion, rash with petechiae/purpuric rash (Figure 2 ) seems to be a symptom of milder COVID‐19 disease. Typically, it spares mucosa and palmoplantar skin. Differential diagnoses include drug‐induced rash and rashes due to other viral diseases.
FIGURE 2.
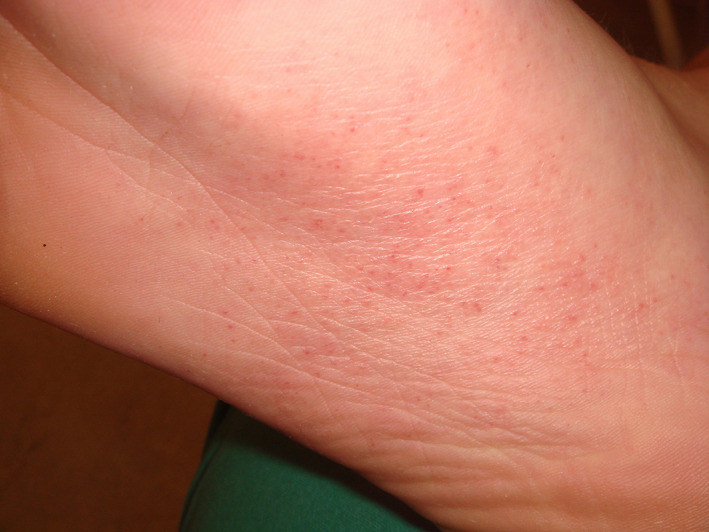
Purpuric rash
6. CHICKENPOX‐LIKE RASH
Chickenpox‐like rashes with small monomorphic vesicles mainly located on the trunk were noted in middle‐aged COVID‐19 patients in up to 9% of cases (Figure 3 ). 15 Recalcati in 2020 observed one case among 88 severe ill Italian COVID‐19 patients. 21
FIGURE 3.
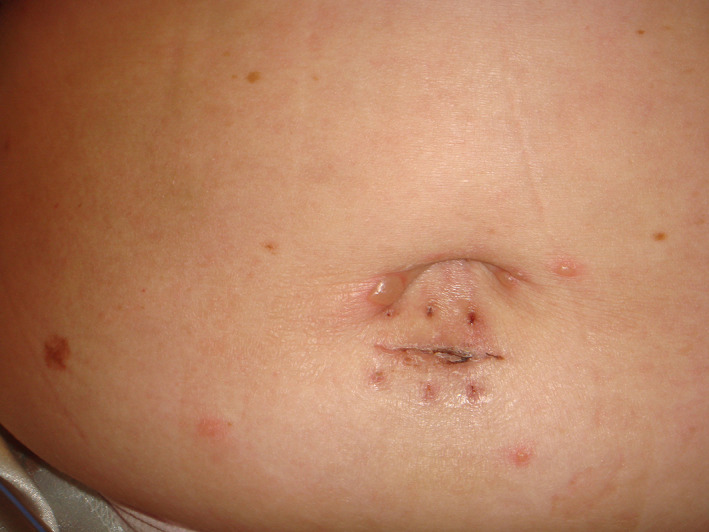
Chickenpox‐like rash
True chickenpox and acute generalized exanthematic pustulosis are passible differential diagnoses.
6.1. Urticarial rash
Urticarial rash can be present before the onset of COVID‐19 symptoms like cough and pyrexia. 28 Two cases have been reported from Belgium—a 39‐year‐old women and a 71‐year‐old man who presented with urticarial rash and pyrexia. Both were tested positive for SARS‐CoV‐2‐RNA. 29 Spanish groups reported an urticarial rash without mucosal involvement in a 32‐year‐old female that started 6 days after the onset of COVID‐19 disease and another group 10 days after onset of COVID‐19 in a 28‐year‐old woman. Lesions may be morbilliform or larger plaques. 30 , 31 Oral antihistamines improved the symptoms. 30
Three severely ill Italian COVID‐19 patients presented a widespread urticarial rash. 21 Among 103 French COVID‐19 patients, only 2 presented with an urticarial rash. 32 The frequency of urticarial rash in a larger Spanish study was 19% and was associated with a more severe course of the disease. 15
There is a case report of an acute urticarial rash in a 2‐month‐old girl from Spain that began on the face and spread all over the body except mucosa and palmoplantar skin. It disappeared after 9 days. 33
In conclusion, urticarial rash in combination with pyrexia is suggestive of COVID‐19 disease. Patients present such lesions in the early phase of infection. Acute idiopathic urticaria and urticarial drug‐induced rash are major differential diagnoses. Syphilis needs consideration. Many viral infections develop rashes with mucosal involvement in contrast to COVID‐19.
6.2. Acute rash reminiscent of symmetrical drug‐related intertriginous and flexural exanthema
A 64‐year‐old women with diabetes and COVID‐19 developed 4 days after the onset of symptoms of the coronavirus infection an erythematous ill‐defined rash on both antecubital fossa, which extended during the following days on the trunk and axillary fold. 34 Symmetrical drug‐related intertriginous and flexural exanthema is the most important differential diagnosis.
6.3. Erythema multiforme‐like rash
Among 27 children with mild disease, 2 developed targetoid lesions reminiscent of erythema multiforme. They did not suffer from herpes simplex infection. 15 In a study from Northern Italy, 2 children developed targetoid lesions on the hands and elbows a few days after the development of chilblain‐like acral eruptions. 21
In conclusion, erythema multiforme‐like lesions are more common in children and associated with a mild COVID‐19 course (Figure 4 ). The major differential diagnosis in that age‐group is herpes simplex‐associated erythema multiforme.
FIGURE 4.
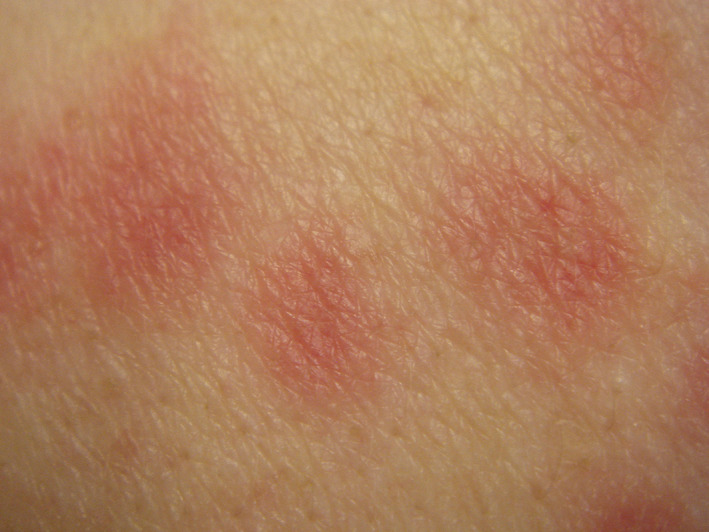
Erythema multiforme‐like rash
6.4. Maculopapular rash
Maculopapular rash was noted in 47% of Spanish COVID‐19 patients, more than half of them reported pruritus. It lasted for about 9 days and was associated with a more severe course of the coronavirus infection. 15 Histopathological findings have rarely been reported. In a series of three elderly patients with erythematous rash from Milan, Italy, microthrombi, lymphocytic vasculitis, and Grover‐like features have been reported. 35 Another case of a 57‐year‐old symptomatic German woman with diffuse‐fixed erythematous blanching asymptomatic maculopapular lesions reported mild spongiosis, basal cell vacuolation, and mild perivascular lymphocytic infiltrate. Whole skin PCR for SARS‐CoV‐2 was negative. 36 A 67‐year‐old Italian woman with COVID‐19 presented with an ill‐defined erythematous pruritic rash, sparing palmoplantar and facial skin, and visible mucosa. Histopathologic examination of a skin biopsy demonstrated a mild superficial perivascular lymphocytic infiltrate in combination with dilated vessels in the upper and mid dermis. 37 A morbilliform nonpurpuric rash was observed in a 58‐year‐old Hispanic man without fever that lasted for 6 days. Erythematous lesions were seen on the legs, thighs, forearms, arms, shoulders, and trunk. They aggregated into confluent larger erythematous patches on the trunk. Face, hands, and feet were spared, and intraoral symptoms were reported. He suffered from pain in hands and legs. The exanthema disappeared with topical steroids. RT‐PCR was positive for SARS‐CoV‐2 RNA. 38
Maculopapular rash is uncommon among COVID‐19‐positive children. A 6‐year‐old boy from Barcelona, Spain, developed a maculopapular rash about 2 weeks after the first COVID‐19 symptoms. It is noteworthy that here the palmoplantar skin became involved. The rash persisted for 5 days. 33
In conclusion, COVID‐19‐associated maculopapular rash in adult sparse palmoplantar skin and mucosa. It is commonly associated with a more severe course of the disease. Differential diagnoses include measles, Epstein‐Barr virus infection, drug‐induced exanthema, and graft vs host disease.
6.5. Mottling
A 15‐day‐term male neonate with clinical sign of sepsis—fever 38.2°C, tachypnoea, lethargy—presented with mottling of the skin. There were no cough, runny nose, or gastrointestinal symptoms. He was tested positive for SARS‐CoV‐2. 39
6.6. Pityriasis rosea‐like eruptions
Pityriasis rosea‐like cutaneous eruption has been reported in an adult patient from Tehran, Iran. 40 A digitate variant has been observed in an elderly man hospitalized because of severe COVID‐19 disease. A skin biopsy revealed mild diffuse epidermal spongiosis, spongiotic vesicles containing lymphocytes, and Langerhans cells. The papillary dermis was slightly edematous. In the upper dermis, a lymphohistiocytic infiltrate was noted. 41
6.7. Unspecified rash
Among 103 COVID‐19 patients from France, 2 presented with an unspecified erythematous rash on face and upper trunk. 32 Among 88 patients from the Lecco Hospital in Lombardy, Italy, an erythematous rash was noted in 14 patients. 10
Unspecified rash warrants a more detailed diagnosis. Viral exanthema and drug‐induced rash are important differential diagnoses.
6.8. Androgenetic alopecia
Androgenetic alopecia is driven by androgens and follows a specific pattern of fronto‐temporal and vertex regression. Regression affects both hair follicles and sebaceous glands. During the process, local fibrotic structures around and beneath hair follicles develop probably due to adipocyte‐myofibroblast transition involving the dermal adipocytes. 42
In a clinical study on 41 Caucasian males with bilateral pneumonia (COVID‐19 disease) and a mean age of 58 years, 29 (71%) had androgenetic alopecia of Hamilton‐Norwood scale >2 and 12 (29%), of them 16 (39%) were classified as severe (Hamilton‐Norwood scale ≥4). 43
There are several pathways by which androgens are involved in COVID‐19. Androgen‐regulated TMPRSS2 protease is a cellular coreceptor required for SARS‐CoV‐2 infection. The viral spike protein is primed by this enzyme. 44 Another link is the androgen‐driven immune modulation, since androgens are immunosuppressive. Indeed, there is a male predominance among adult COVID‐19 patients. 2 , 45
Genetic factors may influence the geographical distribution of COVID‐19. The adrenal‐permissive phenotype of HSD3B1 gene encodes 3β‐hydroxysteroid dehydrogenase‐1, which is involved in the transformation of dehydroepiandrosterone into active and more powerful androgens. Italy and Spain have the highest frequency of HSD3B1 allele in the general population, as per the 1000 Genomes Project. 46 This might explain why androgenetic alopecia has first been reported from Spain during COVID‐19 pandemic.
7. CONCLUSIONS
Cutaneous manifestations of COVID‐19 pandemic gain increasing attention since they might be useful in the early diagnosis, triage of COVID‐19‐positive patients and their risk stratification (Table 1 ). Chilblain‐like acral eruptions and purpuric and erythema multiforme‐like lesions have been associated with children and young adult patients who are either asymptomatic or develop a mild disease. In contrast, acro‐ischemic lesion and maculopapular rash are often seen among adult patients who run a more severe course. Urticaria with pyrexia has diagnostic significance since this combination is an early symptom of an otherwise not confirmed SARS‐CoV‐2 infection. Careful registering possible cutaneous manifestations of the COVID‐19 pandemic is warranted.
TABLE 1.
Cutaneous sign of COVID‐19 disease
| Vascular complications | Acro‐ischemia |
| Livedo‐like | |
| Necrosis | |
| Chilblain‐like eruptions | |
| Maculopapular eruption | Morbiliform |
| Plaques | |
| Pityriasis rosea‐like eruptions | |
| Urticarial rash | |
| Vesicular eruption | Vesicle |
| Bullous eruption | |
| Chickenpox‐like rash | |
| Petechiae/purpuric eruptions | |
| Erythema multiforme‐like rash | |
| Palmar erythema | |
| Perifollicular eruption | |
| Pruritus | |
| Mucosal lesions | Enanthema |
| Androgenetic alopecia |
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Wollina U, Karadağ AS, Rowland‐Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID‐19 patients: A review. Dermatologic Therapy. 2020;33:e13549. 10.1111/dth.13549
REFERENCES
- 1.Johns Hopkins Corona Resource Center. https://coronavirus.jhu.edu. Accessed May 3, 2020.
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kruglikov IL, Scherer PE. The role of adipocyte‐like cells in the severity of COVID‐19 infections. Obesity (Silver Spring). 2020. 10.1002/oby.22856. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xiao AT, Tong YX, Zhang S. False‐negative of RT‐PCR and prolonged nucleic acid conversion in COVID‐19: rather than recurrence. J Med Virol. 2020. 10.1002/jmv.25855. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D, Yin Y, Hu C, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS‐CoV‐2, discharged from two hospitals in Wuhan. China Crit Care. 2020;24(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109(5):531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. [DOI] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16387. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11. Mungmungpuntipantip R, Wiwanitkit V. COVID‐19 and cutaneous manifestations. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16483. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lei Y, Huang X, Bamu S, et al. Clinical features of imported cases of coronavirus disease 2019 in Tibetan patients in the plateau area. Infect Dis Poverty. 2020. 10.21203/rs.3.rs-22978/v1. [DOI] [Google Scholar]
- 13. Freeman EE, McMahon DE, Fitzgerald ME, et al. The AAD COVID‐19 registry: crowdsourcing dermatology in the age of COVID‐19. J Am Acad Dermatol. 2020. 10.1016/j.jaad.2020.04.045. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. [DOI] [PubMed] [Google Scholar]
- 15. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020. 10.1111/bjd.19163. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID‐19: transient livedo reticularis. J Am Acad Dermatol. 2020. 10.1016/j.jaad.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiaohong Y, Tingyuan L, Zhicheng H, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies. Zhonghua bing li xue za zhi. 2020;20. 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 18. Alramthan A, Aldaraji W. A case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020. 10.1111/ced.14243. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duong TA, Velter C, Rybojad M, et al. Did Whatsapp® reveal a new cutaneous COVID‐19 manifestation? J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16534. [Epublished ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia‐Lara G, Laura Linares‐González L, Ródenas‐Herranz T, Ruiz‐Villaverde R. Chilblain‐like lesions in pediatrics dermatological outpatients during the COVID‐19 outbreak. Dermatol Ther. 10.1111/dth.13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16533. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piccolo V, Neri I, Filippeschi C, et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16526. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P, Aguirre T. Chilblain‐like lesions on feet and hands during the COVID‐19 pandemic. Int J Dermatol. 2020. 10.1111/ijd.14937. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avellana Moreno R, Villa E, Avellana Moreno V, Estela Villa C, Aparicio M, Fontanella A. Cutaneous manifestation of COVID‐19 in images: a case report. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16531. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diaz‐Guimaraens B, Dominguez‐Santas M, Suarez‐Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020. 10.1001/jamadermatol.2020.1741. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;S1931‐5244(20):30070‐0. 10.1016/j.trsl.2020.04.007. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16472. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Damme C, Berlingin E, Saussez S, Accaputo O. Acute urticaria with pyrexia as the first manifestations of a COVID‐19. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16523. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernandez‐Nieto D, Ortega‐Quijano D, Segurado‐Miravalles G, Pindado‐Ortega C, Prieto‐Barrios M, Jimenez‐Cauhe J. Comment on: cutaneous manifestations in COVID‐19: a first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16470. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Estébanez A, Pérez‐Santiago L, Silva E, Guillen‐Climent S, García‐Vázquez A, Ramón MD. Cutaneous manifestations in COVID‐19: a new contribution. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16474. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hedou M, Carsuzaa F, Chary E, Hainaut E, Cazenave‐Roblot F, Masson RM. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16519. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morey‐Olivé M, Espiau M, Mercadal‐Hally M, Lera‐Carballo E, García‐Patos V. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed). 2020. 10.1016/j.anpede.2020.04.002. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahé A, Birckel E, Krieger S, Merklen C, Bottlaender L. A distinctive skin rash associated with coronavirus disease 2019? J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16471. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico‐pathological findings in three COVID‐19‐positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020. 10.2340/00015555-3490. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahouach B, Harant S, Ullmer A, et al. Cutaneous lesions in a patient with COVID‐19: are they related? Br J Dermatol. 2020. 10.1111/bjd.19168. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in Covid‐19 induced viral rash. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16569. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020. 10.1016/j.jdcr.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (London). 2020;52(6):427‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16579. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020. 10.1001/jamadermatol.2020.1704. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42. Wollina U, Abdel NM, Kruglikov I. Dermal adipose tissue in hair follicle cycling: possible applications in alopecia? Georgian Med News. 2017;265:41‐45. [PubMed] [Google Scholar]
- 43. Goren A, Vaño‐Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID‐19 patients in Spain—a potential clue to the role of androgens in COVID‐19 severity. J Cosmet Dermatol. 2020. 10.1111/jocd.13443. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 44. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271.e8‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li LQ, Huang T, Wang YQ, et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J Med Virol. 2020. 10.1002/jmv.25757. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sabharwal N, Sharifi N. HSD3B1 genotypes conferring adrenal‐restrictive and adrenal‐permissive phenotypes in prostate cancer and beyond. Endocrinology. 2019;160:2180‐2188. [DOI] [PMC free article] [PubMed] [Google Scholar]


