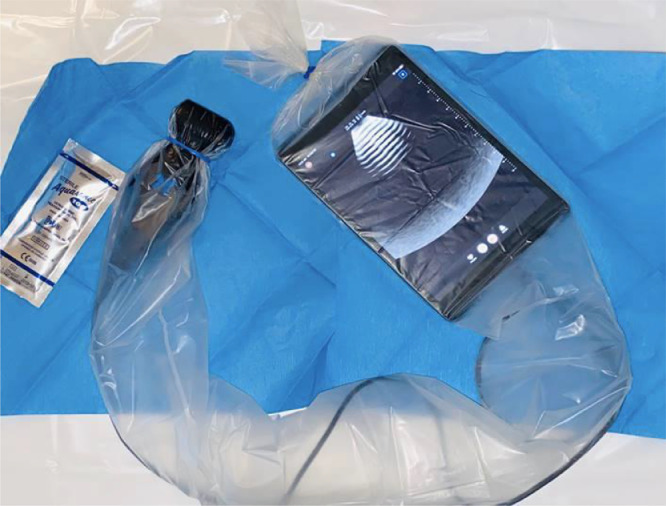
Coronavirus 2019 (COVID-19) is highly contagious and has spread around the world at an unprecedented rate. The American College of Radiology has discouraged the use of routine imaging studies to reduce risk of contamination.1 Ultrasound is useful for ventilatory and hemodynamics optimization, and has been shown to be superior to the stethoscope and chest radiography,2 and comparable to computed tomography in the diagnosis of many pathologies.3 Handheld ultrasound devices fit into a single-use plastic cover (Fig 1 ) and can be easily decontaminated, making them ideal for minimizing viral contamination and sperad during the COVID-19 pandemic.
Fig. 1.
A portable ultrasound device and connected tablet can be placed in a single-use plastic sheath prior to entering a patient's room to minimize the risk of viral contamination and spread.
Table 1 shows the duration that many common hospital pathogens are viable on surfaces. While many pathogens are viable for much longer than SARS-CoV-2, their level of contagiousness is markedly diminished. A survey by Westerway et al4 found that a majority of ultrasound users did not appropriately disinfect traditional ultrasound machines, with only 47% using proper disinfectant solution and only 15% and 47% disinfecting the keyboard and cords, respectively. Single use gels should also be used given that community gel has been a source of contamination. As many in-hospital providers are burdened with increasing patient volumes during the current pandemic, there could be even less adherence to best practices for decontamination.
Table 1.
Survival time of common hospital pathogens on dry inaminate surfaces.
| Type of pathogen | Duration of persistence |
|---|---|
| Bacteria | |
| Acinetobacter spp. | 3 days to 5 months |
| Clostiridium difficile | 5 months |
| Escherichia coli | 1.5 hours to 16 months |
| Enterococcus spp. | 5 days to 4 months |
| Haemophilus influenza | 12 days |
| Klebsiella spp. | 2 hours to >30 months |
| Listeria spp. | 1 day to months |
| Mycobacterium tuberculosis | Up to 4 months |
| Proteus vulgaris | Up to 2 days |
| Pseudomonas aeruginosa | Up to 16 months |
| Serratia marcescens | 3 days to 2 months |
| Staphylococcus aureus | Up to 7 months |
| Streptococcus pneumoniae | 1 to 20 days |
| Streptococcus pyogenes | 3 days to 6.5 months |
| Fungi | |
| Candida albicans | Up to 120 days |
| Torulopsis glabrata | 102 to 150 days |
| Viruses | |
| Adenovirus | 7 days to 3 months |
| HAV | 2 hours to 60 days |
| HBV | Greater than one week |
| HIV | Greater than 7 days |
| HSV | 4.5 hours to 8 weeks |
| Influenza virus | 1 to 2 days |
| Norovirus | 8 hours to 7 days |
| MERS-CoV | Up to 48 hours |
| Respiratory syncytial virus | Up to 6 hours |
| SARS-CoV | 3 to 10 days |
| SARS-CoV-2 | Up to 3 days |
Because of their compact size and profile, handheld devices can be easily decontaminated with a single disinfectant wipe. In contast, decontamination of traditional ultrasound machines can be challenging if not impossible due to greater surface area, and components such as keyboards, knobs, and cords. This process can be time consuming and costly, as many hospitals are rationing disinfectant supplies. In addition, many handheld ultrasound devices now have teleguidance capabilities that allow experts to guide a novice user through an exam remotely thereby minimizing exposure, conserving personal protective equipment, and reducing patient transport for imaging studies. Nurses and respiratory therapists can also be easily trained to perform focused ultrasound assessments.5 , 6
During the current pandemic, disease containment and provider safety are high priorities. We must embrace emerging technologies such as handheld ultrasound devices to allow us to achieve these aims while providing high quality care to our patients.
Footnotes
Conflicts of Interests: L.E.G. reports none. E.A.B. reports none. M.G.C. reports none.
Authors’ contributions: L.E.G., E.A.B., and M.G.C. wrote and reviewed the article.
References
- 1.American College of Radiology, editor. Vol. 2020. American College of Radiology; 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection). Edited by. [Google Scholar]
- 2.Picano E, Scali MC, Ciampi Q, Lichtenstein D. Lung ultrasound for the cardiologist. JACC Cardiovasc Imaging. 2018;11:1692–1705. doi: 10.1016/j.jcmg.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care. 2014;20:315–322. doi: 10.1097/MCC.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 4.Westerway SC, Basseal JM. The ultrasound unit and infection control - Are we on the right track? Ultrasound. 2017;25:53–57. doi: 10.1177/1742271X16688720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tulleken AM, Gelissen H, Lust E, et al. UltraNurse: teaching point-of-care ultrasound to intensive care nurses. Intensive Care Med. 2019;45:727–729. doi: 10.1007/s00134-018-05512-x. [DOI] [PubMed] [Google Scholar]
- 6.Karthika M, Wong D, Nair SG, Pillai LV, Mathew CS. Lung ultrasound: the emerging role of respiratory therapists. Respir Care. 2019;64:217–229. doi: 10.4187/respcare.06179. [DOI] [PubMed] [Google Scholar]
- 7.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]