Key Points
Question
Can a prognostic model using lifestyle risk factors accurately predict the incidence of the first major chronic disease (ie, congestive heart failure, chronic obstructive pulmonary disease, diabetes, myocardial infarction, lung cancer, or stroke) at a population level?
Findings
In this cohort study, sex-specific prognostic models were developed and validated, demonstrating high overall predictive performance, discrimination, and calibration during development, internal validation, and external validation.
Meaning
Using routinely collected risk factor information, the Chronic Disease Population Risk Tool exhibited reproducibility and geographic transportability for predicting the 10-year incidence of the first major chronic disease at the population level.
Abstract
Importance
Predicting chronic disease incidence for the population provides a comprehensive picture to health policy makers of their jurisdictions’ overall future chronic disease burden. However, no population-based risk algorithm exists for estimating the risk of first major chronic disease.
Objective
To develop and validate the Chronic Disease Population Risk Tool (CDPoRT), a population risk algorithm that predicts the 10-year incidence of the first major chronic disease in the adult population.
Design, Setting, and Participants
In this cohort study, CDPoRT was developed and validated with 6 cycles of the Canadian Community Health Survey, linked to administrative data from January 2000 to December 2014. Development and internal validation (bootstrap and split sample) of CDPoRT occurred in Ontario, Canada, from June 2018 to April 2019 followed by external validation in Manitoba from May 2019 to July 2019. The study cohorts included 133 991 adults (≥20 years) representative of the Ontario and Manitoba populations who did not have a history of major chronic disease.
Exposures
Predictors were routinely collected risk factors from the Canadian Community Health Survey, such as sociodemographic factors (eg, age), modifiable lifestyle risk factors (ie, alcohol consumption, cigarette smoking, unhealthy diet, and physical inactivity), and other health-related factors (eg, body mass index).
Main Outcomes and Measures
Six major chronic diseases were considered, as follows: congestive heart failure, chronic obstructive pulmonary disease, diabetes, myocardial infarction, lung cancer, and stroke. Sex-specific CDPoRT algorithms were developed with a Weibull model. Model performance was evaluated with measures of overall predictive performance (eg, Brier score), discrimination (eg, Harrell C index), and calibration (eg, calibration curves).
Results
The Ontario cohort (n = 118 747) was younger (mean [SD] age, 45.6 [16.1] vs 46.3 [16.4] years), had more immigrants (23 808 [20.0%] vs 1417 [10.7%]), and had a lower mean (SD) body mass index (26.9 [5.1] vs 27.7 [5.4]) than the Manitoba cohort (n = 13 244). During development, the full and parsimonious CDPoRT models had similar Brier scores (women, 0.087; men, 0.091), Harrell C index values (women, 0.779; men, 0.783), and calibration curves. A simple version consisting of cigarette smoking, age, and body mass index performed slightly worse than the other versions (eg, Brier score for women, 0.088; for men, 0.092). Internal validation showed consistent performance across models, and CDPoRT performed well during external validation. For example, the female parsimonious version had C index values for bootstrap, split sample, and external validation of 0.778, 0.776, and 0.752, respectively.
Conclusions and Relevance
In this study, CDPoRT provided accurate, population-based risk estimates for the first major chronic disease.
This study reports the development and validation of a population risk algorithm that predicts the 10-year incidence of the first major chronic disease in an adult population.
Introduction
The high prevalence of chronic disease is a worldwide health issue, with 6.7 billion people worldwide living with a noncommunicable disease resulting in substantial years of life lost.1,2 Chronic disease prevalence has increased over time,2 which can be attributed to an aging population and improvements in chronic disease management.2,3,4 As a result of this increase and an increase in multimorbidity (the presence of multiple chronic conditions),5 high costs are borne by the health care system. The total direct and indirect costs of chronic conditions are estimated to be 19.6% of the total US gross domestic product ($3.7 trillion).6 A tool that predicts the future burden of multiple chronic diseases on the health care system would be invaluable to support health policy makers in their planning and prevention efforts.
Primary prevention is an ideal strategy to address the chronic disease burden.7 Well-established evidence shows that 4 modifiable lifestyle risk factors, ie, alcohol consumption, cigarette smoking, unhealthy diet, and physical inactivity, are associated with more than two-thirds of the incidence of cancer, cardiovascular disease, chronic respiratory disease, and diabetes combined.7 Despite the evidence, preventing or delaying chronic disease onset with primary prevention strategies is difficult.7 One problem is that no straightforward way exists to predict the incidence of chronic disease in the population, which has been stated as a need from health policy makers.8 One solution for population prediction is with a population risk algorithm.8,9 These algorithms predict the risk of an outcome (eg, cardiovascular disease) like clinical risk algorithms do (eg, Framingham Risk Score),10 but they are designed to make predictions representative of the overall population (instead of individual-level prediction) by using routinely collected risk factor information (eg, smoking) from a population-level data source (eg, the National Household Interview Survey).9
There have been related attempts to predict future chronic disease burden. An existing clinical risk score that predicts the incidence of multiple chronic diseases was developed in a specific private health care insurance patient population,11 so its generalizability to the general population is unknown. There are also population risk algorithms that predict the incidence of individual chronic diseases (eg, diabetes).9,12,13,14,15 However, to our knowledge, no risk algorithm exists that can predict the incidence of the first major chronic disease at the population level.
To meet this need, this study’s objective was to create the Chronic Disease Population Risk Tool (CDPoRT). The tool uses routinely collected, self-reported information on lifestyle risk factors from population health surveys to predict the first incidence of 1 of 6 major chronic diseases (ie, congestive heart failure, chronic obstructive pulmonary disease, diabetes, lung cancer, myocardial infarction, and stroke including transient ischemic attack) during a 10-year period in the general adult population. Furthermore, CDPoRT was validated with a variety of techniques to quantify its reproducibility and transportability.
Methods
This section summarizes the methods, with additional details available in a published analytic protocol.16 Overall, the protocol was adhered to with some modifications. First, the maximum follow-up was reduced from 15 to 10 years to eliminate model instability caused by very few individuals with 15 years of follow-up. Second, an additional exclusion omitted respondents with missing predictor values (exceptions were body mass index [calculated as weight in kilograms divided by height in meters squared] and income). Third, the Weibull model replaced the Royston-Parmar model because the log cumulative baseline hazard function approximated a straight line, indicating a hazard function with a Weibull distribution (eFigure 1 in the Supplement). Fourth, simple and parsimonious versions of CDPoRT were developed and validated along with the proposed model (ie, full version). The versions show the contributions of the predictors toward model performance. Fifth, a sensitivity analysis was added to understand how CDPoRT performed within the competing risks framework. Sixth, a sensitivity analysis in which each chronic disease was modeled individually and then combined to understand the contributions of each disease to overall chronic disease risk was not completed because of computational nonconvergence.
The study was approved by the research ethics boards of the University of Toronto, Sunnybrook Health Sciences Centre, and University of Manitoba. Informed patient consent was waived because ICES and Manitoba Centre for Health Policy are prescribed entities under the Personal Health Information Protection Act and Personal Health Information Act, respectively. This report was written in accordance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline for prediction model development and validation.
Data Sources
The Canadian Community Health Survey represents 98% of the Canadian population aged 12 years and older17 and contains information on sociodemographic characteristics, health status, and health determinants. The survey was linked to administrative data from 2 Canadian provinces. For development and internal validation in Ontario, the data were linked with unique encoded identifiers and analyzed at ICES. For external validation in Manitoba, data held at the Manitoba Centre for Health Policy were used.
Participants
All Canadian Community Health Survey respondents in Ontario from the first 6 cycles (1.1 [2000], 2.1 [2003], 3.1 [2005], 4.1 [2007], 2009/2010, and 2011/2012) who consented to administrative data linkage were eligible. Respondents were excluded if they were younger than 20 years at interview; had a history of congestive heart failure, chronic obstructive pulmonary disease, diabetes, lung cancer, myocardial infarction, or stroke according to self-report or diagnosis from administrative data; or had missing predictor information (except body mass index and income). The earliest response was used for individuals with multiple survey responses. The Manitoba validation cohort consisted of respondents from Canadian Community Health Survey cycles 3.1, 4.1, 2009/2010, and 2011/2012 who met the same exclusion criteria.
Chronic Disease Outcomes
The chronic diseases were congestive heart failure, chronic obstructive pulmonary disease, diabetes, lung cancer, myocardial infarction, and stroke including transient ischemic attack. They were chosen according to their prevalence,5 established links with lifestyle risk factors,7 associations with morbidity and mortality,1,2,18,19 and importance to the intended users of CDPoRT.8 All diseases were identified from the administrative data with validated algorithms,20,21,22,23,24,25 with vital statistics supplementing additional outcomes based on cause of death.
Predictors
Sixteen candidate predictors were identified from the Canadian Community Health Survey in accordance with well-established associations,18,26,27,28 subject matter expertise, user input, the group’s experiences with population risk algorithms,9,12,14,29,30,31,32 and variable availability across cycles. The self-reported predictors were collected once as of the interview. The predictors were sociodemographic predictors (eg, age, ethnicity, immigration status, household income, education, marital status), modifiable lifestyle risk factors (eg, alcohol consumption, cigarette smoking, daily fruit and vegetable consumption, physical activity), and other health-related factors (eg, asthma, body mass index, high blood pressure, household secondhand smoke, self-rated health, life stress). Ethnicity was categorized as white or visible minority according to Canadian Community Health Survey response categories. Ethnicity was included as a predictor because the incidence of risk of chronic diseases varies by ethnicity.33,34,35
Study Design
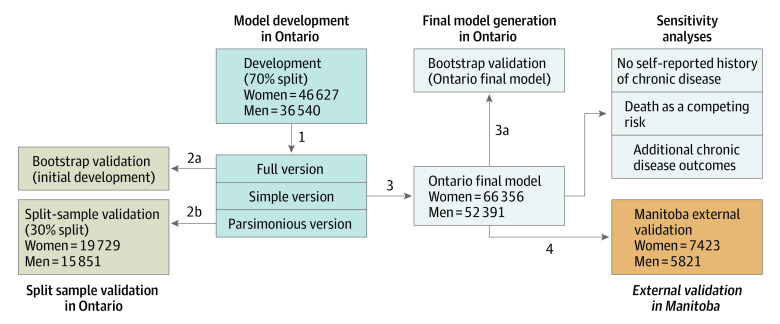
Sex-specific CDPoRT prognostic models were created because the biological underpinnings of chronic disease vary by sex.36,37,38,39,40 The prognostic models were developed and internally validated in Ontario and externally validated in Manitoba (Figure 1). For model development in Ontario, CDPoRT was developed in a 70% random sample of the respondents (step 1). Full, simple, and parsimonious versions of CDPoRT were developed (details in the “Model Specification” section). For the split sample validation in Ontario, internal validation via the bootstrap (1000 samples with replacement) was conducted with the 70% split (step 2a). The remaining 30% was used for split sample validation (step 2b). For final model generation in Ontario, in accordance with inefficiencies of the split sample approach,41,42,43 the entire Ontario cohort was combined to estimate the final Ontario CDPoRT models (step 3). Internal validation via bootstrapping was also conducted (step 3). For external validation in Manitoba, the full, simple, and parsimonious versions of the final Ontario CDPoRT models were validated externally within Manitoba (step 4).
Figure 1. Steps Used in the Development and Validation of the Chronic Disease Population Risk Tool.
Model Specification
A subset of the 16 candidate predictors was selected for the full version based on predictive performance. Predictive performance was evaluated with measures of overall predictive performance (eg, Nagelkerke R2, Brier score), discrimination (eg, Harrell C index, time-specific discrimination slopes), and calibration (time-specific calibration curves, calibration intercepts, and calibration slopes); definitions of each measure are shown in eTable 1 in the Supplement. As an additional check of predictor importance, an analysis of variance with partial χ2 test with prespecified statistical significance level of P < .05 was also used to confirm predictor importance. All time-specific statistics were calculated at 10 years. Candidate predictors that did not improve predictive performance were removed. Prespecified forms of the predictors were used (eTable 2 in the Supplement), and after sequential selection, alternative specifications were examined. For both sexes, cigarette smoking was respecified. Age was also respecified as a restricted cubic spline for both sexes. Education was respecified in the female model, whereas income was respecified in the male model. The final predictor specifications of the full version (eTable 2 in the Supplement) were used in the simple and parsimonious versions. Two-way interactions and cross-classification were also examined between age, smoking, and body mass index.
The simple version included only influential predictors. These were predictors that considerably reduced discrimination (ie, Harrell C index <0.7 [poor discrimination]44) or calibration (ie, major deviation of the calibration plot) when excluded from the full model. The parsimonious version struck a balance between the simple and full versions because it had more predictors than the simple version and fewer than the full version, but had predictive performance similar to that of the full version. The parsimonious version was constructed with forward selection, starting from the simple version in which the remaining predictors were added individually until predictive performance was similar to that of the full version.
Statistical Analysis
Between January 2000 and December 2014, respondents were observed from the Canadian Community Health Survey interview until chronic disease incidence with censoring on death, 10 years of follow-up, or study end. The models were estimated with a Weibull model. Proportional hazards were assessed with scaled Schoenfeld residuals. By incorporating Canadian Community Health Survey weights, all estimates were representative of the population. Variances were calculated from bootstrap survey weights via the percentile bootstrap.45,46
A sensitivity analysis was performed among individuals who did not self-report a previous chronic disease but were excluded because of diagnosis from the administrative data. This analysis shows how CDPoRT performs when administrative data cannot be accessed to exclude individuals with chronic disease. A second sensitivity analysis modeled death as a competing risk to determine how the subdistribution hazard ratios compared with the cause-specific ones. A third sensitivity analysis examined CDPoRT’s performance when 9 additional chronic diseases were considered as outcomes (ie, cardiac arrhythmia, chronic coronary syndrome, Crohn disease or colitis, dementia, osteoporosis, arthritis, rheumatoid arthritis, osteoarthritis, and renal failure).5
Data were cleaned with SAS Enterprise Guide version 7.1 (SAS Institute), whereas R version 3.1.2 (R Project for Statistical Computing) was used for model development and internal validation. SAS was used for external validation. Statistical significance was set at P < .05, and tests were 1-tailed.
Results
The baseline characteristics of the 70% Ontario development cohort (n = 83 167), the 30% Ontario split sample validation cohort (n = 35 580), and the Manitoba external validation cohort (n = 13 244) are provided in Table 1. Cohort exclusions appear in eFigure 2 in the Supplement. Major case-mix differences were that the Manitoba cohort was older (mean [SD] age, 46.3 [16.4] years vs 45.6 [16.1] years), had fewer immigrants (1417 [10.7%] vs 23 808 [20.0%]), and had higher mean (SD) body mass index (27.7 [5.4] vs 26.9 [5.1]) compared with the combined Ontario cohort. The 10-year risk of first chronic disease was greater in Manitoba (women, 12.5%; men, 13.1%) vs Ontario (women, 11.2%; men, 11.8%).
Table 1. Baseline Characteristics of the Development and Validation Cohorts Stratified by Sex.
| Variable | No. (%) | |||||
|---|---|---|---|---|---|---|
| Development (70% split) | Split sample validation (30% split) | All Ontario | Manitoba external validation | |||
| Women | ||||||
| No. | 46 627 | 19 729 | 66 356 | 7423 | ||
| Follow-up time, mean (SD), y | 6.93 (2.88) | 6.90 (2.82) | 6.92 (2.85) | 7.40 (2.76) | ||
| Age, y | ||||||
| Mean (SD) | 46.59 (16.74) | 46.55 (16.82) | 46.58 (16.76) | 47.19 (17.72) | ||
| 20-34 | 13 338 (28.6) | 5711 (28.9) | 19 049 (28.7) | 2262 (30.5) | ||
| 35-44 | 9898 (21.2) | 4197 (21.3) | 14 095 (21.2) | 1300 (17.5) | ||
| 45-54 | 8127 (17.4) | 3383 (17.1) | 11 510 (17.3) | 1273 (17.1) | ||
| 55-64 | 7566 (16.2) | 3155 (16.0) | 10 721 (16.2) | 1239 (16.7) | ||
| 65-74 | 4675 (10.0) | 1935 (9.8) | 6610 (10.0) | 732 (9.9) | ||
| 75-84 | 2431 (5.2) | 1098 (5.6) | 3529 (5.3) | 482 (6.5) | ||
| ≥85 | 592 (1.3) | 250 (1.3) | 842 (1.3) | 135 (1.8) | ||
| Alcohol consumption | ||||||
| None | 29 299 (62.8) | 12 429 (63.0) | 41 728 (62.9) | 5066 (68.2) | ||
| Light | 8280 (17.8) | 3524 (17.9) | 11 804 (17.8) | 1090 (14.7) | ||
| Moderate | 7736 (16.6) | 3209 (16.3) | 10 945 (16.5) | 1051 (14.2) | ||
| Heavy | 1312 (2.8) | 567 (2.9) | 1879 (2.8) | 216 (2.9) | ||
| Cigarette smoking | ||||||
| Never | 19 032 (40.8) | 7978 (40.4) | 27 010 (40.7) | 2942 (39.6) | ||
| Always occasional | 629 (1.3) | 284 (1.4) | 913 (1.4) | 103 (1.4) | ||
| Former daily, now occasional | 1300 (2.8) | 545 (2.8) | 1845 (2.8) | 216 (2.9) | ||
| Daily | 7926 (17.0) | 3369 (17.1) | 11 295 (17.0) | 1327 (17.9) | ||
| Former occasional | 7439 (16.0) | 3207 (16.3) | 10 646 (16.0) | 1122 (15.1) | ||
| Former daily | 10 301 (22.1) | 4346 (22.0) | 14 647 (22.1) | 1713 (23.1) | ||
| Fruit and vegetable consumption, times/d | ||||||
| <3 | 11 152 (23.9) | 4623 (23.4) | 15 775 (23.8) | 1605 (21.6) | ||
| 3-6 | 21 806 (46.8) | 9276 (47.0) | 31 082 (46.8) | 2584 (34.8) | ||
| >6 | 13 669 (29.3) | 5830 (29.6) | 19 499 (29.4) | 1150 (15.5) | ||
| Physical activity, quartile | ||||||
| 1, lowest | 10 928 (23.4) | 4600 (23.3) | 15 528 (23.4) | 1568 (21.1) | ||
| 2 | 12 785 (27.4) | 5394 (27.3) | 18 179 (27.4) | 1986 (26.8) | ||
| 3 | 11 554 (24.8) | 4901 (24.8) | 16 455 (24.8) | 1885 (25.4) | ||
| 4, highest | 11 360 (24.4) | 4834 (24.5) | 16 194 (24.4) | 1984 (26.7) | ||
| Visible minority | 5606 (12.0) | 2375 (12.0) | 7981 (12.0) | 1119 (15.1) | ||
| Immigration status | ||||||
| Canadian born | 37 158 (79.7) | 15 822 (80.2) | 52 980 (79.8) | 6642 (89.5) | ||
| Recent immigrant | 1901 (4.1) | 763 (3.9) | 2664 (4.0) | 230 (3.1) | ||
| Nonrecent immigrant | 7568 (16.2) | 3144 (15.9) | 10 712 (16.1) | 551 (7.4) | ||
| Household income, quintile | ||||||
| 1, lowest | 5525 (11.8) | 2334 (11.8) | 7859 (11.8) | 1433 (19.3) | ||
| 2 | 6391 (13.7) | 2620 (13.3) | 9011 (13.6) | 1444 (19.5) | ||
| 3 | 8868 (19.0) | 3795 (19.2) | 12 663 (19.1) | 1443 (19.4) | ||
| 4 | 11 082 (23.8) | 4893 (24.8) | 15 975 (24.1) | 1288 (17.4) | ||
| 5, highest | 11 417 (24.5) | 4674 (23.7) | 16 091 (24.2) | 1208 (16.3) | ||
| Unknown income | 3344 (7.2) | 1413 (7.2) | 4757 (7.2) | 607 (8.2) | ||
| Education | ||||||
| <Secondary school | 6121 (13.1) | 2657 (13.5) | 8778 (13.2) | 1271 (17.1) | ||
| Secondary school | 9080 (19.5) | 3944 (20.0) | 13 024 (19.6) | 1435 (19.3) | ||
| Any postsecondary education | 31 426 (67.4) | 13 128 (66.5) | 44 554 (67.1) | 4717 (63.5) | ||
| Marital status | ||||||
| Single, never married | 8887 (19.1) | 3780 (19.2) | 12 667 (19.1) | 1447 (19.5) | ||
| Domestic partner, ie, married or common law | 28 032 (60.1) | 11 807 (59.8) | 39 839 (60.0) | 4540 (61.2) | ||
| Widowed, separated, or divorced | 9708 (20.8) | 4142 (21.0) | 13 850 (20.9) | 1436 (19.3) | ||
| Asthma | 3925 (8.4) | 1664 (8.4) | 5589 (8.4) | 561 (7.6) | ||
| BMI | ||||||
| Mean (SD) | 26.45 (5.47) | 26.45 (5.50) | 26.45 (5.48) | 27.16 (5.77) | ||
| <18.5 | 656 (1.4) | 299 (1.5) | 955 (1.4) | 97 (1.3) | ||
| 18.5 to <25.0 | 19 446 (41.7) | 8210 (41.6) | 27 656 (41.7) | 2765 (37.2) | ||
| 25.0 to <30.0 | 13 883 (29.8) | 5876 (29.8) | 19 759 (29.8) | 2384 (32.1) | ||
| 30.0 to <35.0 | 6041 (13.0) | 2576 (13.1) | 8617 (13.0) | 1084 (14.6) | ||
| 35.0 to <40.0 | 2044 (4.4) | 863 (4.4) | 2907 (4.4) | 401 (5.4) | ||
| ≥40.0 | 1015 (2.2) | 438 (2.2) | 1453 (2.2) | 232 (3.1) | ||
| Unknown | 3542 (7.6) | 1467 (7.4) | 5009 (7.5) | 460 (6.2) | ||
| High blood pressure | 6992 (15.0) | 2976 (15.1) | 9968 (15.0) | 1239 (16.7) | ||
| Household secondhand smoke | 4048 (8.7) | 1703 (8.6) | 5751 (8.7) | 764 (10.3) | ||
| Self-rated health | ||||||
| Poor or fair | 3858 (8.3) | 1659 (8.4) | 5517 (8.3) | 661 (8.9) | ||
| Good | 11 805 (25.3) | 4967 (25.2) | 16 772 (25.3) | 2032 (27.4) | ||
| Very good or excellent | 30 964 (66.4) | 13 103 (66.4) | 44 067 (66.4) | 4730 (63.7) | ||
| Life stress | ||||||
| Not at all stressful | 4432 (9.5) | 1859 (9.4) | 6291 (9.5) | 709 (9.6) | ||
| Not very stressful | 11 144 (23.9) | 4625 (23.4) | 15 769 (23.8) | 1921 (25.9) | ||
| A bit stressful | 20 137 (43.2) | 8534 (43.3) | 28 671 (43.2) | 3265 (44) | ||
| Quite a bit or extremely stressful | 10 914 (23.4) | 4711 (23.9) | 15 625 (23.5) | 1528 (20.6) | ||
| Men | ||||||
| No. | 36 540 | 15 851 | 52 391 | 5821 | ||
| Follow-up time, mean (SD), y | 6.93 (2.82) | 6.87 (2.78) | 6.91 (2.80) | 7.35 (2.79) | ||
| Age, y | ||||||
| Mean (SD) | 44.35 (15.14) | 44.43 (15.17) | 44.38 (15.15) | 45.11 (15.60) | ||
| 20-34 | 10 922 (29.9) | 4628 (29.2) | 15 550 (29.7) | 1769 (30.4) | ||
| 35-44 | 9053 (24.8) | 4027 (25.4) | 13 080 (25.0) | 1210 (20.8) | ||
| 45-54 | 6934 (19.0) | 3055 (19.3) | 9989 (19.1) | 1134 (19.5) | ||
| 55-64 | 5531 (15.1) | 2347 (14.8) | 7878 (15.0) | 984 (16.9) | ||
| 65-74 | 2927 (8.0) | 1261 (8.0) | 4188 (8.0) | 488 (8.4) | ||
| 75-84 | 1006 (2.8) | 456 (2.9) | 1462 (2.8) | 204 (3.5) | ||
| ≥85 | 167 (0.5) | 77 (0.5) | 244 (0.5) | 32 (0.5) | ||
| Alcohol consumption | ||||||
| None | 14 714 (40.3) | 6408 (40.4) | 21 122 (40.3) | 2786 (47.9) | ||
| Light | 5851 (16.0) | 2466 (15.6) | 8317 (15.9) | 806 (13.8) | ||
| Moderate | 11 216 (30.7) | 4816 (30.4) | 16 032 (30.6) | 1544 (26.5) | ||
| Heavy | 4759 (13.0) | 2161 (13.6) | 6920 (13.2) | 685 (11.8) | ||
| Cigarette smoking | ||||||
| Never | 10 450 (28.6) | 4504 (28.4) | 14 954 (28.5) | 1762 (30.3) | ||
| Always occasional | 761 (2.1) | 313 (2.0) | 1074 (2.0) | 125 (2.1) | ||
| Former daily, now occasional | 1199 (3.3) | 534 (3.4) | 1733 (3.3) | 192 (3.3) | ||
| Daily | 8107 (22.2) | 3598 (22.7) | 11 705 (22.3) | 1246 (21.4) | ||
| Former occasional | 6435 (17.6) | 2814 (17.8) | 9249 (17.7) | 925 (15.9) | ||
| Former daily | 9588 (26.2) | 4088 (25.8) | 13 676 (26.1) | 1571 (27.0) | ||
| Fruit and vegetable consumption, times/d | ||||||
| <3 | 14 163 (38.8) | 6304 (39.8) | 20 467 (39.1) | 2042 (35.1) | ||
| 3-6 | 16 714 (45.7) | 7049 (44.5) | 23 763 (45.4) | 1691 (29.1) | ||
| >6 | 5663 (15.5) | 2498 (15.8) | 8161 (15.6) | 458 (7.9) | ||
| Physical activity, quartile | ||||||
| 1, lowest | 8795 (24.1) | 3789 (23.9) | 12 584 (24.0) | 1238 (21.3) | ||
| 2 | 9698 (26.5) | 4200 (26.5) | 13 898 (26.5) | 1513 (26.0) | ||
| 3 | 9019 (24.7) | 4026 (25.4) | 13 045 (24.9) | 1331 (22.9) | ||
| 4, highest | 9028 (24.7) | 3836 (24.2) | 12 864 (24.6) | 1739 (29.9) | ||
| Visible minority | 4608 (12.6) | 2041 (12.9) | 6649 (12.7) | 835 (14.3) | ||
| Immigration status | ||||||
| Canadian born | 29 256 (80.1) | 12 703 (80.1) | 41 959 (80.1) | 5185 (89.1) | ||
| Recent immigrant | 1461 (4.0) | 613 (3.9) | 2074 (4.0) | 188 (3.2) | ||
| Nonrecent immigrant | 5823 (15.9) | 2535 (16.0) | 8358 (16.0) | 448 (7.7) | ||
| Household income, quintile | ||||||
| 1, lowest | 3009 (8.2) | 1335 (8.4) | 4344 (8.3) | 726 (12.5) | ||
| 2 | 3836 (10.5) | 1673 (10.6) | 5509 (10.5) | 970 (16.7) | ||
| 3 | 6167 (16.9) | 2681 (16.9) | 8848 (16.9) | 1171 (20.1) | ||
| 4 | 9837 (26.9) | 4242 (26.8) | 14 079 (26.9) | 1310 (22.5) | ||
| 5, highest | 11 874 (32.5) | 5166 (32.6) | 17 040 (32.5) | 1349 (23.2) | ||
| Unknown income | 1817 (5.0) | 754 (4.8) | 2571 (4.9) | 295 (5.1) | ||
| Education | ||||||
| <Secondary school | 4811 (13.2) | 2130 (13.4) | 6941 (13.2) | 1146 (19.7) | ||
| Secondary school | 6896 (18.9) | 3078 (19.4) | 9974 (19.0) | 1156 (19.9) | ||
| Any postsecondary education | 24 833 (68.0) | 10 643 (67.1) | 35 476 (67.7) | 3519 (60.5) | ||
| Marital status | ||||||
| Single, never married | 9501 (26.0) | 4132 (26.1) | 13 633 (26.0) | 1565 (26.9) | ||
| Domestic partner, ie, married or common law | 22 795 (62.4) | 9865 (62.2) | 32 660 (62.3) | 3590 (61.7) | ||
| Widowed, separated, or divorced | 4244 (11.6) | 1854 (11.7) | 6098 (11.6) | 666 (11.4) | ||
| Asthma | 1971 (5.4) | 928 (5.9) | 2899 (5.5) | 343 (5.9) | ||
| BMI | ||||||
| Mean (SD) | 27.44 (4.59) | 27.39 (4.62) | 27.43 (4.60) | 27.99 (5.08) | ||
| <18.5 | 238 (0.7) | 105 (0.7) | 343 (0.7) | 34 (0.6) | ||
| 18.5 to <25.0 | 11 004 (30.1) | 4833 (30.5) | 15 837 (30.2) | 1568 (26.9) | ||
| 25.0 to <30.0 | 16 090 (44.0) | 6989 (44.1) | 23 079 (44.1) | 2588 (44.5) | ||
| 30.0 to <35.0 | 6386 (17.5) | 2723 (17.2) | 9109 (17.4) | 1165 (20.0) | ||
| 35.0 to <40.0 | 1480 (4.1) | 615 (3.9) | 2095 (4.0) | 303 (5.2) | ||
| ≥40.0 | 522 (1.4) | 231 (1.5) | 753 (1.4) | 125 (2.1) | ||
| Unknown | 820 (2.2) | 355 (2.2) | 1175 (2.2) | 38 (0.7) | ||
| High blood pressure | 4798 (13.1) | 2151 (13.6) | 6949 (13.3) | 846 (14.5) | ||
| Household secondhand smoke | 3527 (9.7) | 1552 (9.8) | 5079 (9.7) | 625 (10.7) | ||
| Self-rated health | ||||||
| Poor or fair | 2688 (7.4) | 1124 (7.1) | 3812 (7.3) | 427 (7.3) | ||
| Good | 9547 (26.1) | 4194 (26.5) | 13 741 (26.2) | 1698 (29.2) | ||
| Very good or excellent | 24 305 (66.5) | 10 533 (66.5) | 34 838 (66.5) | 3696 (63.5) | ||
| Life stress | ||||||
| Not at all stressful | 4198 (11.5) | 1951 (12.3) | 6149 (11.7) | 637 (10.9) | ||
| Not very stressful | 8890 (24.3) | 3758 (23.7) | 12 648 (24.1) | 1610 (27.7) | ||
| A bit stressful | 15 660 (42.9) | 6699 (42.3) | 22 359 (42.7) | 2501 (43.0) | ||
| Quite a bit or extremely stressful | 7792 (21.3) | 3443 (21.7) | 11 235 (21.4) | 1073 (18.4) | ||
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
CDPoRT Development
The full versions of the CDPoRT for women and men consisted of 11 predictors, with their coefficients and hazard ratios listed in Table 2. No interactions or cross-classifications improved the predictive performance of the full versions. Age, smoking, and body mass index had the greatest influence on predictive performance, and thereby were retained in the simple version for both sexes (Table 2); for example, they had high predictor importance based on large χ2 values of 1117 for age, 631 for smoking, and 453 for body mass index for women and 1166 for age, 337 for smoking, and 314 for body mass index for men. For both sexes, the parsimonious version contained the same 9 predictors, ie, alcohol consumption, cigarette smoking, fruit and vegetable consumption, age, visible minority, asthma, body mass index, high blood pressure, and self-rated health (Table 2). There were no gross violations of the proportionality assumption.
Table 2. Chronic Disease Population Risk Tool for Women and Men Proportional Hazards Coefficients and Hazard Ratios for the Full, Simple, and Parsimonious Versions, by Ontario Cohort.
| Variable | Cohort | |||
|---|---|---|---|---|
| Development | Entire Ontario | |||
| ln[HR] (95% CI) | HR | ln[HR] (95% CI) | HR | |
| Full version for women | ||||
| Weibull parameters | ||||
| Scale, σ | 0.8924 | NA | 0.8869 | NA |
| Shape, γ | 1.1206 | NA | 1.1275 | NA |
| Intercept | −4.311 (−4.71 to −3.91) | 0.0134 | −4.345 (−4.68 to −4.00) | 0.0130 |
| Alcohol consumption | ||||
| Light | NA | NA | NA | NA |
| Heavy | 0.41 (0.12 to 0.70) | 1.51 | 0.24 (−0.01 to 0.49) | 1.27 |
| Moderate | 0.09 (−0.07 to 0.24) | 1.09 | 0.10 (−0.03 to 0.23) | 1.11 |
| None | 0.34 (0.22 to 0.47) | 1.41 | 0.32 (0.22 to 0.41) | 1.37 |
| Cigarette smoking | ||||
| Never | NA | NA | NA | NA |
| Always occasional | 0.36 (−0.09 to 0.81) | 1.44 | 0.27 (−0.11 to 0.65) | 1.31 |
| Former daily, now occasional | 0.61 (0.34 to 0.89) | 1.84 | 0.61 (0.38 to 0.84) | 1.84 |
| Daily | 1.05 (0.93 to 1.18) | 2.87 | 1.05 (0.95 to 1.15) | 2.86 |
| Former occasional | −0.16 (−0.30 to −0.02) | 0.85 | −0.16 (−0.27 to −0.04) | 0.86 |
| Former daily | 0.20 (0.09 to 0.32) | 1.23 | 0.20 (0.10 to 0.30) | 1.22 |
| Fruit and vegetable consumption, times/d | ||||
| <3 | NA | NA | NA | NA |
| 3-6 | −0.06 (−0.17 to 0.05) | 0.94 | −0.06 (−0.15 to 0.03) | 0.94 |
| >6 | −0.12 (−0.25 to 0.00) | 0.88 | −0.12 (−0.22 to −0.01) | 0.89 |
| Age, spline term | ||||
| 1 | 0.13 (0.11 to 0.16) | 1.14 | 0.13 (0.11 to 0.15) | 1.14 |
| 2 | −0.26 (−0.34 to −0.18) | 0.77 | −0.25 (−0.32 to −0.18) | 0.78 |
| 3 | 0.51 (0.34 to 0.69) | 1.67 | 0.51 (0.36 to 0.66) | 1.66 |
| Visible minority | 0.37 (0.23 to 0.51) | 1.44 | 0.34 (0.22 to 0.46) | 1.41 |
| Postsecondary education | −0.06 (−0.16 to 0.03) | 0.94 | −0.10 (−0.19 to −0.02) | 0.90 |
| Marital status | ||||
| Domestic partner | NA | NA | NA | NA |
| Single, never married | 0.05 (−0.11 to 0.21) | 1.06 | 0.07 (−0.06 to 0.21) | 1.08 |
| Widowed, separated, or divorced | 0.09 (−0.02 to 0.20) | 1.09 | 0.08 (−0.01 to 0.18) | 1.09 |
| Asthma | 0.36 (0.22 to 0.50) | 1.43 | 0.37 (0.25 to 0.50) | 1.45 |
| BMI | ||||
| <18.5 | NA | NA | NA | NA |
| 18.5 to <25.0 | −0.33 (−0.76 to 0.10) | 0.72 | −0.18 (−0.56 to 0.19) | 0.83 |
| 25.0 to <30.0 | 0.32 (0.20 to 0.45) | 1.38 | 0.37 (0.27 to 0.47) | 1.45 |
| 30.0 to <35.0 | 0.61 (0.47 to 0.75) | 1.84 | 0.61 (0.50 to 0.73) | 1.85 |
| 35.0 to <40.0 | 1.01 (0.80 to 1.22) | 2.74 | 1.03 (0.86 to 1.20) | 2.81 |
| ≥40.0 | 1.24 (1.01 to 1.47) | 3.46 | 1.16 (0.97 to 1.36) | 3.20 |
| Unknown | 0.47 (0.32 to 0.62) | 1.59 | 0.43 (0.31 to 0.56) | 1.54 |
| High blood pressure | 0.35 (0.24 to 0.46) | 1.42 | 0.33 (0.23 to 0.42) | 1.39 |
| Self-rated health | ||||
| Good | NA | NA | NA | NA |
| Poor or fair | 0.23 (0.09 to 0.37) | 1.26 | 0.19 (0.08 to 0.31) | 1.21 |
| Very good or excellent | −0.14 (−0.24 to −0.03) | 0.87 | −0.19 (−0.27 to −0.10) | 0.83 |
| Life stress | ||||
| Not at all stressful | NA | NA | NA | NA |
| A bit stressful | −0.02 (−0.17 to 0.12) | 0.98 | 0.00 (−0.13 to 0.13) | 1.00 |
| Not very stressful | −0.13 (−0.29 to 0.02) | 0.88 | −0.10 (−0.23 to 0.02) | 0.90 |
| Quite a bit or extremely stressful | −0.03 (−0.20 to 0.13) | 0.97 | 0.02 (−0.13 to 0.16) | 1.02 |
| Simple version for women | ||||
| Weibull parameters | NA | NA | NA | NA |
| Scale, σ | 0.9000 | NA | 0.8940 | NA |
| Shape, γ | 1.1111 | NA | 1.1186 | NA |
| Intercept | −4.083 (−4.42 to −3.75) | 0.0169 | −4.210 (−4.49 to −3.93) | 0.0148 |
| Cigarette smoking | ||||
| Never | NA | NA | NA | NA |
| Always occasional | 0.29 (−0.17 to 0.74) | 1.33 | 0.18 (−0.20 to 0.56) | 1.20 |
| Former daily, now occasional | 0.51 (0.23 to 0.78) | 1.66 | 0.50 (0.27 to 0.73) | 1.65 |
| Daily | 0.99 (0.87 to 1.12) | 0.73 | 1.00 (0.90 to 1.10) | 0.74 |
| Former occasional | −0.31 (−0.45 to −0.17) | 2.70 | −0.30 (−0.42 to −0.18) | 2.72 |
| Former daily | 0.04 (−0.07 to 0.16) | 1.04 | 0.05 (−0.04 to 0.14) | 1.05 |
| Age, spline term | ||||
| 1 | 0.13 (0.11 to 0.15) | 1.14 | 0.12 (0.10 to 0.14) | 1.13 |
| 2 | −0.25 (−0.33 to −0.17) | 0.78 | −0.24 (−0.30 to −0.17) | 0.79 |
| 3 | 0.52 (0.35 to 0.69) | 1.68 | 0.49 (0.35 to 0.64) | 1.64 |
| BMI | ||||
| <18.5 | NA | NA | NA | NA |
| 18.5 to <25.0 | −0.21 (−0.63 to 0.21) | 2.12 | −0.07 (−0.44 to 0.30) | 2.14 |
| 25.0 to <30.0 | 0.39 (0.26 to 0.51) | 3.42 | 0.43 (0.33 to 0.52) | 3.55 |
| 30.0 to <35.0 | 0.75 (0.62 to 0.89) | 4.32 | 0.76 (0.65 to 0.87) | 4.08 |
| 35.0 to <40.0 | 1.23 (1.02 to 1.43) | 1.47 | 1.27 (1.10 to 1.43) | 1.53 |
| ≥40.0 | 1.46 (1.25 to 1.68) | 0.81 | 1.41 (1.23 to 1.59) | 0.93 |
| Unknown | 0.54 (0.39 to 0.69) | 1.71 | 0.52 (0.39 to 0.64) | 1.67 |
| Parsimonious version for women | ||||
| Weibull parameters | ||||
| Scale, σ | 0.8922 | NA | 0.8864 | NA |
| Shape, γ | 1.1208 | NA | 1.1282 | NA |
| Intercept | −4.384 (−4.75 to −4.01) | 0.0125 | −4.424 (−4.73 to −4.11) | 0.0120 |
| Alcohol consumption | ||||
| Light | NA | NA | NA | NA |
| Heavy | 0.42 (0.13 to 0.71) | 1.52 | 0.26 (0.01 to 0.51) | 1.29 |
| Moderate | 0.08 (−0.07 to 0.24) | 1.09 | 0.10 (−0.03 to 0.23) | 1.10 |
| None | 0.35 (0.23 to 0.47) | 1.42 | 0.33 (0.23 to 0.43) | 1.39 |
| Cigarette smoking | ||||
| Never | NA | NA | NA | NA |
| Always occasional | 0.38 (−0.07 to 0.82) | 1.46 | 0.29 (−0.09 to 0.66) | 1.33 |
| Former daily, now occasional | 0.62 (0.34 to 0.89) | 1.85 | 0.61 (0.38 to 0.84) | 1.84 |
| Daily | 1.07 (0.95 to 1.2) | 2.93 | 1.08 (0.97 to 1.18) | 2.93 |
| Former occasional | −0.16 (−0.3 to −0.02) | 0.85 | −0.16 (−0.28 to −0.04) | 0.85 |
| Former daily | 0.20 (0.08 to 0.32) | 1.23 | 0.20 (0.10 to 0.30) | 1.22 |
| Fruit and vegetable consumption, times/d | ||||
| <3 | NA | NA | NA | NA |
| 3-6 | −0.07 (−0.17 to 0.04) | 0.94 | −0.07 (−0.17 to 0.02) | 0.93 |
| >6 | −0.14 (−0.26 to −0.01) | 0.87 | −0.14 (−0.24 to −0.03) | 0.87 |
| Age, spline term | ||||
| 1 | 0.13 (0.11 to 0.16) | 1.14 | 0.13 (0.11 to 0.15) | 1.14 |
| 2 | −0.26 (−0.34 to −0.18) | 0.77 | −0.25 (−0.31 to −0.18) | 0.78 |
| 3 | 0.52 (0.35 to 0.69) | 1.68 | 0.51 (0.36 to 0.66) | 1.66 |
| Visible minority | 0.37 (0.23 to 0.51) | 1.44 | 0.34 (0.22 to 0.46) | 1.41 |
| Asthma | 0.36 (0.22 to 0.5) | 1.43 | 0.38 (0.26 to 0.50) | 1.46 |
| BMI | ||||
| <18.5 | NA | NA | NA | NA |
| 18.5 to <25.0 | −0.32 (−0.74 to 0.11) | 0.73 | −0.18 (−0.55 to 0.19) | 0.83 |
| 25.0 to <30.0 | 0.33 (0.2 to 0.45) | 1.39 | 0.37 (0.27 to 0.47) | 1.45 |
| 30.0 to <35.0 | 0.61 (0.47 to 0.75) | 1.85 | 0.62 (0.50 to 0.73) | 1.86 |
| 35.0 to <40.0 | 1.02 (0.8 to 1.23) | 2.76 | 1.04 (0.87 to 1.21) | 2.83 |
| ≥40.0 | 1.24 (1.02 to 1.47) | 3.47 | 1.17 (0.98 to 1.36) | 3.22 |
| Unknown | 0.47 (0.32 to 0.62) | 1.60 | 0.44 (0.31 to 0.57) | 1.55 |
| High blood pressure | 0.35 (0.24 to 0.46) | 1.42 | 0.33 (0.24 to 0.42) | 1.39 |
| Self-rated health | ||||
| Good | NA | NA | NA | NA |
| Poor or fair | 0.24 (0.11 to 0.38) | 1.27 | 0.21 (0.10 to 0.33) | 1.24 |
| Very good or excellent | −0.14 (−0.25 to −0.04) | 0.87 | −0.19 (−0.28 to −0.10) | 0.82 |
| Full version for men | ||||
| Weibull parameters | ||||
| Scale, σ | 0.8422 | NA | 0.8496 | NA |
| Shape, γ | 1.1874 | NA | 1.1770 | NA |
| Intercept | −2.405 (−3.35 to −1.46) | 0.0903 | −3.183 (−3.92 to −2.45) | 0.0415 |
| Alcohol consumption | ||||
| Light | NA | NA | NA | NA |
| Heavy | −0.21 (−0.39 to −0.03) | 0.81 | −0.12 (−0.26 to 0.02) | 0.89 |
| Moderate | −0.05 (−0.19 to 0.09) | 0.95 | −0.01 (−0.13 to 0.11) | 0.99 |
| None | 0.19 (0.06 to 0.33) | 1.21 | 0.18 (0.06 to 0.30) | 1.20 |
| Cigarette smoking | ||||
| Never | NA | NA | NA | NA |
| Always occasional | 0.14 (−0.35 to 0.63) | 1.15 | 0.05 (−0.33 to 0.43) | 1.05 |
| Former daily, now occasional | 0.33 (0.06 to 0.61) | 1.40 | 0.35 (0.12 to 0.58) | 1.42 |
| Daily | 0.86 (0.73 to 1.00) | 2.37 | 0.83 (0.72 to 0.94) | 2.30 |
| Former occasional | 0.04 (−0.13 to 0.22) | 1.04 | 0.01 (−0.13 to 0.16) | 1.01 |
| Former daily | 0.20 (0.08 to 0.33) | 1.23 | 0.16 (0.05 to 0.27) | 1.18 |
| Fruit and vegetable consumption, times/d | ||||
| <3 | NA | NA | NA | NA |
| 3-6 | −0.13 (−0.23 to −0.02) | 0.88 | −0.08 (−0.16 to 0.01) | 0.93 |
| >6 | −0.22 (−0.39 to −0.06) | 0.80 | −0.16 (−0.30 to −0.03) | 0.85 |
| Age, spline term | ||||
| 1 | 0.24 (0.18 to 0.29) | 1.27 | 0.19 (0.15 to 0.23) | 1.21 |
| 2 | −0.63 (−0.90 to −0.37) | 0.53 | −0.42 (−0.63 to −0.21) | 0.66 |
| 3 | 1.57 (0.74 to 2.40) | 4.81 | 0.93 (0.26 to 1.60) | 2.54 |
| 4 | −1.04 (−1.84 to −0.24) | 0.35 | −0.48 (−1.15 to 0.18) | 0.62 |
| Visible minority | 0.31 (0.16 to 0.45) | 1.36 | 0.27 (0.15 to 0.39) | 1.31 |
| Household income | ||||
| Not low | NA | NA | NA | NA |
| Low | 0.12 (−0.06 to 0.29) | 1.12 | 0.11 (−0.04 to 0.27) | 1.12 |
| Unknown | 0.08 (−0.13 to 0.30) | 1.09 | 0.13 (−0.04 to 0.31) | 1.14 |
| Asthma | 0.33 (0.14 to 0.51) | 1.39 | 0.27 (0.11 to 0.44) | 1.32 |
| BMI | ||||
| <18.5 | NA | NA | NA | NA |
| 18.5 to <25.0 | 0.53 (−0.04 to 1.10) | 1.69 | 0.42 (−0.07 to 0.92) | 1.53 |
| 25.0 to <30.0 | 0.04 (−0.09 to 0.18) | 1.04 | 0.14 (0.03 to 0.25) | 1.15 |
| 30.0 to <35.0 | 0.51 (0.36 to 0.66) | 1.66 | 0.57 (0.44 to 0.69) | 1.77 |
| 35.0 to <40.0 | 0.87 (0.67 to 1.07) | 2.38 | 0.98 (0.81 to 1.16) | 2.67 |
| ≥40.0 | 1.16 (0.85 to 1.46) | 3.18 | 1.19 (0.94 to 1.44) | 3.28 |
| Unknown | 0.36 (0.14 to 0.59) | 1.44 | 0.36 (0.17 to 0.55) | 1.43 |
| High blood pressure | 0.39 (0.27 to 0.52) | 1.48 | 0.36 (0.26 to 0.46) | 1.43 |
| Self-rated health | ||||
| Good | NA | NA | NA | NA |
| Poor or fair | 0.12 (−0.03 to 0.27) | 1.12 | 0.11 (−0.01 to 0.24) | 1.12 |
| Very good or excellent | −0.33 (−0.43 to −0.22) | 0.72 | −0.29 (−0.38 to −0.20) | 0.75 |
| Life stress | ||||
| Not at all stressful | NA | NA | NA | NA |
| A bit stressful | −0.01 (−0.17 to 0.14) | 0.99 | −0.03 (−0.16 to 0.10) | 0.97 |
| Not very stressful | −0.13 (−0.29 to 0.03) | 0.88 | −0.08 (−0.21 to 0.05) | 0.92 |
| Quite a bit or extremely stressful | −0.11 (−0.28 to 0.06) | 0.89 | −0.13 (−0.27 to 0.01) | 0.88 |
| Simple version for men | ||||
| Weibull parameters | ||||
| Scale, σ | 0.8522 | NA | 0.8571 | NA |
| Shape, γ | 1.1735 | NA | 1.1667 | NA |
| Intercept | −2.629 (−3.52 to −1.74) | 0.0721 | −3.343 (−4.03 to −2.65) | 0.0353 |
| Cigarette smoking | ||||
| Never | NA | NA | NA | NA |
| Always occasional | 0.10 (−0.41 to 0.61) | 1.11 | 0.03 (−0.37 to 0.42) | 1.03 |
| Former daily, now occasional | 0.27 (0.01 to 0.54) | 1.31 | 0.34 (0.12 to 0.56) | 1.40 |
| Daily | 0.87 (0.74 to 1.00) | 2.38 | 0.84 (0.73 to 0.95) | 2.33 |
| Former occasional | −0.03 (−0.19 to 0.14) | 0.97 | −0.04 (−0.18 to 0.10) | 0.96 |
| Former daily | 0.14 (0.01 to 0.26) | 1.15 | 0.12 (0.01 to 0.22) | 1.12 |
| Age, spline term | ||||
| 1 | 0.23 (0.18 to 0.29) | 1.26 | 0.18 (0.14 to 0.23) | 1.20 |
| 2 | −0.59 (−0.86 to −0.32) | 0.56 | −0.39 (−0.60 to −0.17) | 0.68 |
| 3 | 1.42 (0.59 to 2.25) | 4.13 | 0.83 (0.16 to 1.51) | 2.30 |
| 4 | −0.89 (−1.70 to −0.08) | 0.41 | −0.39 (−1.06 to 0.28) | 0.68 |
| BMI | ||||
| <18.5 | NA | NA | NA | NA |
| 18.5 to <25.0 | 0.66 (0.09 to 1.23) | 1.94 | 0.53 (0.04 to 1.02) | 1.70 |
| 25.0 to <30.0 | 0.55 (0.40 to 0.70) | 1.03 | 0.12 (0.48 to 0.72) | 1.13 |
| 30.0 to <35.0 | 1.00 (0.81 to 1.20) | 1.73 | 0.60 (0.93 to 1.27) | 1.82 |
| 35.0 to <40.0 | 1.42 (1.14 to 1.70) | 2.73 | 1.10 (1.19 to 1.65) | 3.01 |
| ≥40.0 | 0.03 (−0.11 to 0.16) | 4.14 | 1.42 (0.01 to 0.23) | 4.13 |
| Unknown | 0.34 (0.10 to 0.57) | 1.40 | 0.32 (0.13 to 0.51) | 1.38 |
| Parsimonious version for men | ||||
| Weibull parameters | ||||
| Scale, σ | 0.8433 | NA | 0.8504 | NA |
| Shape, γ | 1.1858 | NA | 1.1759 | NA |
| Intercept | −2.485 (−3.39 to −1.58) | 0.0833 | −3.279 (−3.99 to −2.57) | 0.0377 |
| Alcohol consumption | ||||
| Light | NA | NA | NA | NA |
| Heavy | −0.21 (−0.39 to −0.03) | 0.81 | −0.12 (−0.26 to 0.03) | 0.89 |
| Moderate | −0.05 (−0.19 to 0.09) | 0.95 | −0.01 (−0.13 to 0.11) | 0.99 |
| None | 0.20 (0.07 to 0.34) | 1.22 | 0.19 (0.07 to 0.30) | 1.21 |
| Cigarette smoking | ||||
| Never | NA | NA | NA | NA |
| Always occasional | 0.15 (−0.34 to 0.63) | 1.16 | 0.06 (−0.32 to 0.45) | 1.06 |
| Former daily, now occasional | 0.34 (0.07 to 0.61) | 1.40 | 0.36 (0.13 to 0.59) | 1.43 |
| Daily | 0.87 (0.73 to 1.00) | 2.38 | 0.84 (0.73 to 0.95) | 2.31 |
| Former occasional | 0.04 (−0.13 to 0.21) | 1.04 | 0.01 (−0.13 to 0.15) | 1.01 |
| Former daily | 0.20 (0.08 to 0.33) | 1.23 | 0.16 (0.06 to 0.27) | 1.18 |
| Fruit and vegetable consumption, times/d | ||||
| <3 | NA | NA | NA | NA |
| 3-6 | −0.13 (−0.24 to −0.02) | 0.88 | −0.08 (−0.16 to 0.01) | 0.92 |
| >6 | −0.23 (−0.39 to −0.06) | 0.80 | −0.17 (−0.30 to −0.03) | 0.85 |
| Age, spline term | ||||
| 1 | 0.23 (0.18 to 0.29) | 1.26 | 0.19 (0.15 to 0.23) | 1.21 |
| 2 | −0.62 (−0.89 to −0.36) | 0.54 | −0.41 (−0.62 to −0.20) | 0.67 |
| 3 | 1.54 (0.71 to 2.37) | 4.66 | 0.91 (0.24 to 1.58) | 2.47 |
| 4 | −1.01 (−1.81 to −0.21) | 0.36 | −0.46 (−1.13 to 0.20) | 0.63 |
| Visible minority | 0.32 (0.18 to 0.46) | 1.38 | 0.29 (0.17 to 0.41) | 1.33 |
| Asthma | 0.32 (0.14 to 0.51) | 1.38 | 0.27 (0.11 to 0.43) | 1.31 |
| BMI | ||||
| <18.5 | NA | NA | NA | NA |
| 18.5 to <25.0 | 0.53 (−0.04 to 1.10) | 1.70 | 0.43 (−0.07 to 0.93) | 1.54 |
| 25.0 to <30.0 | 0.04 (−0.09 to 0.17) | 1.04 | 0.14 (0.03 to 0.25) | 1.15 |
| 30.0 to <35.0 | 0.50 (0.35 to 0.65) | 1.65 | 0.57 (0.44 to 0.69) | 1.76 |
| 35.0 to <40.0 | 0.87 (0.67 to 1.07) | 2.39 | 0.99 (0.81 to 1.16) | 2.68 |
| ≥40.0 | 1.15 (0.85 to 1.45) | 3.16 | 1.19 (0.95 to 1.44) | 3.29 |
| Unknown | 0.36 (0.13 to 0.58) | 1.43 | 0.36 (0.17 to 0.54) | 1.43 |
| High blood pressure | 0.39 (0.27 to 0.51) | 1.48 | 0.36 (0.26 to 0.45) | 1.43 |
| Self-rated health | ||||
| Good | NA | NA | NA | NA |
| Poor or fair | 0.12 (−0.02 to 0.26) | 1.13 | 0.12 (−0.01 to 0.24) | 1.12 |
| Very good or excellent | −0.33 (−0.43 to −0.22) | 0.72 | −0.29 (−0.38 to −0.20) | 0.75 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; ln, natural log; NA, not applicable.
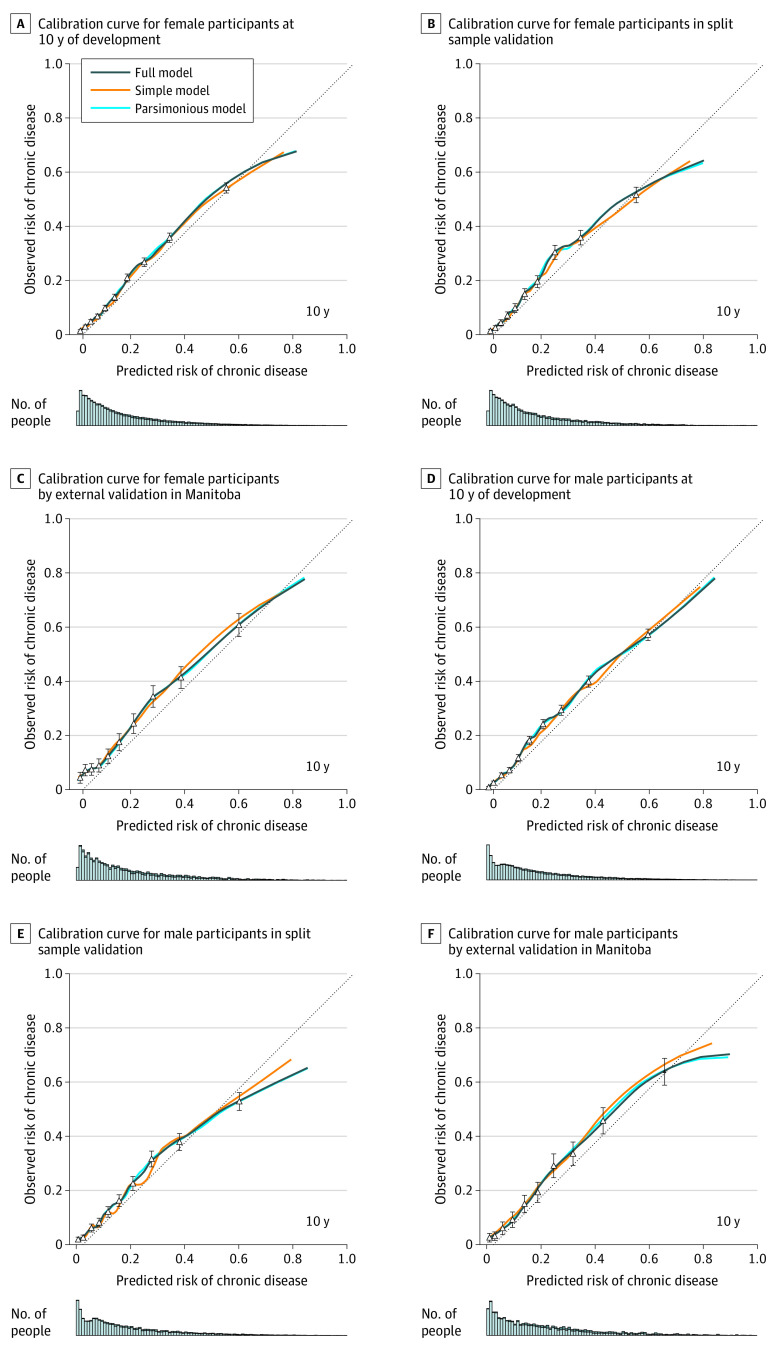
The predictive performances of the development models are detailed in Table 3. In general, the full version had the best overall predictive performance (for women, Brier score, 0.087; for men, Brier score, 0.091) and discrimination (for women, discrimination slope, 0.109; for men, discrimination slope, 0.112) with the parsimonious model performing comparably (for women, Brier score: 0.087; discrimination slope, 0.109; for men, Brier score, 0.091; discrimination slope, 0.111). The simple version performed well but had worse predictive performance than the other versions (for women, Brier score: 0.088; for men, 0.092). The calibration curves (Figure 2A) indicate strong calibration up to a predicted probability of 60%, at which point the model overpredicted chronic disease risk, as indicated by the deviation of the curve from the 45° line. However, this discrepancy occurred among a very small proportion of the cohort (2.9% women and 4.1% men) and thus had minimal influence on overall calibration, as indicated by the small number of people in the bar graph below the calibration curve. By sex, the calibration curves between CDPoRT versions exhibited similar shapes.
Table 3. CDPoRT Predictive Performance for the Full, Simple, and Parsimonious Versions for Women and Men, by Development and Validation Settingsa.
| CDPoRT version | Measures of predictive performance | Development (70%) | Bootstrap validation (development)b | Split sample validation (30%) | Ontario final model | Bootstrap validation (Ontario final model)b | Manitoba external validation | Sensitivity analysis | |
|---|---|---|---|---|---|---|---|---|---|
| No chronic disease history | Additional chronic diseases | ||||||||
| Full version for women, df = 30 | Nagelkerke R2 | 0.160 | 0.159 | NA | 0.160 | 0.159 | NA | NA | NA |
| Brier score | 0.087 | NA | 0.119 | 0.087 | NA | 0.128 | 0.090 | 0.322 | |
| Harrell C index | 0.779 | 0.778 | 0.777 | 0.767 | 0.779 | 0.752 | 0.774 | 0.596 | |
| Discrimination slope | 0.109 | NA | 0.096 | 0.089 | NA | 0.103 | 0.105 | 0.035 | |
| Calibration in the large | 0 | 0.021 | −0.006 | 0 | 0.013 | 0.032 | −0.018 | 0.284 | |
| Calibration slope | 1 | 0.994 | 0.994 | 1 | 0.996 | 0.893 | 0.952 | 0.317 | |
| Simple version for women, df = 14 | Nagelkerke R2 | 0.144 | 0.143 | NA | 0.143 | 0.143 | NA | NA | NA |
| Brier score | 0.088 | NA | 0.122 | 0.089 | NA | 0.13 | 0.092 | 0.319 | |
| Harrell C index | 0.767 | 0.767 | 0.766 | 0.767 | 0.766 | 0.739 | 0.764 | 0.590 | |
| Discrimination slope | 0.092 | NA | 0.080 | 0.089 | NA | 0.081 | 0.087 | 0.033 | |
| Calibration in the large | 0 | 0.010 | −0.005 | 0 | 0.007 | 0.033 | −0.016 | 0.280 | |
| Calibration slope | 1 | 0.997 | 1.010 | 1 | 0.998 | 0.894 | 0.972 | 0.293 | |
| Parsimonious version for women, df = 24 | Nagelkerke R2 | 0.160 | 0.158 | NA | 0.159 | 0.158 | NA | NA | NA |
| Brier score | 0.087 | NA | 0.119 | 0.088 | NA | 0.128 | 0.090 | 0.322 | |
| Harrell C index | 0.779 | 0.778 | 0.776 | 0.779 | 0.778 | 0.752 | 0.774 | 0.596 | |
| Discrimination slope | 0.109 | NA | 0.095 | 0.104 | NA | 0.102 | 0.104 | 0.035 | |
| Calibration in the large | 0 | 0.017 | −0.005 | 0 | 0.012 | 0.03 | −0.018 | 0.284 | |
| Calibration slope | 1 | 0.995 | 0.994 | 1 | 0.997 | 0.885 | 0.951 | 0.316 | |
| Full version for men df = 30 | Nagelkerke R2 | 0.178 | 0.176 | NA | 0.169 | 0.168 | NA | NA | NA |
| Brier score | 0.091 | NA | 0.091 | 0.091 | NA | 0.129 | 0.092 | 0.297 | |
| Harrell C index | 0.783 | 0.782 | 0.769 | 0.780 | 0.779 | 0.775 | 0.775 | 0.618 | |
| Discrimination slope | 0.112 | NA | 0.103 | 0.106 | NA | 0.121 | 0.106 | 0.049 | |
| Calibration in the large | 0 | 0.021 | −0.008 | 0 | 0.015 | 0.013 | −0.024 | 0.244 | |
| Calibration slope | 1 | 0.994 | 0.865 | 1 | 0.996 | 1.007 | 0.946 | 0.331 | |
| Simple version for men df = 15 | Nagelkerke R2 | 0.159 | 0.176 | NA | 0.155 | 0.154 | NA | NA | NA |
| Brier score | 0.092 | NA | 0.091 | 0.092 | NA | 0.129 | 0.093 | 0.294 | |
| Harrell C index | 0.773 | 0.773 | 0.765 | 0.771 | 0.771 | 0.766 | 0.768 | 0.614 | |
| Discrimination slope | 0.093 | NA | 0.095 | 0.092 | NA. | 0.099 | 0.093 | 0.045 | |
| Calibration in the large | 0 | 0.010 | −0.003 | 0 | 0.006 | 0.021 | −0.023 | 0.242 | |
| Calibration slope | 1 | 0.997 | 0.924 | 1 | 0.998 | 1.004 | 0.971 | 0.319 | |
| Parsimonious version for men df = 25 | Nagelkerke R2 | 0.177 | 0.176 | NA | 0.168 | 0.167 | NA | NA | NA |
| Brier score | 0.091 | NA | 0.091 | 0.091 | NA | 0.129 | 0.092 | 0.297 | |
| Harrell C index | 0.783 | 0.782 | 0.769 | 0.780 | 0.779 | 0.775 | 0.775 | 0.618 | |
| Discrimination slope | 0.111 | NA | 0.103 | 0.105 | NA | 0.120 | 0.105 | 0.049 | |
| Calibration in the large | 0 | 0.018 | −0.003 | 0 | 0.011 | 0.017 | −0.024 | 0.244 | |
| Calibration slope | 1 | 0.995 | 0.866 | 1 | 0.997 | 1.006 | 0.948 | 0.332 | |
As a reference, the predictive performance measures of the female model with all predictors were Nagelkerke R2 = 0.154, Brier score = 0.088, Harrell C index = 0.775, and discrimination slope = 0.106. The predictive performance measures of the male model with all predictors were Nagelkerke R2 = 0.169, Brier score = 0.093, Harrell C index = 0.779, and discrimination slope = 0.107.
Some statistics were not calculated during bootstrap validation (rms package).
Figure 2. Calibration Curves for the Chronic Disease Population Risk Tool.
Error bars indicate 95% CIs.
Bootstrap Validation of the Ontario Development Cohort and Split Sample Validation
For both sexes, the baseline characteristics between the 70% sample and 30% sample were similar (standardized differences, <0.03). For model performance, the bootstrap validation and split sample validation results were similar for women and men (Table 3). For example, the C-indices for the bootstrap validation and split sample validation for the parsimonious model were 0.778 and 0.776 in women and 0.782 and 0.769 in men. The shapes of the calibration curves were also similar (Figure 2B).
Ontario Final Model
The Ontario final models were estimated (Table 2), and each version exhibited similar overall predictive performance and discrimination compared with their development and split sample validation counterparts. For example, in the female parsimonious model, the Brier score was 0.088 (final) vs 0.087 (development) and the discrimination slope was 0.104 (final) vs 0.109 (development). The calibration curves showed a well-fit model for predicted probabilities up to 60%, with overfitting above this point (eFigure 3 in the Supplement). Bootstrap validation suggested high reproducibility.
External Validation in Manitoba
For a given female CDPoRT version, the Brier score and calibration in the large were generally greater in the Manitoba external validation vs Ontario development and validation (eg, full version, Brier score: 0.128 vs 0.087 and 0.119; calibration in the large: 0.032 vs 0 and −0.006) (Table 3). Harrell C index and the calibration slope tended to be smaller, whereas the discrimination slope was similar in Manitoba vs Ontario (eg, parsimonious version, Harrell C index: 0.752 vs 0.779 and 0.778; calibration slope: 0.885 vs 1 vs 0.995; discrimination slope: 0.102 vs 0.109). For a given male CDPoRT version, Harrell C index, discrimination slope, calibration in the large, and the calibration slope were similar vs the Ontario settings (full version: Harrell C index: 0.775 vs 0.783 vs 0.769; discrimination slope: 0.121 vs 0.112 vs 0.103; calibration in the large: 0.013 vs 0 vs −0.008; calibration slope: 1.007 vs 1 vs 0.865). For both sexes, the Manitoba calibration plots had a shape similar to that of the Ontario calibration plots (Figure 2C).
Sensitivity Analysis
The first sensitivity analysis examined the performance of CDPoRT among individuals who did not self-report a history of chronic disease in Ontario (n = 135 085; 75 880 [56.2%] women). The predictive performance was similar to the other results (eg, full version for women, Brier score: 0.090; full version for men, Brier score: 0.092) (Table 3; eFigure 4 in the Supplement). The second sensitivity analysis treating death as a competing risk found the subdistribution hazard ratios similar to the cause-specific hazard ratios (eTable 3 in the Supplement). The third sensitivity analysis conducted among individuals free of the original 6 chronic diseases and 9 others at baseline (n = 67 235; 34 729 [51.7%] women) found the model showed poor predictive performance (eg, full version for women, Brier score, 0.322; full version for men, Brier score, 0.316) (Table 3) and underpredicted chronic disease incidence when predicting 9 additional chronic diseases.
Discussion
According to the predictive performance displayed during development and validation, CDPoRT exhibited good discrimination and calibration for population-based predictions of the first incidence of multiple chronic diseases, using routinely collected data with sensitivity analyses showing the tool’s robustness. Internal validation assessed CDPoRT’s reproducibility, whereas external validation assessed the tool’s transportability. According to these results, the application of CDPoRT in different settings should translate to robust performance.
The important predictors were age, smoking, and body mass index, which are widely known risk factors for single chronic diseases.47 As a major risk factor for lung cancer,48 cardiovascular disease,49 chronic obstructive pulmonary disease,50 and diabetes,51 cigarette smoking is a major predictor. Smoking has been found to be a major predictor in similar population risk algorithms for stroke,12 all-cause mortality,30 and cardiovascular disease.13 Body mass index was the most prognostic predictor for a similar diabetes tool.9 CDPoRT is unique vs other population risk algorithms because it accurately predicted the risk of the first of multiple chronic diseases in accordance with shared risk factors at the population level instead of for individual chronic diseases. A potential application of CDPoRT is to allow policy makers to identify and understand which population segments are at increased risk for a major chronic disease and to assess the contribution of socioeconomic, behavioral, and demographic risk factors to inform appropriate prevention strategies. Although CDPoRT does predict chronic disease burden, this burden is an underestimate because the tool does not consider all chronic diseases or subsequent chronic disease incidence (ie, multimorbidity).5
Three versions of CDPoRT were developed and validated, which enables transportability of the tool to other settings and under different data access conditions. Although the full version of CDPoRT had 11 predictors, the parsimonious version performed similarly with 9 predictors. This is in agreement with previous work that found diminishing improvements in predictive performance when including additional predictors.52 In accordance with these findings, we recommend the parsimonious CDPoRT version because it includes the predictors with the most predictive power but has fewer predictors overall to safeguard against overfitting. The parsimonious version requires fewer inputs, making it more user-friendly and less sensitive to changes in survey questions over time. The simple version is advantageous when limited data are available. The full version may be advantageous in predicting chronic disease risk for specific population subgroups. CDPoRT calculates chronic disease risk by using the coefficients listed in Table 2 and the formula in the eAppendix in the Supplement. CDPoRT was built for population-based prediction of chronic disease risk (ie, the general population). CDPoRT does not currently account for the extra variability that occurs at the individual level, so additional validation is needed to assess its performance at the individual level. The additional variability could be accounted for with new predictors (eg, biological, clinical factors) or more complex models with interaction terms.
There were case-mix differences between the Ontario and Manitoba cohorts. Thus, external validation provided insight into CDPoRT’s generalizability based on geographic transportability. Overall, CDPoRT’s predictive performance in Manitoba was similar, albeit slightly worse, in terms of discrimination and calibration vs its performance in Ontario, which suggests CDPoRT is transportable to other settings.
Limitations
This study has limitations. Although CDPoRT was built with routinely collected survey data representative of most Canadian residents, indigenous peoples were not sampled. This is an important consideration because indigenous persons have a greater reported risk of chronic disease than nonindigenous persons in Canada.53,54,55,56 This may have been a contributing factor in the slight underperformance of CDPoRT in Manitoba because the indigenous population is proportionately larger there (15%) vs Ontario (2%).57 On-reserve survey data could be used to calibrate and update CDPoRT.
Another limitation is the measurement quality of the lifestyle risk factors. For example, fruit and vegetable consumption represented diet, whereas other food groups (eg, meats, dairy) and constituents (eg, sodium, fat) were not captured. However, greater frequencies of daily fruit and vegetable consumption are correlated with better dietary habits.58 Only physical activity associated with leisure was captured, whereas other forms (eg, transportation, work) were not. This may explain why physical inactivity was not predictive despite being a major risk factor for the chronic diseases under study.59,60,61 Capturing lifestyle behaviors across the entire population is difficult, time consuming, and costly, but this study capitalized on existing data by linking population-level surveys to administrative data.
The calibration curves consistently demonstrated overfitting for higher predicted risks. These individuals tended to be older and have suboptimal lifestyle habits (eg, daily smoking, high body mass index). They appear to represent survivors who, despite these habits, do not develop a chronic disease. CDPoRT may be missing a predictor that improves calibration in this region. For example, the Canadian Community Health Survey lacks genetic information, such as family history of chronic disease, which has been an important predictor in other models.62 Capturing genetic risk of chronic disease may offset the overfitting for this small subgroup because these individuals might not have a lower genetic risk for chronic disease. Despite overfitting in this subgroup, CDPoRT accurately predicts chronic disease risk for most of the population (approximately 95%), using routinely collected data that are less costly and easier to assess than clinical data.
Conclusions
To our knowledge, CDPoRT is the first population risk algorithm that accurately predicts the first incidence of major chronic disease in adults, using routinely collected information on modifiable lifestyle risk factors during 10 years. CDPoRT has great potential applications for health policy makers in planning initiatives and supporting decision-making at the population level.
eAppendix. Calculating CDPoRT Probabilities
eFigure 1. Log Baseline Cumulative Hazard Function of the Royston-Parmar Model Using 6 Knots and All Predictors, by Sex
eFigure 2. Cohort Inclusion and Exclusion Criteria for the Ontario and Manitoba Cohort
eFigure 3. Calibration Curves at 10 Years for the Sensitivity Analysis of Women and Men in Ontario Who Did Not Self-report a History of Chronic Disease, by Model Version
eFigure 4. Calibration Curves at 10 Years for the Sensitivity Analysis of 9 Additional Chronic Disease Outcomes for Women and Men, by Model Version
eTable 1. Definitions for Measures of Predictive Performance
eTable 2. Starting and Final Predictor Specifications, by Sex
eTable 3. Fine-Gray Model of Time-to-First Chronic Disease With Death as a Competing Risk Using the Parsimonious Version of CDPoRT, by Sex
eReferences.
References
- 1.Naghavi M, Abajobir AA, Abbafati C, et al. ; GBD 2016 Causes of Death Collaborators . Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151-1210. doi: 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211-1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodenheimer T, Wagner EHE, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775-1779. doi: 10.1001/jama.288.14.1775 [DOI] [PubMed] [Google Scholar]
- 4.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health Aff (Millwood). 2009;28(1):75-85. doi: 10.1377/hlthaff.28.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pefoyo AJK, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. doi: 10.1186/s12889-015-1733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waters H, Graf M The costs of chronic disease in the US. Published August 28, 2018. Accessed April 24, 2020. https://milkeninstitute.org/reports/costs-chronic-disease-us
- 7.Beaglehole R, Bonita R, Horton R, et al. ; Lancet NCD Action Group; NCD Alliance . Priority actions for the non-communicable disease crisis. Lancet. 2011;377(9775):1438-1447. doi: 10.1016/S0140-6736(11)60393-0 [DOI] [PubMed] [Google Scholar]
- 8.Rosella LC, Mowat D, Fransoo R, et al. Evaluating the process and outcomes of a knowledge translation approach to supporting use of the Diabetes Population Risk Tool (DPoRT) in public health practice. Can J Program Eval. 2018;33(1):21-48. doi: 10.3138/cjpe.31160 [DOI] [Google Scholar]
- 9.Rosella LC, Manuel DG, Burchill C, Stukel TA; PHIAT-DM Team . A population-based risk algorithm for the development of diabetes: development and validation of the Diabetes Population Risk Tool (DPoRT). J Epidemiol Community Health. 2011;65(7):613-620. doi: 10.1136/jech.2009.102244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38(1):46-51. doi: 10.1016/0002-9149(76)90061-8 [DOI] [PubMed] [Google Scholar]
- 11.May HT, Lappé DL, Knowlton KU, Muhlestein JB, Anderson JL, Horne BD. Prediction of long-term incidence of chronic cardiovascular and cardiopulmonary diseases in primary care patients for population health monitoring: the Intermountain Chronic Disease Model (ICHRON). Mayo Clin Proc. December 2018. doi: 10.1016/j.mayocp.2018.06.029 [DOI] [PubMed] [Google Scholar]
- 12.Manuel DG, Tuna M, Perez R, et al. Predicting stroke risk based on health behaviours: development of the Stroke Population Risk Tool (SPoRT). PLoS One. 2015;10(12):e0143342. doi: 10.1371/journal.pone.0143342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manuel DG, Tuna M, Bennett C, et al. Development and validation of a cardiovascular disease risk-prediction model using population health surveys: the Cardiovascular Disease Population Risk Tool (CVDPoRT). CMAJ. 2018;190(29):E871-E882. doi: 10.1503/cmaj.170914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher S, Hsu A, Mojaverian N, et al. Dementia Population Risk Tool (DemPoRT): study protocol for a predictive algorithm assessing dementia risk in the community. BMJ Open. 2017;7(10):e018018. doi: 10.1136/bmjopen-2017-018018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta S, Jackson R, Pylypchuk R, Poppe K, Wells S, Kerr AJ. Development and validation of alternative cardiovascular risk prediction equations for population health planning: a routine health data linkage study of 1.7 million New Zealanders. Int J Epidemiol. 2018;47(5):1571-1584. doi: 10.1093/ije/dyy137 [DOI] [PubMed] [Google Scholar]
- 16.Ng R, Sutradhar R, Wodchis WP, Rosella LC. Chronic Disease Population Risk Tool (CDPoRT): a study protocol for a prediction model that assesses population-based chronic disease incidence. Diagn Progn Res. 2018;2(1):19. doi: 10.1186/s41512-018-0042-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Béland Y. Canadian Community Health Survey: methodological overview. Health Rep. 2002;13(3):9-14. [PubMed] [Google Scholar]
- 18.World Health Organization Global status report on noncommunicable diseases, 2014. Accessed April 24, 2020. https://www.who.int/nmh/publications/ncd-status-report-2014/en/
- 19.Global Burden of Disease Global Burden of Disease Study 2016 (GBD 2016): population estimates, 1950-2016. Accessed April 24, 2020. http://ghdx.healthdata.org/record/ihme-data/gbd-2016-population-estimates-1950-2016
- 20.Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33(3):160-166. [PubMed] [Google Scholar]
- 21.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD. 2009;6(5):388-394. doi: 10.1080/15412550903140865 [DOI] [PubMed] [Google Scholar]
- 22.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512-516. doi: 10.2337/diacare.25.3.512 [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin JR, Kreiger N, Marrett LD, Holowaty EJ. Cancer incidence registration and trends in Ontario. Eur J Cancer. 1991;27(11):1520-1524. doi: 10.1016/0277-5379(91)90041-B [DOI] [PubMed] [Google Scholar]
- 24.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290-296. doi: 10.1067/mhj.2002.123839 [DOI] [PubMed] [Google Scholar]
- 25.Tu K, Wang M, Young J, et al. Validity of administrative data for identifying patients who have had a stroke or transient ischemic attack using EMRALD as a reference standard. Can J Cardiol. 2013;29(11):1388-1394. doi: 10.1016/j.cjca.2013.07.676 [DOI] [PubMed] [Google Scholar]
- 26.Public Health Agency of Canada How healthy Are Canadians? Accessed April 24, 2020. https://www.canada.ca/en/public-health/services/publications/healthy-living/how-healthy-canadians.html
- 27.Busse R, Blumel M, Scheller-Kreinsen D, Zentner A. Tackling Chronic Disease in Europe: Strategies, Interventions, and Challenges. European Observatory on Health Systems and Policies; 2010. [Google Scholar]
- 28.Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45-52. doi: 10.1016/S0140-6736(14)60648-6 [DOI] [PubMed] [Google Scholar]
- 29.Taljaard M, Tuna M, Bennett C, et al. Cardiovascular Disease Population Risk Tool (CVDPoRT): predictive algorithm for assessing CVD risk in the community setting: a study protocol. BMJ Open. 2014;4(10):e006701. doi: 10.1136/bmjopen-2014-006701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manuel DG, Perez R, Sanmartin C, et al. Measuring burden of unhealthy behaviours using a multivariable predictive approach: life expectancy lost in Canada attributable to smoking, alcohol, physical inactivity, and diet. PLoS Med. 2016;13(8):e1002082. doi: 10.1371/journal.pmed.1002082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosella LC, Kornas K, Yao Z, et al. Predicting high health care resource utilization in a single-payer public health care system: development and validation of the High Resource User Population Risk Tool (HRUPoRT). Med Care. 2017;00(00):1-9. doi: 10.1097/MLR.0000000000000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebenbaum M, Espin-Garcia O, Li Y, Rosella LC. Development and validation of a population based risk algorithm for obesity: the Obesity Population Risk Tool (OPoRT). PLoS One. 2018;13(1):e0191169. doi: 10.1371/journal.pone.0191169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152. [PubMed] [Google Scholar]
- 34.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138-2145. doi: 10.1001/archinte.168.19.2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quiñones AR, Botoseneanu A, Markwardt S, et al. Racial/ethnic differences in multimorbidity development and chronic disease accumulation for middle-aged adults. PLoS One. 2019;14(6):e0218462. doi: 10.1371/journal.pone.0218462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249-1257. doi: 10.2337/dc11-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145-2154. doi: 10.1161/CIRCULATIONAHA.110.968792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet. 2015;386(9998):1075-1085. doi: 10.1016/S0140-6736(15)00156-7 [DOI] [PubMed] [Google Scholar]
- 39.Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385(9971):899-909. doi: 10.1016/S0140-6736(14)60446-3 [DOI] [PubMed] [Google Scholar]
- 40.Irigaray P, Newby JA, Clapp R, et al. Lifestyle-related factors and environmental agents causing cancer: an overview. Biomed Pharmacother. 2007;61(10):640-658. doi: 10.1016/j.biopha.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 41.Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016;69:245-247. doi: 10.1016/j.jclinepi.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steyerberg EW, Harrell FE Jr, Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774-781. doi: 10.1016/S0895-4356(01)00341-9 [DOI] [PubMed] [Google Scholar]
- 43.Steyerberg EW. Validation in prediction research: the waste by data splitting. J Clin Epidemiol. 2018;103:131-133. doi: 10.1016/j.jclinepi.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 44.Harrell FE. Regression Modeling Strategies. Springer International Publishing; 2015. doi: 10.1007/978-3-319-19425-7 [DOI] [Google Scholar]
- 45.Kovacevic MS, Mach L, Roberts G Bootstrap variance estimation for predicted individual and population-average risks. Accessed April 24, 2020. https://pdfs.semanticscholar.org/cbe8/e6d3fb8b548d0dd925acd1eef3a974a28a83.pdf?_ga=2.250068805.2110187797.1587737357-2126011429.1585581526
- 46.Yeo D, Mantel H, Liu TP. Bootstrap variance estimation for the national population health survey In Proceedings of the Survey Research Methods Section. American Statistical Association; 1999. [Google Scholar]
- 47.Ng R, Sutradhar R, Yao Z, Wodchis WP, Rosella LC. Smoking, drinking, diet and physical activity: modifiable lifestyle risk factors and their associations with age to first chronic disease. Int J Epidemiol. 2019;(April):dyz078. doi: 10.1093/ije/dyz078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.US Department of Health, Education, and Welfare Smoking and health: report of the Advisory Committee to the Surgeon General of the Public Health Service. Accessed May 2, 2020. https://www.govinfo.gov/content/pkg/GPO-SMOKINGANDHEALTH/pdf/GPO-SMOKINGANDHEALTH.pdf
- 49.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-Century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi: 10.1056/NEJMsa1211128 [DOI] [PubMed] [Google Scholar]
- 50.Jayes L, Haslam PL, Gratziou CG, et al. ; Tobacco Control Committee of the European Respiratory Society . SmokeHaz: systematic reviews and meta-analyses of the effects of smoking on respiratory health. Chest. 2016;150(1):164-179. doi: 10.1016/j.chest.2016.03.060 [DOI] [PubMed] [Google Scholar]
- 51.Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(12):958-967. doi: 10.1016/S2213-8587(15)00316-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Austin PC, Pencinca MJ, Steyerberg EW. Predictive accuracy of novel risk factors and markers: a simulation study of the sensitivity of different performance measures for the Cox proportional hazards regression model. Stat Methods Med Res. 2017;26(3):1053-1077. doi: 10.1177/0962280214567141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dyck R, Osgood N, Lin TH, Gao A, Stang MR. Epidemiology of diabetes mellitus among First Nations and non–First Nations adults. CMAJ. 2010;182(3):249-256. doi: 10.1503/cmaj.090846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ospina MB, Voaklander D, Senthilselvan A, et al. Incidence and prevalence of chronic obstructive pulmonary disease among aboriginal peoples in Alberta, Canada. PLoS One. 2015;10(4):e0123204. doi: 10.1371/journal.pone.0123204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reading J. Confronting the growing crisis of cardiovascular disease and heart health among aboriginal peoples in Canada. Can J Cardiol. 2015;31(9):1077-1080. doi: 10.1016/j.cjca.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 56.McGahan CE, Linn K, Guno P, et al. Cancer in First Nations people living in British Columbia, Canada: an analysis of incidence and survival from 1993 to 2010. Cancer Causes Control. 2017;28(10):1105-1116. doi: 10.1007/s10552-017-0950-7 [DOI] [PubMed] [Google Scholar]
- 57.Statistics Canada Aboriginal identity population, by province and territory (2006. census). Published January 15, 2008. Accessed March 28, 2016. https://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo60a-eng.htm
- 58.Garriguet D. Diet quality in Canada. Health Rep. 2009;20(3):41-52. [PubMed] [Google Scholar]
- 59.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30(7):529-542. doi: 10.1007/s10654-015-0056-z [DOI] [PubMed] [Google Scholar]
- 60.Li J, Siegrist J. Physical activity and risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int J Environ Res Public Health. 2012;9(2):391-407. doi: 10.3390/ijerph9020391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tardon A, Lee WJ, Delgado-Rodriguez M, et al. Leisure-time physical activity and lung cancer: a meta-analysis. Cancer Causes Control. 2005;16(4):389-397. doi: 10.1007/s10552-004-5026-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Damen JA, Pajouheshnia R, Heus P, et al. Performance of the Framingham risk models and pooled cohort equations for predicting 10-year risk of cardiovascular disease: a systematic review and meta-analysis. BMC Med. 2019;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Calculating CDPoRT Probabilities
eFigure 1. Log Baseline Cumulative Hazard Function of the Royston-Parmar Model Using 6 Knots and All Predictors, by Sex
eFigure 2. Cohort Inclusion and Exclusion Criteria for the Ontario and Manitoba Cohort
eFigure 3. Calibration Curves at 10 Years for the Sensitivity Analysis of Women and Men in Ontario Who Did Not Self-report a History of Chronic Disease, by Model Version
eFigure 4. Calibration Curves at 10 Years for the Sensitivity Analysis of 9 Additional Chronic Disease Outcomes for Women and Men, by Model Version
eTable 1. Definitions for Measures of Predictive Performance
eTable 2. Starting and Final Predictor Specifications, by Sex
eTable 3. Fine-Gray Model of Time-to-First Chronic Disease With Death as a Competing Risk Using the Parsimonious Version of CDPoRT, by Sex
eReferences.