Abstract
Parkinson’s disease is a chronic progressive neurodegenerative disorder characterized by resting tremor, slowness of movements, rigidity, gait disturbance and postural instability. Most investigations on Parkinson’s disease focused on the basal ganglia, whereas the cerebellum has often been overlooked. However, increasing evidence suggests that the cerebellum may have certain roles in the pathophysiology of Parkinson’s disease. Anatomical studies identified reciprocal connections between the basal ganglia and cerebellum. There are Parkinson’s disease–related pathological changes in the cerebellum. Functional or morphological modulations in the cerebellum were detected related to akinesia/rigidity, tremor, gait disturbance, dyskinesia and some non-motor symptoms. It is likely that the major roles of the cerebellum in Parkinson’s disease include pathological and compensatory effects. Pathological changes in the cerebellum might be induced by dopaminergic degeneration, abnormal drives from the basal ganglia and dopaminergic treatment, and may account for some clinical symptoms in Parkinson’s disease. The compensatory effect may help maintain better motor and non-motor functions. The cerebellum is also a potential target for some parkinsonian symptoms. Our knowledge about the roles of the cerebellum in Parkinson’s disease remains limited, and further attention to the cerebellum is warranted.
Keywords: Parkinson’s disease, cerebellum, compensation, pathological changes
Introduction
Parkinson’s disease is one of the most common progressive neurological degenerative disorders. It is characterized by motor dysfunction including resting tremor, slowness of movements (bradykinesia), difficulty in initiating movements (akinesia), rigidity, gait disturbance and postural instability as well as the more recently emphasized non-motor dysfunctions. The pathological hallmark of Parkinson’s disease is progressive dopamine neuronal loss within the substantia nigra and other brain structures combined with the appearance of intracytoplasmic inclusions composed of α-synuclein aggregates known as Lewy bodies (Forno, 1981;Jankovic, 2008). Since the discovery of markedly decreased dopamine concentrations in the striatum in the 1960s (Hornykiewicz, 2006), the basal ganglia are the major clinical and research targets in Parkinson’s disease. More recently, the importance of additional degeneration including other catecholaminergic systems became clear. In contrast, although it was previously recognized as important in the coordination of voluntary movement, gait, posture and motor functions (Ghez and Fahn, 1985), the influences of the cerebellum in Parkinson’s disease were often overlooked. However, increasing anatomical, pathophysiological and clinical evidence suggested that the cerebellum may contribute substantially to the clinical symptoms of Parkinson’s disease. For example, parkinsonian resting tremor is abolished by stimulating or lesioning the ventral intermediate nucleus of the thalamus, which is a target of cerebellar efferents (Benabidet al., 1991). A PET study found that akinesia in Parkinson’s disease is correlated with abnormally increased regional cerebral blood flow values in the cerebellum. Deep brain stimulation of the subthalamic nucleus of the basal ganglia improves the motor signs, which are associated with reduced regional cerebral blood flow in the cerebellum (Payouxet al., 2004). Here, we review related anatomical, clinical and neurophysiological findings, and discuss the possible roles of the cerebellum in the pathophysiology of Parkinson’s disease.
Anatomical connections between the basal ganglia and cerebellum
The cerebellum and basal ganglia are two major subcortical structures that influence multiple aspects of motor, cognitive and affective behaviour (Alexanderet al., 1986;Stricket al., 2009). Both structures form multi-synaptic loops with the cerebral cortex; the cerebellum is known to influence motor and cognitive operations through the cerebello-thalamo-cortical circuit (Middleton and Strick, 2001). The cerebellum and basal ganglia have distinct loops connecting with largely overlapping cortical areas (Middleton and Strick, 2000). Thus, basal ganglia and cerebellar loops were long assumed to be entirely separate anatomically and to perform distinct functional operations. Interactions between these two regions were traditionally thought to occur at the level of the cerebral cortex (Jones, 1985;Percheronet al., 1996). However, recent studies elucidated that there are anatomical connections between the cerebellum and basal ganglia.
Ichinoheet al. (2000) demonstrated the existence of a disynaptic connection between the cerebellum and striatum in rats. Later, using transneuronal transport of rabies virus in monkeys,Hoshiet al.(2005) showed that one of the output nuclei of the cerebellum, the dentate nucleus, projects to the striatum via a disynaptic connection, and to the external globus pallidus via a trisynaptic connection. The disynaptic projection to the striatum originates from both the motor and non-motor domains of the dentate. These findings demonstrate that the cerebellum has a strong disynaptic projection to the striatum by way of the thalamus, and may influence the pathways involved in basal ganglia processing.
In a more recent study,Bostanet al. (2010) used retrograde transneuronal transport of the rabies virus to determine the origin of multi-synaptic inputs to the cerebellum. They found that the subthalamic nucleus has a disynaptic projection to the cerebellar cortex by way of the pontine nuclei and that this connection is topographically organized. Most of the subthalamic nucleus neurons projecting to the Crus II posterior are located in its associative territory, which receives input from the frontal eye fields and regions of the prefrontal cortex. In contrast, most of the subthalamic nucleus neurons projecting to the hemispheric expansion of lobule VIIB are located in its sensorimotor territory, which receives input from the primary motor cortex and premotor areas. These results suggest that the subthalamic nucleus–cerebellar connection is involved in integrating basal ganglia and cerebellar functions in both motor and non-motor domains. The anatomical findings from the above studies together prove that the cerebellum and basal ganglia have substantial two-way communications between each other and are linked together to form an integrated functional network (Fig. 1). The discovery of this reciprocal connection between the basal ganglia and cerebellum provides an anatomical basis to explain the role of the cerebellum in Parkinson’s disease.
Figure 1.
Structural connections between the basal ganglia and cerebellum. The solid line indicates the projection from the dentate nucleus to the striatum, while the dotted line indicates the projection from the subthalamic nucleus to the cerebellar cortex. STN = subthalamic nucleus. Information fromBostanet al. (2010).
Parkinson’s disease–associated pathological changes in the cerebellum
The presence of dopaminergic innervation and dopamine D1–3 receptors in the cerebellum has been proven (Hurleyet al., 2003;Giompres and Delis, 2005). The cerebellum receives a dopaminergic projection from the ventral tegmental area/substantia nigra pars compacta (Panagopouloset al., 1991;Ikaiet al., 1992;Melchitzkyet al., 2000). Pathological changes in the cerebellum following dopaminergic degeneration were reported in patients with Parkinson’s disease and animal models.Rollandet al. (2007) showed that degeneration of nigrostriatal dopaminergic neurons causes dysfunction of both the basal ganglia–thalamic and cerebello-thalamic pathways in 6-hydroxydopamine-lesioned rats and MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) monkeys. Neuronal degeneration in the cerebellum was shown in an MPTP mouse model (Takadaet al., 1993), characterized by the loss of Nissl-stained Purkinje cells and aggravated by the number of repeated MPTP injections. An MPTP insult also induced the loss of calcium-binding positive Purkinje cells in monkeys (Vignolaet al., 1994). A recent study found that persistent hyperactivation of Purkinje cells correlated with the level of dopaminergic neuronal loss in the substantia nigra in chronic parkinsonian monkeys (Hemanet al., 2012).
The deposition of α-synuclein-containing Lewy bodies and mitochondrial dysfunction are among the major pathological changes in Parkinson’s disease (Schapiraet al., 1990;Braaket al., 2003). α-Synuclein is also present in regions not directly associated with Parkinson’s disease, including the cerebellum (Solanoet al., 2000). With immunohistochemical examinations,Piaoet al. (2003) found that α-synuclein-positive doughnut-shaped structures occasionally presented in the cerebellar molecular layer in some patients with Parkinson’s disease.Fuchset al. (2008) reported a correlation between Parkinson’s disease and decreased α-synuclein messenger RNA levels in the cerebellum.Westerlundet al. (2008) found significantly reduced α-synuclein protein levels in the cerebellum of patients with Parkinson’s disease. Because this decrease appeared to be independent of theSNCA (α-synuclein gene) genotype, the authors suggested that the cerebellar α-synuclein deficiency may be a more general aspect of Parkinson’s disease or related to Parkinson’s disease medication. Previous studies on α-synuclein levels in Parkinson’s disease reported contradictory findings, that is, increased α-synuclein levels in the mid-brain (Chiba-Faleket al., 2006) or substantia nigra (Gründemannet al., 2008), decreased α-synuclein levels in the substantia nigra (Neystatet al., 1999;Kingsburyet al., 2004;Fuchset al., 2008) or cerebellum (Fuchset al., 2008;Westerlundet al., 2008) or unchanged α-synuclein levels (Tanet al., 2005) in Parkinson’s disease compared with control subjects. The reason for these inconsistent findings remains unclear and might relate to different stages of the disorder, genetic variability or medical treatment. The decreased α-synuclein levels might indicate that the cerebellum may also be a target of α-synuclein pathology. However, because Lewy bodies have not been detected in the cerebellum and the exact physiological function of α-synuclein and its changes in Parkinson’s disease remain unclear, the meaning of decreased α-synuclein levels in the cerebellum in Parkinson’s disease needs further investigation.
In contrast, mitochondrial dysfunction in the cerebellum has not been proven.Deviet al. (2008) showed that accumulation of wild-type α-synuclein in the mitochondria of human dopaminergic neurons led to reduced mitochondrial complex I activity and increased production of reactive oxygen species. Mitochondria from the substantia nigra and striatum, but not the cerebellum, from patients with Parkinson’s disease showed significant accumulation of α-synuclein and decreased complex I activity.
Hurleyet al. (2003) examined dopaminergic neurotransmission in the cerebellum from post-mortem brain tissue obtained from healthy subjects and patients with Parkinson’s disease who were receiving treatment with dopaminergic drugs. They found that dopamine D1–3 receptors, tyrosine hydroxylase and dopamine transporter messenger RNA were detected in the uvula and nodulus (lobules 9 and 10, respectively) of the vermis from control subjects. In Parkinson’s disease, the level of dopamine D1 and D3 receptor messenger RNA was reduced in lobule 9 and the level of tyrosine hydroxylase messenger RNA was reduced in lobule 10. The reduced dopamine D1 and D3 receptors and tyrosine hydroxylase messenger RNA in cerebellum suggests that this brain area may have a role in Parkinson’s disease symptoms.
Structural changes in the cerebellum
With the deformation-based morphometry method,Borghammeret al. (2010) revealed significant contraction in the left cerebellum in patients with early-stage Parkinson’s disease compared with control subjects. Using the voxel-based morphometry method,Benningeret al. (2009) found that in patients with mild-to-moderate Parkinson’s disease with and without resting tremor, grey matter volume is decreased in the right quadrangular lobe and declive of the cerebellum in Parkinson’s disease with tremor compared with those without. Other studies also found cognitive- (Camicioliet al., 2009;Pereiraet al., 2009;Nishioet al., 2010) or olfactory-related (Zhanget al., 2011) structural changes in the cerebellum in patients with Parkinson’s disease. Therefore, there are specific Parkinson’s disease–related morphological changes in the cerebellum.
The cerebellum and parkinsonian akinesia/rigidity
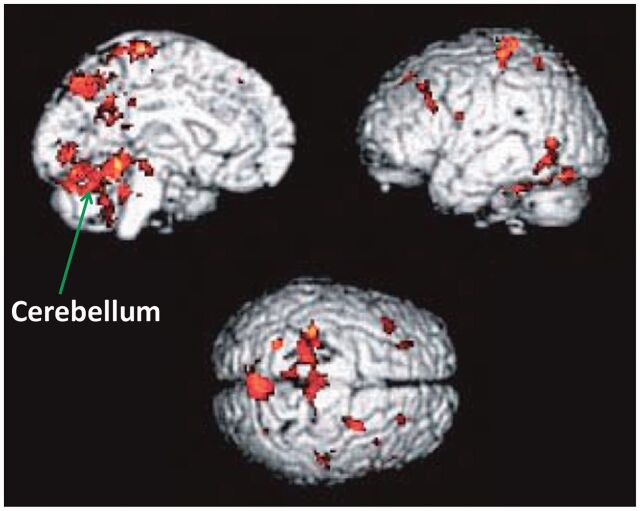
Parkinson’s disease is not a homogenous disease and has two predominant forms: akinesia and rigidity (akinesia/rigidity subtype) and prominent resting tremor (tremor subtype). Akinesia can be defined as a delay or a failure in movement initiation (Hallettet al., 1991), particularly for self-initiated movements. Functional neuroimaging studies using PET or blood oxygen level–dependent functional MRI frequently demonstrated increased activation in the cerebellum in patients with Parkinson’s disease during performance of various upper limb movements (Rascolet al., 1997;Catalanet al., 1999;Wu and Hallett, 2005,2008;Yuet al., 2007;Wuet al., 2010b) (Fig. 2). For example, during externally or internally timed simple finger movements (Rascolet al., 1997;Cerasaet al., 2006;Yuet al., 2007), motor timing (Jahanshahiet al., 2010), complex sequential movements (Catalanet al., 1999), bimanual two-hand coordinated tasks (Wuet al., 2010b) or two different motor tasks simultaneously (Wu and Hallett, 2008), patients with Parkinson’s disease OFF medication showed hyperactivation in the cerebellum.
Figure 2.
Brain areas more activated in patients with Parkinson’s disease than in normal subjects during automatic execution of sequential movements. Modified fromWu and Hallett (2005), with permission from Oxford University Press.
Increased activation of the cerebellum in Parkinson’s disease appears not only during motor execution, but also during the motor learning process. Functional MRI studies of motor learning commonly found that patients with Parkinson’s disease have dysfunction in frontostriatal motor circuits, especially with decreased activity in the prefrontal cortex, but with increased activation in the parietal and premotor cortices and cerebellum (Werheidet al. 2003;Mallolet al., 2007;Bédard and Sanes, 2009). A PET study on trial-and-error sequence learning found that mildly affected patients with Parkinson’s disease could perform as well as control subjects, but activated four times as much neural tissue as the controls, including several brain cortical regions and bilateral cerebellum (Mentiset al., 2003). When patients with Parkinson’s disease try to automatize movement, they appear to require greater activity in the cerebellum and premotor and parietal cortices compared with normal subjects (Wu and Hallett, 2005). The hyperactivation in the cerebellum not only occurs during motor tasks, but also at rest. A functional MRI study showed increased spontaneous neural activity in the cerebellum in the resting state in patients with akinesia/rigidity Parkinson’s disease (Wuet al., 2009a).
Because multiple neural areas are involved in performing any tasks, examining network connectivity certainly provides valuable information. It was suggested that connectivity analysis may be a more sensitive method to detect changes in Parkinson’s disease than activation amplitude (Palmeret al., 2010). In addition to local activity, the connectivity pattern of the cerebellum in Parkinson’s disease also shows characteristic changes. A specific metabolic pattern (Parkinson’s disease–related spatial covariance pattern) in patients with akinesia/rigidity Parkinson’s disease was identified by18F-fluorodeoxyglucose PET. This is characterized by hypermetabolism in the striatum, thalamus, pons and cerebellum, together with hypometabolism in the supplementary motor cortex, premotor cortex and parieto-occipital association areas (Maet al., 2007;Eidelberg, 2009;Poston and Eidelberg, 2009). A functional MRI study also investigated the pattern of functional connectivity in the motor network in the resting state in patients with Parkinson’s disease ON and OFF levodopa, based on the graph theory (Wuet al., 2009b). Patients OFF levodopa had decreased degrees of connectivity in the supplementary motor cortex, left dorsolateral prefrontal cortex and left putamen, and increased connectivity degrees in the left cerebellum, left premotor cortex and left parietal cortex. Administration of levodopa somewhat normalized these connectivity patterns in patients.
Jahanshahiet al.(2010) investigated brain activation and effective connectivity correlated with motor timing in patients with Parkinson’s disease ON and OFF apomorphine. They found that patients with Parkinson’s disease had significantly greater activation in the bilateral cerebellum, right thalamus and left midbrain/substantia nigra compared with the control subjects during motor timing exercises. Effective connectivity analysis showed that activity in the caudate nucleus was associated with increased activity in the lentiform nucleus and cerebellum OFF medication, and with increased activity in the prefrontal cortex ON medication. With the psychophysiological interaction method,Wuet al. (2011) investigated the effective connectivity of the brain networks while performing self-initiated movement in patients with akinesia/rigidity Parkinson’s disease, and found that the striatum–cortical and striatum–cerebellar connectivities were weakened, whereas the connections between cortico-cerebellar motor regions were strengthened.
The nature of the hyperactivation or strengthened connectivity in the cerebellum in Parkinson’s disease remains unclear. One likely explanation, that has often been cited, is that this phenomenon presents a compensatory effect. Together with the hyperactivation or strengthened connectivity in the cerebellum are hypoactivations in some other regions, such as the supplementary motor cortex and striatum (Sabatiniet al., 2000;Haslingeret al., 2001;Buhmannet al., 2003;Wu and Hallett 2005;Wuet al., 2010b), and weakened striato-thalamo-cortical and striato-cerebellar connectivity (Wu and Hallett, 2008;Wuet al., 2011) in patients with Parkinson’s disease compared with healthy control subjects. The supplementary motor cortex is one of the main receiving regions of the basal ganglia motor circuit (Schell and Strick, 1984). The decreased activity in the supplementary motor cortex is postulated as a consequence of insufficient striato-thalamo-cortical facilitation in Parkinson’s disease (DeLong, 1990). The impaired striato-cerebellar connection is likely a reflection of abnormal signals from the basal ganglia to influence cerebellar function (Bostanet al., 2010). Because the supplementary motor cortex is a critical component in effectively initiating movements, and particularly for those that are internally generated (Deiberet al., 1991, 1996;Playfordet al., 1992;Jahanshahiet al., 1995;Jenkinset al., 2000;Tanji and Hoshi, 2001), an impaired striato-thalamo-cortical pathway secondary to dopamine depletion is likely to be an important explanation underlying akinesia in Parkinson’s disease.
The dysfunction of the striato-thalamo-cortical circuit should induce deterioration in motor performance. However, in several studies that showed cerebellar hyperactivation, patients could execute the motor tasks at the same level as the healthy controls (Rascolet al., 1997;Catalanet al., 1999;Wu and Hallett, 2005,2008;Yuet al., 2007;Wuet al., 2010a,b). The cerebellum is important in preparing or executing movements (Sasakiet al., 1979;Ikedaet al., 1994;Ohishiet al., 2003;Purzneret al., 2007), and is also involved in generating accurate timing of movements (Raoet al., 1997;Kawashimaet al., 2000;Dreher and Grafman, 2002), which is important for self-paced movements. Thus, it is likely that the increased activity or connectivity in the cerebello-thalamo-cortical loop is to compensate for hypofunction in the striato-thalamo-cortical circuit to maintain motor function at a near normal level.
Some findings support the compensatory role of the cerebellum in Parkinson’s disease.Yuet al. (2007) observed a significant negative correlation between the blood oxygen level–dependent activation in the ipsilateral cerebellum and the contralateral putamen while performing a right hand pressing task. In a study on motor urgency in Parkinson’s disease (Ballangeret al., 2008), during performance of externally cued movements in an urgent situation, the cerebellum was more activated in patients with Parkinson’s disease. Because an urgent situation dramatically improved motor performance, the associated recruitment of the cerebellum is possibly a compensation for basal ganglia dysfunction to increase movement velocity in patients with Parkinson’s disease.Palmeret al. (2009) found that when performing a visually guided sinusoidal force task at different speeds (0.25, 0.5 and 0.75 Hz), the activity in the basal ganglia linearly increased with movement speed. In patients with Parkinson’s disease, the activity of this network at low speeds was similar to that in controls at higher speeds. To perform the task at higher speeds, patients additionally recruited the bilateral cerebellum and primary motor cortex.
In rats unilaterally lesioned in the striatum with 6-hydroxydopamine, after 5 weeks, regional cerebral blood flow–related tissue radioactivity was decreased in the striatum and external globus pallidus and increased in the subthalamic nucleus, thalamus, internal globus pallidus, primary motor cortex and cerebellum. During walking, perfusion decreased in lesioned compared with sham-lesioned rats across the ipsilateral striato-pallido-thalamo-cortical motor circuit. Compensatory increases were seen bilaterally in the ventromedial thalamus and red nucleus, in the contralateral subthalamic nucleus, anterior substantia nigra, subiculum, motor cortex and midline cerebellum. Enhanced recruitment of associative sensory areas was noted cortically and subcortically (Yanget al., 2007).
The activity or connectivity was shown to be positively correlated with the Unified Parkinson’s Disease Rating Scale in the cerebello-thalamo-cortical loop, but negatively correlated with Unified Parkinson’s Disease Rating Scale in the striato-thalamo-cortical circuit (Wuet al., 2009a,2010b). Recruitment of the cerebello-thalamo-cortical circuit increases concomitant with Parkinson’s disease progression (Senet al., 2010). These findings might indicate that as the disorder progresses, dysfunction of the striato-thalamo-cortical circuit becomes more severe, while at the same time, the apparent compensatory effect in the cerebello-thalamo-cortical loop becomes more important.
The neurodegenerative process in Parkinson’s disease begins several years before the onset of any clinical symptoms (Braaket al., 2003). The motor symptoms of Parkinson’s disease usually present after ∼70% of dopaminergic neurons have degenerated (Fearnley and Lees, 1991;Leeet al., 2000). Presumably, the compensatory effect in the cerebellum and other brain regions accounts for delaying the onset of motor symptoms and preserving relatively normal function.
The enhanced activity or connectivity in the cerebellum was observed in some other neurological disorders. For example, in stroke damaging the pyramidal tract, increased activity or connectivity in the contralesional cerebellum was frequently reported, and may be related to restoration of motor function (Cholletet al., 1991;Weilleret al., 1992;Smallet al., 2002;Jaillardet al., 2005;Wanget al., 2010). Thus, the compensatory efforts of the cerebellum are likely not specifically related to the dopamine deficiency, but also appear in other neurological conditions.
However, the idea of a compensatory effect of the cerebellum in Parkinson’s disease is still speculative and requires further proof. It is also possible that the increased activity in the cerebellum is not only a compensation but may also reflect a primary pathophysiological change of Parkinson’s disease, as a consequence of the inability to inhibit contextually inappropriate circuits secondary to abnormal basal ganglia outflow (Mink, 1996;Turneret al., 2003;Graftonet al., 2006). The cerebellum receives a disynaptic projection from the subthalamic nucleus (Bostanet al., 2010). The subthalamic nucleus was described as the ‘driving force of the basal ganglia’ (Kitai and Kita, 1987). Both normal and abnormal signals from the subthalamic nucleus should exert influence on cerebellar processing. In Parkinson’s disease, neural activity in the subthalamic nucleus is higher than healthy control subjects and is characterized by abnormal bursting and oscillatory activity (Schrocket al., 2009). Output neurons from the subthalamic nucleus are excitatory and use glutamate as a neurotransmitter (Smithet al., 1998). Animal parkinsonism models have shown increased glutamatergic output of the subthalamic nucleus (Robledo and Feger, 1990;Parent and Hazrati, 1995). The subthalamic nucleus projects to the cerebellar cortex likely by way of the pontine nuclei (Bostanet al., 2010). The projection from the pontine nuclei to the cerebellum was shown also to be largely glutamatergic (Beitzet al., 1986). Thus, abnormal signals from the subthalamic nucleus should increase cerebellar activation. A recent finding that high-frequency stimulation of the subthalamic nucleus increases neuronal activation in the deep cerebellar nuclei in rats supports this assumption (Moers-Hornikxet al., 2011). The altered cerebello-thalamo-cortical input to the cerebral cortex might then contribute to the clinical symptoms in Parkinson’s disease.
The cerebellum and parkinsonian tremor
The classic type of tremor in Parkinson’s disease is resting tremor. Parkinsonian resting tremor is mainly caused by central mechanisms because peripheral deafferentation does not suppress it (Pollock and Davis, 1930;Deuschlet al., 2000; McAuley and Marsden, 2000). The pathophysiology of Parkinson’s disease tremor certainly differs from that underlying akinesia/rigidity (Paulus and Jellinger, 1991;Zaidelet al., 2009). Akinesia/rigidity is related to dopamine depletion and is typically responsive to dopamine treatment. The striatal dopamine depletion and dysfunction of basal ganglia seem to be more important in akinesia/rigidity than in tremor. Although still debatable (Niet al., 2010), increasing evidence suggests that the cerebello-thalamo-cortical circuit is an important underlying pathophysiology of the parkinsonian resting tremor. For example, electrophysiological recordings in the thalamus of MPTP-treated monkeys showed that during tremor, the mean firing rate of neurons in the pallidal and cerebellar territories increases (Guehlet al., 2003).
For amelioration of parkinsonian tremor, deep brain stimulation of the thalamic ventral intermediate nucleus, the cerebellar territory of the thalamus, was considered to be the optimal target (Lenzet al., 1995;Papavassiliouet al., 2008). An early PET study found that suppressing tremor by stimulating the ventral intermediate nucleus was specifically associated with decreased regional cerebral blood flow in the cerebellum in patients with Parkinson’s disease (Deiberet al., 1993). This finding is supported by later studies (Fukudaet al., 2004). In contrast,Lozzaet al. (2002) found a negative correlation between tremor scores and cerebral metabolic rate of glucose in the bilateral putamen and cerebellar vermis. The reasons contributing to these inconsistent findings are unclear, but might relate to different experimental situations. InDeiber’s study (1993), the regional cerebral blood flow was measured when the patients were OFF anti-parkinsonian medications and during deep brain stimulation; while inLozza’s study (2002), the experiment was performed when the patients were ON anti-parkinsonian medications and without deep brain stimulation.
Another effective intervention to relieve parkinsonian tremor is deep brain stimulation of the subthalamic nucleus (Kracket al., 1997). Imaging studies during deep brain stimulation of the subthalamic nucleus reported regional cerebral blood flow, cerebral metabolic rate of glucose or metabolic changes in the cerebellum (Asanumaet al., 2006;Nagaokaet al., 2007; Ciliaet al., 2008;Gedayet al., 2009). However, the clinical improvements in these studies following deep brain stimulation of the subthalamic nucleus were not restricted to tremor, but also included other motor signs, such as rigidity or bradykinesia. Thus, whether these neural changes in the cerebellum were tremor-related need further clarification.
With magnetoencephalography,Timmermannet al. (2003) showed tremor-related oscillatory network, with abnormal coupling in a cerebello-diencephalic-cortical loop and cortical motor and sensory areas contralateral to the tremor hand, which is supported by subsequent magnetoencephalography studies (Timmermannet al., 2004;Polloket al., 2009). A specific metabolic brain network associated with Parkinson’s disease resting tremor, Parkinson’s disease tremor-related pattern has been identified with fluorodeoxyglucose PET (Mureet al., 2011). The Parkinson’s disease tremor-related pattern is characterized by covarying metabolic increases in the cerebellum, motor cortex and putamen. This network correlates specifically with clinical tremor ratings, but not with akinesia/rigidity. Its activity is elevated in tremor-dominant versus akinesia-dominant patients. The Parkinson’s disease tremor-related pattern differs from the Parkinson’s disease–related spatial covariance pattern in akinesia/rigidity patients that was described previously (Eckertet al., 2007;Maet al., 2007). Relief of tremor symptoms by deep brain stimulation of the ventral intermediate nucleus selectively reduces the activity of Parkinson’s disease tremor-related pattern. By contrast, subthalamic nucleus deep brain stimulation reduces abnormal activity of both tremor- and akinesia-related brain networks. These findings suggest that Parkinson’s disease tremor is mediated by a distinct metabolic network involving primarily cerebello-thalamo-cortical pathways.
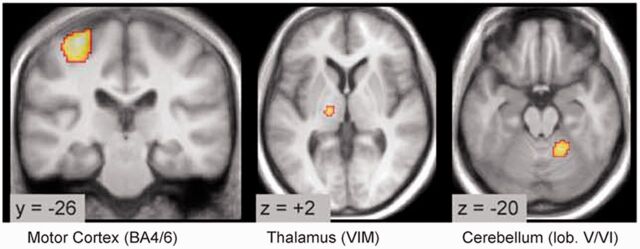
A recent study combined functional MRI and electromyography methods to investigate tremor-related activity and connectivity in the basal ganglia and the cerebello-thalamo-cortical circuit (Helmichet al., 2011). They found that the basal ganglia are transiently activated at the onset of tremor episodes, whereas tremor amplitude-related activity correlates with the cerebello-thalamo-cortical circuit (Fig. 3). The patients with tremor-dominant Parkinson’s disease had increased functional connectivity between the basal ganglia and the cerebello-thalamo-cortical circuit. These results indicate that resting tremor may result from a pathological interaction between the basal ganglia and the cerebello-thalamo-cortical circuit. Tremor generation in the cerebello-thalamo-cortical circuit is likely triggered by activity in the basal ganglia.Lewiset al. (2011) supposed that the primary dysfunction in the cerebello-thalamo-cortical circuit, particularly the vermis/paravermis region, may be responsible for the occurrence of resting tremor.
Figure 3.
Tremor-related cerebral activity in tremor-dominant Parkinson’s disease. Location of cerebral regions where activity cofluctuated with tremor amplitude. Activity was localized to the motor cortex, ventral intermediate nucleus of the thalamus and cerebellum (side contralateral to the tremor). BA = Brodmann area; lob = lobule; VIM = ventral intermediate nucleus. Reprinted fromHelmichet al. (2011), with permission from John Wiley and Sons.
It has long been thought that resting tremor may be generated by neural mechanisms compensating for akinesia/rigidity (Hallett and Khoshbin, 1980;Rivlin-Etzionet al., 2006;Zaidelet al., 2009;Helmichet al., 2011). Whether compensation or pathological changes in the cerebello-thalamo-cortical circuit contribute to parkinsonian resting tremor still needs further investigation.
The cerebellum and parkinsonian gait
Gait disturbance is one of the cardinal symptoms in Parkinson’s disease, which is characterized by small shuffling steps and general movement slowness (Aita, 1982;Morriset al., 1998). Compared with motor deficits in the upper limbs, neural correlates underlying gait disturbance in Parkinson’s disease were much less investigated. In a single-photon emission computed tomography study during gait on a treadmill,Hanakawaet al. (1999) found that in controls, a gait-induced increase in brain activity was observed in the medial and lateral premotor areas, primary sensorimotor areas, anterior cingulate cortex, superior parietal cortex, visual cortex, dorsal brainstem, basal ganglia and cerebellum. The patients with Parkinson’s disease had hypoactivation in the left medial frontal area, right precuneus and left anterior lobe of the cerebellar hemisphere, but hyperactivity in the left temporal cortex, right insula, left cingulate cortex and cerebellar vermis (Fig. 4). The authors suggested that the hypoactivation in the left cerebellar hemisphere may be related to a loss of lateral gravity shift in parkinsonian gait, which in turn might result in small shuffling steps. The hyperactivation in the vermis might possibly be a compensatory effect. These assumptions, of course, still need further verification.
Figure 4.
Relative underactivity (A) and overactivity (B) induced by treadmill walking in patients with Parkinson’s disease. InA, relative underactivity in Parkinson’s disease includes the left cerebellar hemisphere (1), precuneus (2) and the left presupplementary motor cortex (3). InB, relative overactivity in Parkinson’s disease was found in the left middle temporal gyrus (1), right insula (2), left cingulate cortex (3) and cerebellar vermis (4 and 5). Modified fromHanakawaet al. (1999), with permission from Oxford University Press.
Deep brain stimulation of the pedunculopontine nucleus may be an effective therapy for some patients with Parkinson’s disease with gait disturbances (Stefaniet al., 2007).Schwederet al. (2010) characterized the anatomical connectivity of the pedunculopontine nucleus in patients with gait freezing Parkinson’s disease using diffusion tensor imaging techniques. They found that the pedunculopontine nucleus showed connectivity with the cerebellum in control subjects and patients with Parkinson’s disease without gait freezing. In contrast, patients with Parkinson’s disease with gait freezing showed absence of pedunculopontine nucleus–cerebellar connectivity, and increased visibility of the decussation of cortico-pontine fibres in the anterior pons. Deep brain stimulation in the pedunculopontine nucleus (Ballangeret al., 2009) induced significant regional cerebral blood flow increments in the thalamus, cerebellum, midbrain and different cortical areas involving the medial sensorimotor cortex extending into the caudal supplementary motor cortex. Pedunculopontine nucleus deep brain stimulation in advanced Parkinson’s disease resulted in blood flow changes in subcortical and cortical areas involved in balance and motor control, including the mesencephalic locomotor region (e.g. pedunculopontine nucleus) and closely interconnected structures within the cerebello-(rubro)-thalamo-cortical circuit.
A recent study used PET with11C-methylpiperidinyl propionate to measure acetylcholinesterase activity (Gilmanet al., 2010), and demonstrated a correlation between the severity of the balance and gait in patients with Parkinson’s disease and decreased acetylcholinesterase activity in the midbrain and cerebellum, but not for any other structure. These findings suggest the involvement of the cortico-pontine-cerebello-thalamo-cortical pathway in the pathophysiology of gait disturbances.
The cerebellum and dyskinesia
Chronic dopamine replacement therapy in patients with Parkinson’s disease is commonly complicated by involuntary movements known as levodopa-induced dyskinesia (Fahn, 2000;Rascolet al., 2000). The neural mechanisms of levodopa-induced dyskinesia are still partially obscure, but levodopa-induced dyskinesia has been considered to be the consequence of an abnormal activity pattern in the striato-thalamo-cortical loops, which in turn induces the excessive disinhibition of thalamocortical neurons and overactivation of cortical motor areas (Lozanoet al., 2000;Bezardet al., 2001;Wagle-Shuklaet al., 2007). Recent studies suggested that the cerebello-thalamo-cortical circuit also contributes to the development of levodopa-induced dyskinesia. Deep brain stimulation of the subthalamic nucleus or globus pallidus, the surgical procedures that alleviate levodopa-induced dyskinesia (Kracket al., 2003;Andersonet al., 2005), was reported to modulate neural activity or metabolism in the cerebellum (Hilkeret al., 2004;Payouxet al., 2004;Asanumaet al., 2006;Graftonet al., 2006;Gedayet al., 2009;Payouxet al., 2009). In a PET study (Nimuraet al., 2004) on patients with advanced Parkinson’s disease undergoing stereotactic pallidal surgery (pallidotomy or deep brain stimulation), the level of binding potential of cerebellar sigma receptors did not correlate with the Hoehn and Yahr stages, or Unified Parkinson’s Disease Rating Scale, but a positive correlation was seen between the binding potential and the preoperative levodopa-induced dyskinesia severity score, giving evidence that the cerebellum may be involved in the genesis of dyskinesia.
Kochet al. (2009) tested the effect of repetitive transcranial magnetic stimulation over the lateral cerebellum on levodopa-induced dyskinesia. The authors found that a single session of cerebellar continuous theta burst stimulation could transiently reduce levodopa-induced dyskinesia. Cerebellar continuous theta burst stimulation reduced short intracortical inhibition and increased long intracortical inhibition in the contralateral primary motor cortexs, inducing a cortical reorganization that is associated with reduced levodopa-induced dyskinesia. A 2-week course of bilateral cerebellar continuous theta burst stimulation induced persistent clinical beneficial effects, reducing peak-dose levodopa-induced dyskinesia for up to 4 weeks after the stimulation. These clinical improvements were paralleled by reducing18F-fluorodeoxyglucose metabolism in the cerebellum (Brusaet al., 2012). There was a global decrease in the metabolism of the bilateral cerebellar hemispheres and a significant corresponding decrease in18F-fluorodeoxyglucose uptake in the bilateral dentate nuclei. These findings demonstrate that the antidyskinetic effect of cerebellar continuous theta burst stimulation is accompanied by modulation of the cerebellar activity, supporting the hypothesis that the cerebell-thalamo-cortical circuit is involved in generating levodopa-induced dyskinesia. A reduced level of dopamine D1 and D3 receptor messenger RNA in the cerebellum in patients with Parkinson’s disease receiving dopaminergic treatment was reported (Hurleyet al., 2003). Whether this change is secondary to long-term exposure to levodopa and a partial reason contributing to dyskinesia still needs further investigation.
The cerebellum and non-motor symptoms in Parkinson’s disease
Many non-motor symptoms, including sensory, autonomic, cognitive and behavioural problems, coexist with the motor signs in Parkinson’s disease (Hillen and Sage, 1996). Non-motor symptoms exist in up to 60% of patients (Shulmanet al., 2001), and can be primary complaints in Parkinson’s disease (Adler, 2005). Cognitive impairment is common in patients with Parkinson’s disease (Aarslandet al., 2001). Hypometabolism in the prefrontal, parietal, temporal and mesolimbic regions was correlated with cognitive impairment in Parkinson’s disease (Huet al., 2000;Rinneet al., 2000;Itoet al., 2002;Mentiset al., 2002;Nagano-Saitoet al., 2004). With fluorodeoxyglucose PET and spatial covariance analysis,Huanget al. (2007a) identified a significant covariance pattern that correlated with cognitive performance, particularly involving executive functioning in Parkinson’s disease. This Parkinson’s disease–related cognitive pattern is characterized by metabolic reductions in frontal and parietal association areas, and increases in the cerebellar vermis and dentate nuclei (Huanget al., 2007a). Parkinson’s disease–related cognitive pattern expression increased with worsening of cognitive impairment (Huanget al., 2008;Eidelberg, 2009), but is not correlated with the decline of striatal dopaminergic function (Huanget al., 2007b). Therefore, the hypermetabolism in the cerebellum might also be a compensatory effort to maintain cognitive function in Parkinson’s disease.
Using the voxel-based morphometry method,Nishioet al. (2010) found reduced regional grey matter volume in the cerebellum, as well as in the cortico-limbic network in non-demented Parkinson’s disease with impaired cognition. Verbal fluency tests are often used to assess cognitive dysfunction in Parkinson’s disease.Pereiraet al. (2009) showed that in non-demented patients with Parkinson’s disease, grey matter density in the temporal, frontal and cerebellar areas correlated with semantic fluency scores.Camicioliet al. (2009) found that executive function is associated with grey matter atrophy in the cerebellum, middle temporal gyri and left precuneus in patients with Parkinson’s disease.
Caoet al. (2011) investigated neural correlates of sensory damage in early Parkinson’s disease. During a passive tactile stimulation task, patients with Parkinson’s disease had hypoactivation in the bilateral sensorimotor cortex, and hyperactivation in the bilateral prefrontal cortex, bilateral cerebellum and contralateral striatum compared with control subjects. In addition, there was significantly decreased connectivity in the supplementary motor cortex and increased striato-prefrontal and cerebello-prefrontal connections in Parkinson’s disease.
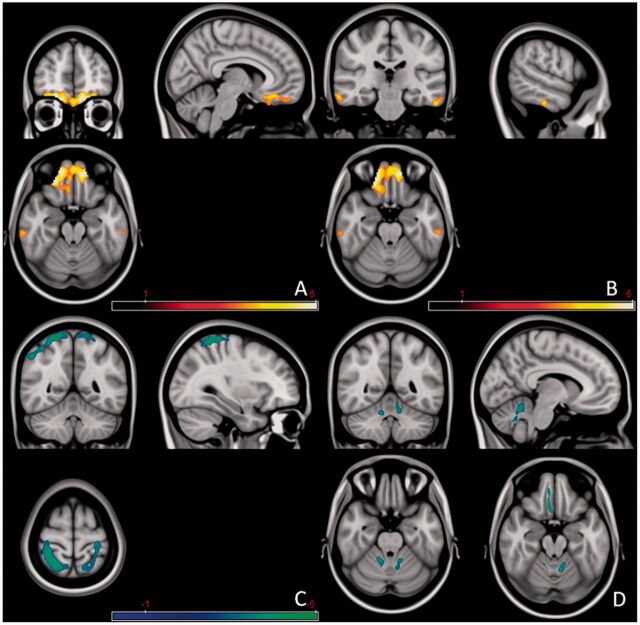
Impaired olfaction is a characteristic and early feature of Parkinson’s disease. Recent studies indicate that olfactory loss is one of the most prevalent motor and non-motor symptoms in patients with early stage Parkinson’s disease (Haehneret al., 2009;Politiset al., 2010). However, the underlying mechanism of olfactory dysfunction remains unknown. A recent diffusion tensor imaging study (Zhanget al., 2011) reported decreased fractional or increased mean diffusivity in the bilateral cerebellum and orbitofrontal cortex in patients with Parkinson’s disease compared with control subjects (Fig. 5). There was a positive correlation between fractional anisotropy values in the white matter of the left cerebellum and the thresholds of olfactory identification, and a negative correlation between mean diffusivity values in the white matter of right cerebellum and the thresholds of olfactory identification. These findings suggest that there is a correlation between cerebellar white matter damage and olfactory dysfunction in patients with Parkinson’s disease. Although the cerebellum is traditionally not considered to be part of the olfaction processing system, activation of the cerebellum was observed during performance of olfactory tasks in healthy participants (Qureshyet al., 2000). Aged subjects had decreased cerebellar activation correlating with decreased olfactory abilities (Ferdon and Murphy, 2003). Moreover, olfactory dysfunction was detected in patients with cerebellar lesions (Mainlandet al., 2005), or patients with ataxias primarily due to cerebellar pathology (Connellyet al., 2003;Moscovichet al., 2012). Therefore, whether functional or structural changes in the cerebellum contribute to olfactory impairment in Parkinson’s disease is worth further investigation.
Figure 5.
Increased mean diffusivity in bilateral orbitofrontal cortices (A) and bilateral inferior temporal gyri (B), decreased mean diffusivity in bilateral parietal lobes and left precentral gyrus (C) and decreased fractional anisotropy in bilateral cerebellum and right rectus gyrus (D) in patients with Parkinson’s disease versus normal controls. The red colour indicates an increase of the value, and blue-green colour indicates a decrease. Modified fromZhanget al. (2011), with permission from Elsevier.
Goerendtet al. (2004) measured brain activation patterns related to processing monetary rewards in unmedicated patients with Parkinson’s disease. Both Parkinson’s disease and healthy groups showed increased search efficiency with increasing reward, but with different patterns of neuronal activation. The increasing reward magnitude correlated with the activity in the prefrontal and rhinal cortices and thalamus in healthy controls, but correlated with activity in the cerebellar vermis in patients with Parkinson’s disease. Because motivational processes are mediated by dopaminergic neural systems and are relatively spared in Parkinson’s disease, the cerebellum might be particularly involved in motivational modulation and its compensatory influence is possibly a reason why the motivational processes are relatively intact in Parkinson’s disease.
The cerebellum as a target for Parkinson’s disease treatment
While cerebellar dysfunction might contribute to some motor and non-motor signs in Parkinson’s disease, a possible approach for treating parkinsonian symptoms is to attempt to normalize cerebellar function. Surgical treatment, such as deep brain stimulation of the subthalamic nucleus (Hilkeret al., 2004;Payouxet al., 2004;Asanumaet al., 2006;Graftonet al., 2006;Gedayet al., 2009) or globus pallidus (Payouxet al., 2009) improves the motor signs and normalizes cerebellar activation. Levodopa administration can also normalize the activity and connectivity in the cerebello-thalamo-cortical circuit (Wuet al., 2009a,b). However, whether it is reduced compensation or alleviation of pathological impairment as a consequence of effective treatment remains unclear. Suppressing cerebellar activity should theoretically answer the question: improvement would mean that the cerebellum is contributing to the manifestations; worsening would mean that the cerebellar activity is compensatory. We suppose that if the main efforts of the cerebellum in Parkinson’s disease are compensatory, suppression of cerebellar activity should be accompanied by further impairments of Parkinson’s disease symptoms.
Lesion or deep brain stimulation of the cerebellar territory of the thalamus can successfully ameliorate parkinsonian resting tremor (Benabidet al., 1991;Lenzet al., 1995). Moreover, the observation that a 2-week course of bilateral cerebellar repetitive transcranial magnetic stimulation could induce a marked and persistent reduction of levodopa-induced dyskinesia that lasted for up to 4 weeks after the end of the stimulation period (Kochet al., 2009) demonstrated that the cerebellum is a potential target to relieve some Parkinson’s disease symptoms. While these two arguments favour cerebellar contribution to the parkinsonism, both tremor and levodopa-induced dyskinesia are not primary symptoms. Observations would be needed particularly on aspects of bradykinesia.
Summary
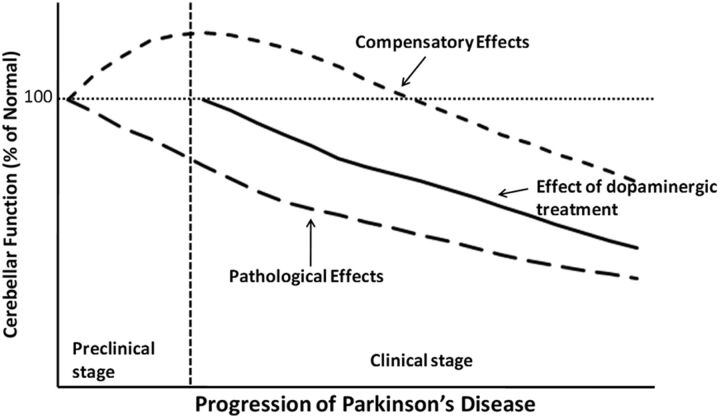
We propose that the major role of the cerebellum in Parkinson’s disease includes two aspects, pathological and compensatory effects. Pathological changes in the cerebellum might be induced by dopaminergic degeneration, abnormal drives from the subthalamic nucleus and dopaminergic treatment, and may account for several clinical symptoms in Parkinson’s disease. Because dopaminergic degeneration develops gradually (Hilkeret al., 2005), presumably, pathological impairments should be more severe as disease progresses. The compensatory effect may help to maintain relatively normal motor and non-motor function. In the mild-to-moderate stages of Parkinson’s disease, recruitment of the cerebello-thalamo-cortical circuit positively correlates with the severity of symptoms or progression (Wuet al., 2009a,2010b;Senet al., 2010). It is likely that the compensatory effect strengthens at a relatively early stage, but may diminish or eventually fail as pathological damages become more severe at the advanced stage (Jankovic, 2005). A hypothetical model of functional changes in the cerebellum during progression of Parkinson’s disease is shown inFig. 6.
Figure 6.
A hypothetical model of functional changes in the cerebellum accompanying the progression of Parkinson’s disease.
Our knowledge on the role of the cerebellum in Parkinson’s disease remains limited. Further investigations are needed to clarify Parkinson’s disease–related pathological alterations in the cerebellum and how cerebellar pathological and compensatory effects evolve as the disorder progresses. A better understanding of the Parkinson’s disease–related functional and morphological changes of the cerebellum will significantly contribute to the pathophysiology of Parkinson’s disease and may help develop new strategies and targets for treatment.
Funding
This work was supported by grants from the National Science Foundation of China [30870693, 81071012 and 81271429, to T.W.].
Acknowledgements
We wish to thank D. G. Schoenberg for skilful editing.
Glossary
Abbreviations
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Risk of dementia in Parkinson’s disease. A community-based, prospective study. Neurology. 2001;56:730–6. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- Adler CH. Nonmotor complications in Parkinson’s disease. Mov Disord. 2005;20(Suppl 11):S23–9. doi: 10.1002/mds.20460. [DOI] [PubMed] [Google Scholar]
- Aita JF. Why patients with Parkinson's disease fall. JAMA. 1982;247:515–16. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Anderson VC, Burchiel KJ, Hogarth P, Farve J, Hammerstad JP. Pallidal vs. subthalamic nucleus deep brain stimulation in Parkinson's disease. Arch Neurol. 2005;62:554–60. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, et al. Network modulation in the treatment of Parkinson's disease. Brain. 2006;129:2667–78. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanger B, Baraduc P, Broussolle E, Le Bars D, Desmurget M, Thobois S. Motor urgency is mediated by the contralateral cerebellum in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2008;79:1110–6. doi: 10.1136/jnnp.2007.141689. [DOI] [PubMed] [Google Scholar]
- Ballanger B, Lozano AM, Moro E, van Eimeren T, Hamani C, Chen R, et al. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson's disease: a [15O] H2O PET study. Hum Brain Mapp. 2009;30:3901–9. doi: 10.1002/hbm.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard P, Sanes JN. On a basal ganglia role in learning and rehearsing visual-motor associations. Neuroimage. 2009;47:1701–10. doi: 10.1016/j.neuroimage.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz AJ, Larson AA, Monaghan P, Altschuler RA, Mullett MM, Madl JE. Immunohistochemical localization of glutamate, glutaminase and aspartate aminotransferase in neurons of the pontine nuclei of the rat. Neuroscience. 1986;17:741–53. doi: 10.1016/0306-4522(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–6. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- Benninger DH, Thees S, Kollias SS, Bassetti CL, Waldvogel D. Morphological differences in Parkinson's disease with and without rest tremor. J Neurol. 2009;256:256–63. doi: 10.1007/s00415-009-0092-2. [DOI] [PubMed] [Google Scholar]
- Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci. 2001;2:577–88. doi: 10.1038/35086062. [DOI] [PubMed] [Google Scholar]
- Borghammer P, Østergaard K, Cumming P, Gjedde A, Rodell A, Hall N, et al. A deformation-based morphometry study of patients with early-stage Parkinson's disease. Eur J Neurol. 2010;17:314–20. doi: 10.1111/j.1468-1331.2009.02807.x. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA. 2010;107:8452–6. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del TK, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brusa L, Ceravolo R, Kiferle L, Monteleone F, Iani C, Schillaci O, et al. Metabolic changes induced by theta burst stimulation of the cerebellum in dyskinetic Parkinson's disease patients. Parkinsonism Relat Disord. 2012;18:59–62. doi: 10.1016/j.parkreldis.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. Pharmacologically modulated fMRI - cortical responsiveness to levodopa in drug-naïve hemiparkinsonian patients. Brain. 2003;126:451–61. doi: 10.1093/brain/awg033. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Gee M, Bouchard TP, Fisher NJ, Hanstock CC, Emery DJ, Martin WR. Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism Relat Disord. 2009;15:187–95. doi: 10.1016/j.parkreldis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Cao H, Xu X, Zhao Y, Long D, Zhang M. Altered brain activation and connectivity in early Parkinson disease tactile perception. AJNR Am J Neuroradiol. 2011;32:1969–74. doi: 10.3174/ajnr.A2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain. 1999;122:483–95. doi: 10.1093/brain/122.3.483. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Hagberg GE, Peppe A, Bianciardi M, Gioia MC, Costa A, et al. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson's disease. Brain Res Bull. 2006;71:259–69. doi: 10.1016/j.brainresbull.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Chiba-Falek O, Lopez GJ, Nussbaum RL. Levels of alpha-synuclein mRNA in sporadic Parkinson disease patients. Mov Disord. 2006;21:1703–8. doi: 10.1002/mds.21007. [DOI] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Cilia R, Marotta G, Landi A, Isaias IU, Mariani CB, Vergani F, et al. Clinical and cerebral activity changes induced by subthalamic nucleus stimulation in advanced Parkinson's disease: a prospective case-control study. Clin Neurol Neurosurg. 2009;111:140–6. doi: 10.1016/j.clineuro.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Connelly T, Farmer JM, Lynch DR, Doty RL. Olfactory dysfunction in degenerative ataxias. J Neurol Neurosurg Psychiatry. 2003;74:1435–7. doi: 10.1136/jnnp.74.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Pollak P, Passingham R, Landais P, Gervason C, Cinotti L, et al. Thalamic stimulation and suppression of parkinsonian tremor. Evidence of a cerebellar deactivation using positron emission tomography. Brain. 1993;116:267–279. doi: 10.1093/brain/116.1.267. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Raethjen J, Baron R, Lindemann M, Wilms H, Krack P. The pathophysiology of parkinsonian tremor: a review. J Neurol. 2000;247(Suppl 5):V33–48. doi: 10.1007/pl00007781. [DOI] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci. 2002;16:1609–19. doi: 10.1046/j.1460-9568.2002.02212.x. [DOI] [PubMed] [Google Scholar]
- Eckert T, Tang C, Eidelberg D. Assessment of the progression of Parkinson’s disease: a metabolic network approach. Lancet Neurol. 2007;6:926–32. doi: 10.1016/S1474-4422(07)70245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32:548–57. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. The spectrum of levodopa-induced dyskinesias. Ann Neurol. 2000;47:S2–9. [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantianigra regional selectivity. Brain. 1991;114:2283–01. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Ferdon S, Murphy C. The cerebellum and olfaction in the aging brain: a functional magnetic resonance imaging study. Neuroimage. 2003;20:12–21. doi: 10.1016/s1053-8119(03)00276-3. [DOI] [PubMed] [Google Scholar]
- Forno S. Pathology of Parkinson's disease. In: Marsden CD, Fahn S, editors. Movement disorders, neurology. 2nd edn. Cornwall: Butterworth Scientific; 1981. p. 21–40.
- Fuchs J, Tichopad A, Golub Y, Munz M, Schweitzer KJ, Wolf B, et al. Genetic variability in the SNCA gene influences α-synuclein levels in the blood and brain. FASEB J. 2008;22:1327–34. doi: 10.1096/fj.07-9348com. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Barnes A, Simon ES, Holmes A, Dhawan V, Giladi N, et al. Thalamic stimulation for parkinsonian tremor: correlation between regional cerebral blood flow and physiological tremor characteristics. Neuroimage. 2004;21:608–15. doi: 10.1016/j.neuroimage.2003.09.068. [DOI] [PubMed] [Google Scholar]
- Geday J, Østergaard K, Johnsen E, Gjedde A. STN-stimulation in Parkinson's disease restores striatal inhibition of thalamocortical projection. Hum Brain Mapp. 2009;30:112–21. doi: 10.1002/hbm.20486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Fahn S. The cerebellum. In: Kandel ER, Schwartz JH, editors. Principles of neural science. 2nd edn. New York: Elsevier; 1985. p. 502-22.
- Gilman S, Koeppe RA, Nan B, Wang CN, Wang X, Junck L, et al. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology. 2010;74:1416–23. doi: 10.1212/WNL.0b013e3181dc1a55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giompres P, Delis F. Dopamine transporters in the cerebellum of mutant mice. Cerebellum. 2005;4:105–11. doi: 10.1080/14734220510007851. [DOI] [PubMed] [Google Scholar]
- Goerendt IK, Lawrence AD, David J, Brooks DJ. Reward processing in health and Parkinson’s disease: neural organization and reorganization. Cereb Cortex. 2004;14:73–80. doi: 10.1093/cercor/bhg105. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Turner RS, Desmurget M, Bakay R, Delong M, Vitek J, Crutcher M. Normalizing motor-related brain activity: Subthalamic nucleus stimulation in Parkinson disease. Neurology. 2006;66:1192–9. doi: 10.1212/01.wnl.0000214237.58321.c3. [DOI] [PubMed] [Google Scholar]
- Gründemann J, Schlaudraff F, Haeckel O, Liss B. Elevated alpha-synuclein mRNA levels in individual UV-laser-microdissected dopaminergic substantia nigra neurons in idiopathic Parkinson's disease. Nucleic Acids Res. 2008;36:e38. doi: 10.1093/nar/gkn084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guehl D, Pessiglione M, Francois C, Yelnik J, Hirsch EC, Feger J, et al. Tremor-related activity of neurons in the ‘motor’ thalamus: changes in firing rate and pattern in the MPTP vervet model of parkinsonism. Eur J Neurosci. 2003;17:2388–400. doi: 10.1046/j.1460-9568.2003.02685.x. [DOI] [PubMed] [Google Scholar]
- Haehner A, Boesveldt S, Berendse HW, Mackay-Sim A, Fleischmann J, Silburn PA, et al. Prevalence of smell loss in Parkinson's disease- a multicenter study. Parkinsonism Relat Disord. 2009;15:490–4. doi: 10.1016/j.parkreldis.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–14. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Hallett M, Cohen LG, Bierner SM. Studies of sensory and motor cortex physiology: with observations on akinesia in Parkinson's disease. Electroencephalogr Clin Neurophysiol. 1991;43(Suppl.):76–85. [PubMed] [Google Scholar]
- Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, et al. Mechanisms underlying gait disturbance in Parkinson's disease: a single photon emission computed tomography study. Brain. 1999;122:1271–82. doi: 10.1093/brain/122.7.1271. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, et al. Event-related Functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain. 2001;124:558–70. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Aarts E, de Lange FP, Bloem BR, Toni I. Increased dependence of action selection on recent motor history in Parkinson’s disease. J Neurosci. 2009;29:6105–13. doi: 10.1523/JNEUROSCI.0704-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol. 2011;69:269–81. doi: 10.1002/ana.22361. [DOI] [PubMed] [Google Scholar]
- Heman P, Barcia C, Gómez A, Ros CM, Ros-Bernal F, Yuste JE, et al. Nigral degeneration correlates with persistent activation of cerebellar Purkinje cells in MPTP-treated monkeys. Histol Histopathol. 2012;27:89–94. doi: 10.14670/HH-27.89. [DOI] [PubMed] [Google Scholar]
- Hilker R, Schweitzer K, Coburger S, Ghaemi M, Weisenbach S, Jacobs AH, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol. 2005:62378–82. doi: 10.1001/archneur.62.3.378. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson's disease. J Cereb Blood Flow Metab. 2004;24:7–16. doi: 10.1097/01.WCB.0000092831.44769.09. [DOI] [PubMed] [Google Scholar]
- Hillen ME, Sage JI. Nonmotor fluctuations in patients with Parkinson’s disease. Neurology. 1996;47:1180–3. doi: 10.1212/wnl.47.5.1180. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. The discovery of dopamine deficiency in the parkinsonian brain. J Neural Transm. 2006;70:9–15. doi: 10.1007/978-3-211-45295-0_3. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–3. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Hu MT, Taylor-Robinson SD, Chaudhuri KR, Bell JD, Labbe C, Cunningham VJ, et al. Cortical dysfunction in non-demented Parkinson’s disease patients. A combined 31P-MRS and 18FDG-PET Study. Brain. 2000;123:340–52. doi: 10.1093/brain/123.2.340. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson's disease. Neuroimage. 2007a;34:714–23. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tang C, Feigin A, Lesser M, Ma Y, Pourfar M, et al. Changes in network activity with the progression of Parkinson's disease. Brain. 2007b;130:1834–46. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MJ, Mash DC, Jenner P. Markers for dopaminergic neurotransmission in the cerebellum in normal individuals and patients with Parkinson's disease examined by RT-PCR. Eur J Neurosci. 2003;18:2668–72. doi: 10.1046/j.1460-9568.2003.02963.x. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Mori F, Shoumura K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 2000;880:191–7. doi: 10.1016/s0006-8993(00)02744-x. [DOI] [PubMed] [Google Scholar]
- Ikai Y, Takada M, Shinonaga Y, Mizuno N. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience. 1992;51:719–28. doi: 10.1016/0306-4522(92)90310-x. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Shibasaki H, Nagamine T, Terada K, Kaji R, Fukuyama H, et al. Dissociation between contingent negative variation and Bereitschafts potential in a patient with cerebellar efferent lesion. Electroencephalogr Clin Neurophysiol. 1994;90:359–64. doi: 10.1016/0013-4694(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Ito K, Nagano-Saito A, Kato T, Arahata T, Nakamura A, Kawasumi Y, Hatano K, et al. Striatal and extrastriatal dysfunction in Parkinson’s disease with dementia: a 6-[(18)]fluoro-L-dopa PET study. Brain. 2002;125:1358–65. doi: 10.1093/brain/awf134. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins H, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CR, Zijlmans J, Katzenschlager R, Lee L, Quinn N, et al. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson's disease. Brain. 2010;133:727–45. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- Jaillard A, Martin CD, Garambois K, Lebas JF, Hommel M. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain. 2005;128:1122–38. doi: 10.1093/brain/awh456. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Progression of Parkinson disease: are we making progress in charting the course? Arch Neurol. 2005;62:351–2. doi: 10.1001/archneur.62.3.351. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamus. New York: Plenum; 1985. [Google Scholar]
- Kawashima R, Okuda J, Umetsu A, Sugiura M, Inoue K, Suzuki K, et al. Human cerebellum plays an important role in memory-timed finger movement: an fMRI study. J Neurophysiol. 2000;83:1079–87. doi: 10.1152/jn.2000.83.2.1079. [DOI] [PubMed] [Google Scholar]
- Kingsbury AE, Daniel SE, Sangha H, Eisen S, Lees AJ, Foster OJ. Alteration in alpha-synuclein mRNA expression in Parkinson’s disease. Mov Disord. 2004;19:162–70. doi: 10.1002/mds.10683. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Kita H. The Basal Ganglia II - structure and function: current concepts. In: Carpenter MB, Jayaraman A, editors. Plenum: New York; 1987. p. 357-73.
- Koch G, Brusa L, Carrillo F, Lo Gerfo E, Torriero S, Oliveri M, et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73:113–9. doi: 10.1212/WNL.0b013e3181ad5387. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349:1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Krack P, Pollak P, Limousin P, Benazzouz A, Benabid AL. Stimulation of subthalamic nucleus alleviates tremor in Parkinson’s disease. Lancet. 1997;350:1676. doi: 10.1016/s0140-6736(97)24049-3. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000;47:493–503. [PubMed] [Google Scholar]
- Lenz FA, Normand SL, Kwan HC, Andrews D, Rowland LH, Jones MW, et al. Statistical prediction of the optimal site for thalamotomy in parkinsonian tremor. Mov Disord. 1995;10:318–28. doi: 10.1002/mds.870100315. [DOI] [PubMed] [Google Scholar]
- Lewis MM, Du G, Sen S, Kawaguchi A, Truong Y, Lee S, et al. Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson's disease. Neuroscience. 2011;177:230–9. doi: 10.1016/j.neuroscience.2010.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Lang AE, Levy R, Hutchison W, Dostrovsky J. Neuronal recordings in Parkinson’s disease patients with dyskinesias induced by apomorphine. Ann Neurol. 2000;47:S141–6. [PubMed] [Google Scholar]
- Lozza C, Marie RM, Baron JC. The metabolic substrates of bradykinesia and tremor in uncomplicated Parkinson’s disease. Neuroimage. 2002;17:688–99. [PubMed] [Google Scholar]
- Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson’s disease: test-retest reproducibility. J Cereb Blood Flow Metab. 2007;27:597–605. doi: 10.1038/sj.jcbfm.9600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainland JD, Johnson BN, Khan R, Ivry RB, Sobel N. Olfactory impairments in patients with unilateral cerebellar lesions are selective to inputs from the contralesional nostril. J Neurosci. 2005;25:6362–71. doi: 10.1523/JNEUROSCI.0920-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallol R, Barrós-Loscertales A, López M, Belloch V, Parcet MA, Avila C. Compensatory cortical mechanisms in Parkinson's disease evidenced with fMRI during the performance of pre-learned sequential movements. Brain Res. 2007;1147:265–71. doi: 10.1016/j.brainres.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA. Tyrosine hydroxylase- and dopamine transporter-immunoreactivity axons in the primate cerebellum. Evidence for a lobular- and laminar-specific dopamine innervation. Neuropsychopharmacology. 2000;22:466–72. doi: 10.1016/S0893-133X(99)00139-6. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, Dhawan V, Nakamura T, Ghilardi MF, Feigin A, Edwards C, et al. Enhancement of brain activation during trial-and-error sequence learning in early PD. Neurology. 2003;60:612–9. doi: 10.1212/01.wnl.0000044154.92143.dc. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, McIntosh AR, Perrine K, Dhawan V, Berlin B, Feigin A, et al. Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson’s disease. Am J Psychiatry. 2002;159:746–754. doi: 10.1176/appi.ajp.159.5.746. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–50. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–12. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink J. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Moers-Hornikx VM, Vles JS, Tan SK, Cox K, Hoogland G, Steinbusch WM, et al. Cerebellar nuclei are activated by high-frequency stimulation of the subthalamic nucleus. Neurosci Lett. 2011;496:111–5. doi: 10.1016/j.neulet.2011.03.094. [DOI] [PubMed] [Google Scholar]
- Morris M, Iansek R, Matyas T, Summers J. Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov Disord. 1998;13:61–69. doi: 10.1002/mds.870130115. [DOI] [PubMed] [Google Scholar]
- Moscovich M, Munhoz RP, Teive HA, Raskin S, Carvalho Mde J, Barbosa ER, Ranvaud R, Liu J, McFarland K, Ashizawa T, Lees AJ, Silveira-Moriyama L. Olfactory impairment in familial ataxias. J Neurol Neurosurg Psychiatry. 2012;83:970–4. doi: 10.1136/jnnp-2012-302770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mure H, Hirano S, Tang CC, Isaias IU, Antonini A, Ma Y, et al. Parkinson's disease tremor-related metabolic network: Characterization, progression, and treatment effects. NeuroImage. 2011;54:1244–53. doi: 10.1016/j.neuroimage.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Kato T, Arahata Y, Washimi Y, Nakamura A, Abe Y, et al. Cognitive- and motor-related regions in Parkinson's disease: FDOPA and FDG PET studies. Neuroimage. 2004;22:553–61. doi: 10.1016/j.neuroimage.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Katayama Y, Kano T, Kobayashi K, Oshima H, Fukaya C, et al. Changes in glucose metabolism in cerebral cortex and cerebellum correlate with tremor and rigidity control by subthalamic nucleus stimulation in Parkinson's disease: a positron emission tomography study. Neuromodulation. 2007;10:206–15. doi: 10.1111/j.1525-1403.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- Neystat M, Lynch T, Przedborski S, Kholodilov N, Rzhetskaya M, Burke RE. (1999) Alpha-synuclein expression in substantia nigra and cortex in Parkinson’s disease. Mov Disord. 1999;14:417–22. doi: 10.1002/1531-8257(199905)14:3<417::aid-mds1005>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ni Z, Pinto AD, Lang AE, Chen R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol. 2010;68:816–24. doi: 10.1002/ana.22221. [DOI] [PubMed] [Google Scholar]
- Nimura T, Ando T, Yamaguchi K, Nakajima T, Shirane R, Itoh M, et al. The role of sigma-receptors in levodopa-induced dyskinesia in patients with advanced Parkinson disease: a positron emission tomography study. J Neurosurg. 2004;100:606–10. doi: 10.3171/jns.2004.100.4.0606. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Hirayama K, Takeda A, Hosokai Y, Ishioka T, Suzuki K, Itoyama Y, Takahashi S, Mori E. Corticolimbic gray matter loss in Parkinson's disease without dementia. Eur J Neurol. 2010;17:1090–7. doi: 10.1111/j.1468-1331.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Ichikawa J, Matsuzaki R, Kyuhou S, Matsuura-Nakao K, et al. Cortical field potentials preceding self-paced forelimb movements and influences of cerebellectomy upon them in rats. Neurosci Lett. 2003;352:5–8. doi: 10.1016/j.neulet.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Eigenraam L, Hoque T, McCaig RG, Troiano A, McKeown MJ. Levodopa-sensitive, dynamic changes in effective connectivity during simultaneous movements in Parkinson's disease. Neuroscience. 2009;158:693–704. doi: 10.1016/j.neuroscience.2008.06.053. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Li J, Wang ZJ, McKeown MJ. Joint amplitude and connectivity compensatory mechanisms in Parkinson's disease. Neuroscience. 2010;166:1110–1118. doi: 10.1016/j.neuroscience.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Panagopoulos NT, Panagopoulos GC, Matsokis NA. Dopaminergic innervation and binding in the rat cerebellum. Neurosci Lett. 1991;130:208–12. doi: 10.1016/0304-3940(91)90398-d. [DOI] [PubMed] [Google Scholar]
- Papavassiliou E, Rau G, Heath S, Abosch A, Barbaro NM, Larson PS, Lamborn K, Starr PA. Thalamic deep brain stimulation for essential tremor: relation of lead location to outcome. Neurosurgery. 2008;62(Suppl 2):884–894. doi: 10.1227/01.neu.0000316290.83360.7e. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Paulus W, Jellinger K. Theneuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol. 1991;50:743–55. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, et al. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurol. 2004;61:1307–13. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- Payoux P, Remy P, Miloudi M, Houeto JL, Stadler C, Bejjani BP, et al. Contrasting changes in cortical activation induced by acute high-frequency stimulation within the globus pallidus in Parkinson's disease. J Cereb Blood Flow Metab. 2009;29:235–43. doi: 10.1038/jcbfm.2008.107. [DOI] [PubMed] [Google Scholar]
- Percheron G, Francois C, Talbi B, Yelnik J, Febelon G. The primate motor thalamus. Brain Res Brain Res Rev. 1996;22:93–181. [PubMed] [Google Scholar]
- Pereira JB, Junqué C, Martí MJ, Ramirez-Ruiz B, Bartrés-Faz D, Tolosa E. Structural brain correlates of verbal fluency in Parkinson's disease. Neuroreport. 2009;20:741–4. doi: 10.1097/WNR.0b013e328329370b. [DOI] [PubMed] [Google Scholar]
- Piao YS, Mori F, Hayashi S, Tanji K, Yoshimoto M, Kakita A, et al. Alpha-synuclein pathology affecting Bergmann glia of the cerebellum in patients with alpha-synucleinopathies. Acta Neuropathol. 2003;105:403–9. doi: 10.1007/s00401-002-0655-0. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Politis M, Wu K, Molloy S, Bain PG, Chaudhuri KR, Piccini P. Parkinson's disease symptoms: the patient's perspective. Mov Disord. 2010;25:1646–51. doi: 10.1002/mds.23135. [DOI] [PubMed] [Google Scholar]
- Pollock LJ, Davis L. Muscle tone in parkinsonian states. Arch Neurol Psychiatry. 1930;23:303–19. [Google Scholar]
- Pollok B, Makhloufi H, Butz M, Gross J, Timmermann L, Wojtecki L, et al. Levodopa affects functional brain networks in parkinsonian resting tremor. Mov Disord. 2009;24:91–8. doi: 10.1002/mds.22318. [DOI] [PubMed] [Google Scholar]
- Poston KL, Eidelberg D. Network biomarkers for the diagnosis and treatment of movement disorders. Neurobiol Dis. 2009;35:141–7. doi: 10.1016/j.nbd.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Purzner J, Paradiso GO, Cunic D, Saint-Cyr JA, Hoque T, Lozano AM, et al. Involvement of the basal ganglia and cerebellar motor pathways in the preparation of self-initiated and externally triggered movements in humans. J Neurosci. 2007;27:6029–36. doi: 10.1523/JNEUROSCI.5441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshy A, Kawashima R, Imran MB, Sugiura M, Goto R, Okada K, et al. Functional mapping of human brain in olfactory processing: a PET study. J Neurophysiol. 2000;84:1656–66. doi: 10.1152/jn.2000.84.3.1656. [DOI] [PubMed] [Google Scholar]
- Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. J Neurosci. 1997;17:5528–35. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, et al. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 1997;120:103–10. doi: 10.1093/brain/120.1.103. [DOI] [PubMed] [Google Scholar]
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med. 2000;342:1484–91. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Portin R, Ruottinen H, Nurmi E, Bergman J, Haaparanta M, Solin O. Cognitive impairment and the brain dopaminergic system in Parkinson’s disease. [18F]fluorodopa positron emission tomographic study. Arch Neurol. 2000;57:470–5. doi: 10.1001/archneur.57.4.470. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Marmor O, Heimer G, Raz A, Nini A, Bergman H. Basal ganglia oscillations and pathophysiology of movement disorders. Curr Opin Neurobiol. 2006;16:629–37. doi: 10.1016/j.conb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Robledo P, Feger J. Excitatory influence of rat subthalamic nucleus to substantia nigra pars reticulate and the pallidal complex: electrophysiological data. Brain Res. 1990;518:47–54. doi: 10.1016/0006-8993(90)90952-8. [DOI] [PubMed] [Google Scholar]
- Rolland AS, Herrero MT, Garcia-Martinez V, Ruberg M, Hirsch EC, François C. Metabolic activity of cerebellar and basal ganglia-thalamic neurons is reduced in parkinsonism. Brain. 2007;130:265–75. doi: 10.1093/brain/awl337. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, et al. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain. 2000;123:394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Gemba H, Hashimoto S, Mizuno N. Influences of cerebellar hemispherectomy on slow potentials in the motor cortex preceding self paced hand movements in the monkey. Neurosci Lett. 1979;15:23–8. doi: 10.1016/0304-3940(79)91523-4. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schell GR, Strick PL. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci. 1984;4:539–560. doi: 10.1523/JNEUROSCI.04-02-00539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfler C, Schocke MF, Seppi K, Esterhammer R, Brenneis C, Jaschke W, et al. Voxel-wise analysis of diffusion weighted imaging reveals disruption of the olfactory tract in Parkinson’s disease. Brain. 2006;129:538–42. doi: 10.1093/brain/awh674. [DOI] [PubMed] [Google Scholar]
- Schrock LE, Ostrem JL, Turner RS, Shimamoto SA, Starr PA. The subthalamic nucleus in primary dystonia: Single-unit discharge characteristics. J Neurophysiol. 2009;102:3740–52. doi: 10.1152/jn.00544.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweder PM, Hansen PC, Green AL, Quaghebeur G, Stein J, Aziz TZ. Connectivity of the pedunculopontine nucleus in parkinsonian freezing of gait. Neuroreport. 2010;21:914–6. doi: 10.1097/WNR.0b013e32833ce5f1. [DOI] [PubMed] [Google Scholar]
- Sen S, Kawaguchi A, Truong Y, Lewis MM, Huang X. Dynamic changes in cerebello-thalamo-cortical motor circuitry during progression of Parkinson's disease. Neuroscience. 2010;166:712–9. doi: 10.1016/j.neuroscience.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord. 2001;16:507–10. doi: 10.1002/mds.1099. [DOI] [PubMed] [Google Scholar]
- Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–57. doi: 10.1093/brain/awf148. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–87. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Solano SM, Miller DW, Augood SJ, Young AB, Penney JB., Jr Expression of alpha-synuclein, parkin, and ubiquitin carboxy-terminal hydrolase L1 mRNA in human brain: genes associated with familial Parkinson’s disease. Ann Neurol. 2000;47:201–10. [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. 2007;130:1596–607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Takada M, Sugimoto T, Hattori T. MPTP neurotoxicity to cerebellar purkinje cells in mice. Neurosci Lett. 1993;150:49–52. doi: 10.1016/0304-3940(93)90105-t. [DOI] [PubMed] [Google Scholar]
- Tan EK, Chandran VR, Fook-Chong S, Shen H, Yew K, Teoh ML, et al. Alpha-synuclein mRNA expression in sporadic Parkinson's disease. Mov Disord. 2005;20:620–3. doi: 10.1002/mds.20391. [DOI] [PubMed] [Google Scholar]
- Tang CC, Poston KL, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson’s disease. J Neurosci. 2010;30:1049–56. doi: 10.1523/JNEUROSCI.4188-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. Behavioral planning in the prefrontal cortex. Curr Opin Neurobiol. 2001;11:164–170. doi: 10.1016/s0959-4388(00)00192-6. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Butz M, Kircheis G, Haussinger D, Schnitzler A. Pathological oscillatory coupling within the human motor system in different tremor syndromes as revealed by magnetoencephalography. Neurol Clin Neurophysiol. 2004;2004:26. [PubMed] [Google Scholar]
- Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- Turner RS, Grafton ST, McIntosh AR, DeLong MR, Hoffman JM. The functional anatomy of parkinsonian bradykinesia. Neuroimage. 2003;19:163–179. doi: 10.1016/s1053-8119(03)00059-4. [DOI] [PubMed] [Google Scholar]
- Vignola C, Nechi D, Scherini E, Bernocchi G. MPTP induced changes in the monkey cerebellum. Immunohistochemistry of calcium binding and cytoskeletal proteins. Neurodegeneration. 1994;3:25–31. [Google Scholar]
- Wagle-Shukla A, Angel MJ, Zadikoff C, Enjati M, Gunraj C, Lang AE, et al. Low-frequency repetitive transcranial magnetic stimulation for treatment of levodopa-induced dyskinesias. Neurology. 2007;68:704–5. doi: 10.1212/01.wnl.0000256036.20927.a5. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133:1224–38. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–72. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Werheid K, Zysset S, Müller A, Reuter M, von Cramon DY. Rule learning in a serial reaction time task: an fMRI study on patients with early Parkinson's disease. Brain Res Cogn Brain Res. 2003;16:273–84. doi: 10.1016/s0926-6410(02)00283-5. [DOI] [PubMed] [Google Scholar]
- Westerlund M, Belin AC, Anvret A, Håkansson A, Nissbrandt H, Lind C, et al. Cerebellar α-synuclein levels are decreased in Parkinson’s disease and do not correlate with SNCA polymorphisms associated with disease in a Swedish material. FASEB J. 2008;22:3509–14. doi: 10.1096/fj.08-110148. [DOI] [PubMed] [Google Scholar]
- Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson’s disease. NeuroImage. 2010a;49:2581–7. doi: 10.1016/j.neuroimage.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128:2250–59. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79:760–6. doi: 10.1136/jnnp.2007.126599. [DOI] [PubMed] [Google Scholar]
- Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, Chan P. Regional homogeneity changes in patients with Parkinson’s disease. Hum Brain Mapp. 2009a;30:1502–10. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang L, Chen Y, Zhao C, Li K, Chan P. Changes of functional connectivity of the motor network in the resting state in Parkinson's disease. Neurosci Lett. 2009b;460:6–10. doi: 10.1016/j.neulet.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. NeuroImage. 2011;55:204–15. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain. 2010b;133:2394–409. doi: 10.1093/brain/awq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sadler TR, Givrad TK, Maarek JM, Holschneider DP. Changes in brain functional activation during resting and locomotor states after unilateral nigrostriatal damage in rats. Neuroimage. 2007;36:755–73. doi: 10.1016/j.neuroimage.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage. 2007;35:222–33. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in parkinsonism. Curr Opin Neurol. 2009;22:387–93. doi: 10.1097/WCO.0b013e32832d9d67. [DOI] [PubMed] [Google Scholar]
- Zhang K, Yu C, Zhang Y, Wu X, Zhu C, Chan P, Li K. Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson's disease. Eur J Radiol. 2011;77:269–73. doi: 10.1016/j.ejrad.2009.07.032. [DOI] [PubMed] [Google Scholar]