This cohort study compares the overall survival, disease progression, and other outcomes of stereotactic radiosurgery with those of whole-brain radiotherapy in patients with brain metastases from small cell lung cancer.
Key Points
Question
What outcomes are associated with stereotactic radiosurgery alone for small cell lung cancer brain metastases, and how do these results compare with the standard whole-brain radiotherapy approach?
Findings
In a cohort study of 710 patients with small cell lung cancer brain metastases who received first-line stereotactic radiosurgery, the median overall survival was 8.5 months, and the median time to central nervous system progression was 8.1 months. After controlling for multiple prognostic factors, whole-brain radiotherapy (vs stereotactic radiosurgery) was associated with superior time to central nervous system progression but offered no overall survival advantage.
Meaning
This study provides a benchmark for stereotactic radiosurgery outcomes and suggests that this treatment alone is a potential option for select patients with small cell lung cancer.
Abstract
Importance
Although stereotactic radiosurgery (SRS) is preferred for limited brain metastases from most histologies, whole-brain radiotherapy (WBRT) has remained the standard of care for patients with small cell lung cancer. Data on SRS are limited.
Objective
To characterize and compare first-line SRS outcomes (without prior WBRT or prophylactic cranial irradiation) with those of first-line WBRT.
Design, Setting, and Participants
FIRE-SCLC (First-line Radiosurgery for Small-Cell Lung Cancer) was a multicenter cohort study that analyzed SRS outcomes from 28 centers and a single-arm trial and compared these data with outcomes from a first-line WBRT cohort. Data were collected from October 26, 2017, to August 15, 2019, and analyzed from August 16, 2019, to November 6, 2019.
Interventions
SRS and WBRT for small cell lung cancer brain metastases.
Main Outcomes and Measures
Overall survival, time to central nervous system progression (TTCP), and central nervous system (CNS) progression-free survival (PFS) after SRS were evaluated and compared with WBRT outcomes, with adjustment for performance status, number of brain metastases, synchronicity, age, sex, and treatment year in multivariable and propensity score–matched analyses.
Results
In total, 710 patients (median [interquartile range] age, 68.5 [62-74] years; 531 men [74.8%]) who received SRS between 1994 and 2018 were analyzed. The median overall survival was 8.5 months, the median TTCP was 8.1 months, and the median CNS PFS was 5.0 months. When stratified by the number of brain metastases treated, the median overall survival was 11.0 months (95% CI, 8.9-13.4) for 1 lesion, 8.7 months (95% CI, 7.7-10.4) for 2 to 4 lesions, 8.0 months (95% CI, 6.4-9.6) for 5 to 10 lesions, and 5.5 months (95% CI, 4.3-7.6) for 11 or more lesions. Competing risk estimates were 7.0% (95% CI, 4.9%-9.2%) for local failures at 12 months and 41.6% (95% CI, 37.6%-45.7%) for distant CNS failures at 12 months. Leptomeningeal progression (46 of 425 patients [10.8%] with available data) and neurological mortality (80 of 647 patients [12.4%] with available data) were uncommon. On propensity score–matched analyses comparing SRS with WBRT, WBRT was associated with improved TTCP (hazard ratio, 0.38; 95% CI, 0.26-0.55; P < .001), without an improvement in overall survival (median, 6.5 months [95% CI, 5.5-8.0] for SRS vs 5.2 months [95% CI, 4.4-6.7] for WBRT; P = .003) or CNS PFS (median, 4.0 months for SRS vs 3.8 months for WBRT; P = .79). Multivariable analyses comparing SRS and WBRT, including subset analyses controlling for extracranial metastases and extracranial disease control status, demonstrated similar results.
Conclusions and Relevance
Results of this study suggest that the primary trade-offs associated with SRS without WBRT, including a shorter TTCP without a decrease in overall survival, are similar to those observed in settings in which SRS is already established.
Introduction
Stereotactic radiosurgery (SRS) has become a well-established first-line therapy for limited brain metastases after multiple phase 3 randomized clinical trials of SRS with and without whole-brain radiotherapy (WBRT) collectively demonstrated no overall survival advantage with the addition of WBRT to SRS despite the superior central nervous system (CNS) control observed with WBRT.1,2,3,4,5 The absence of an overall survival advantage to justify the toxic effects of WBRT on cognitive function and quality of life1,4,6 made SRS alone the preferred treatment for limited brain metastases in most settings.7 However, patients with small cell lung cancer were excluded from the landmark randomized clinical trials2,3,4,5,8 that established SRS alone as a first-line strategy, making small cell lung cancer an exception where WBRT has remained the standard of care for limited and even solitary brain metastases.9 Historical reservations regarding SRS alone in small cell lung cancer have included concerns for short interval CNS progression that could potentially lead to a decrease in overall survival with WBRT omission, as well as the paucity of data on first-line SRS in this setting.10
In recent years, interest in the potential role of SRS in small cell lung cancer has been growing because of multiple factors, including the expanded use of surveillance brain magnetic resonance imaging (MRI), evolving controversies surrounding prophylactic cranial irradiation (PCI), integration of immunotherapy into management, and improvements in prognosis.9,11,12,13,14 These developments are expected to increase the identification of patients with small cell lung cancer and limited brain metastases who may be candidates for first-line SRS and to magnify survivorship considerations such as the long-term cognitive and quality-of-life advantages of avoiding early WBRT administration.15 In this context, a number of smaller studies, which have typically included a mix of both salvage and first-line SRS, and population-based analyses have been reported,16,17,18,19 and several clinical trials have been launched.20,21 Currently, however, the role of first-line SRS in contemporary small cell lung cancer management remains unclear.
This cohort study, First-line Radiosurgery for Small-Cell Lung Cancer (FIRE-SCLC), was a multicenter retrospective analysis of patients with small cell lung cancer brain metastases who were treated with SRS without prior PCI or WBRT. The analysis included a comparison of SRS outcomes with the outcomes from a cohort of patients treated with first-line WBRT. We hypothesized that SRS alone could deliver acceptable outcomes in clinical end points, such as overall survival, CNS control, and neurologic-specific mortality, and that the potential advantages in CNS control associated with WBRT would not translate into a decrease in overall survival with SRS alone, similar to other settings in which SRS is already well-established.1
Methods
The FIRE-SCLC retrospective cohort study included data from 28 centers in 6 countries (Japan, the US, Canada, Taiwan, Germany, and Switzerland) and 98 patients with small cell lung cancer who participated in the JLGK0901 prospective single-arm trial of SRS for 1 to 10 brain metastases from mixed histologies.22 The 28 participating centers obtained approval for the study from their respective institutional review boards, which granted informed consent exemptions because of the minimal risk of harm associated with analysis of deidentified data sets. The study was registered through the International Radiosurgery Research Foundation. Data for this study were collected from October 26, 2017, to August 15, 2019.
The primary analysis included patients with biopsy-confirmed small cell lung cancer who were treated with first-line SRS (without prior PCI or WBRT) for brain metastases. Stereotactic radiosurgery was described according to a consensus definition,23 and all platforms of SRS delivery were acceptable. After undergoing SRS, patients underwent follow up with clinical and radiographic surveillance per institutional standards. Data were collected on treatment center, treatment year, age, sex, Karnofsky Performance Status (KPS) score, presence of brain metastases at diagnosis, number of brain metastases treated, time until first CNS progression after SRS, type of first CNS progression (local, distant, or both), leptomeningeal progression, salvage therapy for CNS progression, vital status at reporting, neurological mortality, and duration of follow-up.
The primary objective of the analysis was to describe the clinical outcomes associated with first-line SRS without prior PCI or WBRT. Overall survival was defined as time from SRS to death and estimated using the Kaplan-Meier method, and comparisons were made using the log-rank test; the hazard ratio (HR) was modeled using Cox proportional hazards regression models. Time to CNS progression (TTCP) was defined as time from SRS to first CNS progression. Death was treated as a competing risk for TTCP; the cumulative incidence was examined, and the HR was estimated using the Fine-Gray method. Multivariable analyses of overall survival and TTCP were adjusted for treatment year (continuous), age (continuous), sex, region (Asia vs North America and Europe), KPS score (≤60, 70-80, 90-100),24 brain metastases at diagnosis (synchronous vs metachronous), and number of brain metastases (continuous). Subset analyses were performed with stratification by number of brain metastases (1, 2-4, 5-10, and ≥11 lesions), with established clinical relevance for SRS in other settings.7,22 Central nervous system control data were not available beyond the first CNS progression; cumulative incidence of local control and of distant CNS failures were estimated at 6 and 12 months; deaths or alternate forms of CNS progression (eg, distant failure in the setting of ongoing local control) were considered as competing risks. Local and distant failures were also calculated using the Kaplan-Meier method, with censoring of deaths and alternate forms of CNS progression. Central nervous system control outcomes were calculated on a per patient basis. Total rates of leptomeningeal progression, neurological mortality, and salvage therapies for brain metastases after SRS were described.
To compare SRS outcomes with contemporary WBRT outcomes, we acquired individual patient data from a large published data set of first-line WBRT for small cell lung cancer.25 The data elements in the WBRT cohort were the same as those in the SRS cohort, with the exception of unavailable data on CNS progression type (ie, local, distant, or both), leptomeningeal progression, salvage therapy, and neurological mortality; data on the number of brain metastases included 1, 2 to 4, and 5 or more.
The secondary objective of the analysis was to compare the SRS and WBRT cohort outcomes for overall survival, TTCP, and the composite end point of CNS progression-free survival (PFS). The CNS PFS was defined as time from SRS to either death or CNS progression and estimated using the Kaplan-Meier method. Multivariable comparisons were adjusted for KPS score, number of brain metastases, synchronicity, age, sex, and treatment year. Subset analyses were performed in patients with data on extracranial metastases outside of the thoracic tumor and regional lymph nodes (present vs absent) and extracranial disease control status (controlled [stable or responding] vs uncontrolled [progressive disease or treatment naive]).26 Because no differences in adjusted overall survival, TTCP, or CNS PFS were observed by region in the SRS cohort and all patients who received WBRT were treated in Germany, region was not included in the primary models comparing SRS and WBRT. Sensitivity analyses, including region in the multivariable models, returned similar results. Differences in overall survival, TTCP, and CNS PFS were also evaluated using propensity score–matched analyses (eMethods in the Supplement) accounting for KPS score, number of brain metastases, synchronicity, age, sex, and treatment year. In addition, semi–competing risk models were used to model the hazard of CNS progression, death without CNS progression, and death after a CNS progression event (eTable 6 in the supplement).27
All hypothesis tests were 2-sided and a P < .05 was considered statistically significant. Because of the hypothesis-generating nature of the comparative analyses, no corrections were made for multiple comparisons.28,29 All analyses were performed in R, version 3.5.2 (R Foundation for Statistical Computing), and SAS, version 9.4 (SAS Institute Inc), by the University of Colorado Cancer Center Biostatistics Core. Data analysis was conducted from August 16, 2019, to November 6, 2019.
Results
SRS Cohort
Data were collected on 710 patients with small cell lung cancer brain metastases who were treated with first-line SRS without prior PCI or WBRT (Table 1). Patients were treated between 1994 and 2018, with 621 (87.5%) receiving treatment in the year 2000 or later (median [interquartile range (IQR)] treatment year, 2011 [2004-2014]). The median (IQR) patient age was 68.5 (62-74) years, and most patients were men (531 [74.8%]) and had a good performance status (437 [61.5%] had a KPS score ≥90). The median (IQR) number of brain metastases treated was 2.5 (1-6), and 540 (76.1%) were treated in Asia and 170 (23.9%) in North America and Europe. A complete list of participating centers is in eTable 1 in the Supplement.
Table 1. Patient Characteristics for the SRS Cohort.
| Variable | No. (%) |
|---|---|
| No. | 710 |
| Age, median (IQR) | 68.5 (62-74) |
| Sex | |
| Men | 531 (74.8) |
| Women | 179 (25.2) |
| KPS score | |
| 90-100 | 437 (61.5) |
| 70-80 | 222 (31.3) |
| ≤60 | 51 (7.2) |
| No. of brain metastases treated | |
| Median (IQR) | 2.5 (1-6) |
| 1 | 232 (32.7) |
| 2-4 | 251 (35.4) |
| 5-10 | 137 (19.3) |
| ≥11 | 90 (12.7) |
| Brain metastasis at diagnosis | |
| No | 472 (66.5) |
| Yes | 238 (33.5) |
| Region | |
| Asia | 540 (76.1) |
| North America and Europe | 170 (23.9) |
| Extracranial metastasesa,b | |
| Absent | 328 (56.1) |
| Present | 257 (43.9) |
| Unreported | 125 |
| Extracranial disease statusa | |
| Controlled | 191 (44.5) |
| Uncontrolled | 238 (55.5) |
| Unreported | 281 |
| Leptomeningeal progression after SRS | |
| No | 379 (89.2) |
| Yes | 46 (10.8) |
| Unreported | 285 |
| Salvage WBRT after SRS | |
| No | 596 (83.9) |
| Yes | 114 (16.1) |
| Salvage SRS after upfront SRS | |
| No | 472 (66.5) |
| Yes | 238 (33.5) |
| Neurological mortality | |
| No | 567 (87.6) |
| Possible or likely | 80 (12.4) |
| Unreported | 63 |
| Vital status at reporting | |
| Alive | 114 (16.1) |
| Deceased | 596 (83.9) |
| Treatment year, median (IQR) | 2011 (2004-2014) |
Abbreviations: IQR, interquartile range; KPS, Karnofsky Performance Status; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Subset analyses limited to patients with known status for extracranial metastases and extracranial disease control status are presented in the Results and eTables 7 and 8 in the Supplement.
As noted in the Methods section, extracranial metastases refers to extracranial disease outside of the primary thoracic tumor and regional lymph nodes.
At the time of analysis, 596 patients (83.9%) were deceased. The median overall survival after SRS was 8.5 months (95% CI, 7.9-9.5) (Figure 1A). When stratified by the number of brain metastases treated, the median overall survival was 11.0 months (95% CI, 8.9-13.4) for 1 lesion, 8.7 months (95% CI, 7.7-10.4) for 2 to 4 lesions, 8.0 months (95% CI, 6.4-9.6) for 5 to 10 lesions, and 5.5 months (95% CI, 4.3-7.6) for 11 or more lesions (P < .001) (Figure 1C). No significant differences in overall survival were observed after SRS for 2 to 4 vs 5 to 10 brain metastases (log-rank P = .30). As shown in Table 2, factors significantly associated with superior overall survival on multivariable analyses included better KPS scores, fewer brain metastases (continuous), synchronous brain metastases, younger age, female sex, and a more recent year of treatment. No significant differences in multivariable adjusted overall survival were observed by region (Asia vs North America and Europe).
Figure 1. Overall Survival (OS) and Time to Central Nervous System Progression (TTCP) After First-line Stereotactic Radiosurgery.

CNS indicates central nervous system. The dashed lines indicate the median values.
Table 2. Univariable and Multivariable Analyses of Overall Survival in the SRS Cohort .
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI)a | P value | HR (95% CI)a | P value | |
| Age, continuous | 1.02 (1.01-1.03) | <.001 | 1.01 (1.00-1.02) | .02 |
| Sex | ||||
| Men | 1 [Reference] | 1 [Reference] | ||
| Women | 0.67 (0.56-0.82) | <.001 | 0.77 (0.63-0.94) | .01 |
| KPS score | ||||
| 90-100 | 1 [Reference] | 1 [Reference] | ||
| 70-80 | 1.67 (1.40-1.99) | <.001 | 1.69 (1.40-2.03) | <.001 |
| ≤60 | 1.85 (1.36-2.51) | <.001 | 1.89 (1.38-2.60) | <.001 |
| No. of brain metastases treated, continuous | 1.04 (1.03-1.05) | <.001 | 1.03 (1.02-1.05) | <.001 |
| Brain metastasis at diagnosis | ||||
| No | 1 [Reference] | 1 [Reference] | ||
| Yes | 0.74 (0.63-0.89) | <.001 | 0.78 (0.65-0.94) | .008 |
| Region | ||||
| North America and Europe | 1 [Reference] | 1 [Reference] | ||
| Asia | 1.30 (1.06-1.59) | .01 | 1.15 (0.92-1.44) | .22 |
| Treatment year, continuous | 0.97 (0.96-0.98) | <.001 | 0.98 (0.97-1.00) | .02 |
Abbreviations: HR, hazard ratio; KPS, Karnofsky Performance Status; SRS, stereotactic radiosurgery.
Hazard for death was modeled using univariable and multivariable Cox proportional hazards regression models.
We performed TTCP analyses for 456 patients with clinical follow-up data on CNS control. Of these patients, 406 (89.0%) had at least 1 follow-up brain MRI after SRS. The median TTCP was 8.1 months (95% CI, 7.1-9.4) (Figure 1B). When stratified by the number of brain metastases treated, the median TTCPs were 11.7 months (95% CI, 8.8-not reached) for 1 lesion, 6.8 months (95% CI, 5.7-8.3) for 2 to 4 lesions, 6.1 months (95% CI, 4.9-7.7) for 5 to 10 lesions, and 4.7 months (95% CI, 3.2-not reached) for 11 or more lesions (P < .001) (Figure 1D). On multivariable analysis, the number of brain metastases (continuous) was the only factor associated with TTCP (eTable 2 in the Supplement). Competing risk estimates for local failure were 4.1% (95% CI, 2.5%-5.7%) at 6 months and 7.0% (95% CI, 4.9%-9.2%) at 12 months, and distant CNS failure estimates were 28.0% (95% CI, 24.4%-31.7%) at 6 months and 41.6% (95% CI, 37.6%-45.7%) at 12 months. Kaplan-Meier estimates for local failure and distant failure are included in eTable 3 in the Supplement. The radiation necrosis rate (any grade) was 5.0%, and no treatment-related deaths were reported. After first-line SRS, 238 of 710 patients (33.5%) subsequently underwent salvage SRS and 114 patients (16.1%) underwent salvage WBRT. In patients with available data, leptomeningeal progression was reported in 46 of 425 patients (10.8%), and neurological mortality was considered possible or likely in 80 of 647 patients (12.4%).
Comparison of the SRS and WBRT Cohorts
In the WBRT data set, 219 patients were evaluable for overall survival and CNS control. Patients were treated with WBRT between 2003 and 2015 (median [IQR] treatment year, 2012 [2010-2014]). At the time of analysis, 206 patients (94.1%) were deceased and 101 patients (46.1%) had at least 1 follow-up brain MRI after WBRT. Baseline differences were found between the WBRT and SRS cohorts, with patients in the WBRT cohort displaying worse KPS score (≤60 score: 30.1% vs 7.2%), more brain metastases (≥5 metastases: 58.9% vs 32.0%), younger age (median [IQR] age: 62 [56.5-70.0] years vs 68.5 [62.0-74.0] years), more women (41.1% vs 25.2%), and treatment in more recent years (median [IQR] year: 2012 [2010-2014] vs 2011 [2004-2014]) (Table 3).
Table 3. Unmatched and Propensity Score–Matched Data Sets for Overall Survival Analysesa.
| Variable | Unmatched | Propensity score–matched | ||||
|---|---|---|---|---|---|---|
| No. (%) | P value | No. (%) | P value | |||
| SRS cohort (n = 710) | WBRT cohort (n = 219) | SRS cohort (n = 187) | WBRT cohort (n = 187) | |||
| Age, median (IQR), y | 68.5 (62.0-74.0) | 62.0 (56.5-70.0) | <.001 | 65.0 (57.5-70.5) | 63.0 (56.5-71.0) | .49 |
| Sex | ||||||
| Men | 531 (74.8) | 129 (58.9) | <.001 | 121 (64.7) | 114 (61.0) | .52 |
| Women | 179 (25.2) | 90 (41.1) | 66 (35.3) | 73 (39.0) | ||
| KPS score | ||||||
| 90-100 | 437 (61.5) | 41 (18.7) | <.001 | 50 (26.7) | 41 (21.9) | .53 |
| 70-80 | 222 (31.3) | 112 (51.1) | 96 (51.3) | 100 (53.5) | ||
| ≤60 | 51 (7.2) | 66 (30.1) | 41 (21.9) | 46 (24.6) | ||
| No. of brain metastases treated | ||||||
| 1 | 232 (32.7) | 47 (21.5) | <.001 | 53 (28.3) | 47 (25.1) | .73 |
| 2-4 | 251 (35.4) | 43 (19.6) | 43 (23.0) | 42 (22.5) | ||
| ≥5 | 227 (32.0) | 129 (58.9) | 91 (48.7) | 98 (52.4) | ||
| Brain metastasis at diagnosis | ||||||
| No | 472 (66.5) | 148 (67.6) | .83 | 119 (63.6) | 131 (70.1) | .23 |
| Yes | 238 (33.5) | 71 (32.4) | 68 (36.4) | 56 (29.9) | ||
| Treatment year, median (IQR) | 2011 (2004-2014) | 2012 (2010-2014) | <.001 | 2012 (2009-2016) | 2012 (2010-2014) | .11 |
Abbreviations: IQR, interquartile range; KPS, Karnofsky Performance Status; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
χ2 tests were used to evaluate the balance between treatment groups for categorical variables, and the Wilcoxon rank-sum test was used for continuous variables.
On unadjusted analysis, a significant difference in overall survival was found between the 2 cohorts, in favor of SRS vs WBRT (median, 8.5 months [95% CI, 7.9-9.5] vs 5.2 months [95% CI, 4.2-6.7]; P < .001) (Figure 2A), which persisted after multivariable adjustment (HR, 1.48 [95% CI, 1.21-1.80]; P < .001). On unadjusted analysis of TTCP, a significant difference was observed in favor of WBRT (median, 8.1 months (95% CI, 7.1-9.4) for SRS vs not reached for WBRT; P < .001) (Figure 2B), which persisted after multivariable adjustment (HR, 0.38 [95% CI, 0.28-0.52]; P < .001). On analyses of CNS PFS, unadjusted comparisons favored SRS vs WBRT (median, 5.0 months vs 3.8 months; P = .03) (eFigure 1A in the Supplement), but no differences were observed after multivariable adjustment (HR, 0.91 [95% CI, 0.74-1.11]; P = .35).
Figure 2. Overall Survival (OS) and Time to Central Nervous System Progression (TTCP) After First-line Stereotactic Radiosurgery (SRS) vs Whole-Brain Radiotherapy (WBRT).
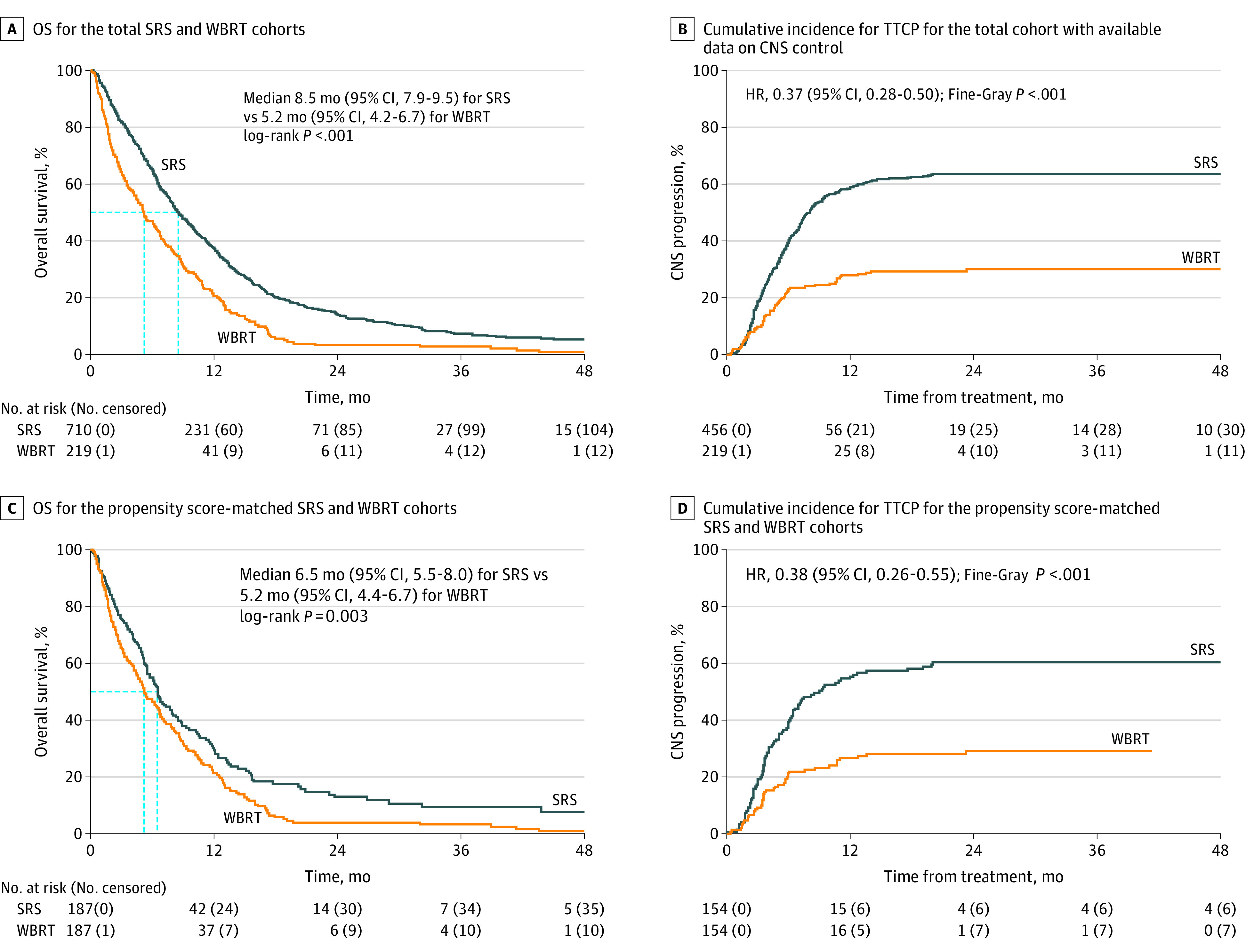
CNS indicates central nervous system. The dashed lines indicate the median values.
After propensity score matching, 187 patients in the SRS cohort and 187 patients in the WBRT cohort with balanced KPS scores, number of brain metastases, synchronicity, age, sex, and treatment year were analyzed for overall survival (Table 3). Overall survival outcomes in the matched data set were more similar than in the unmatched analyses but remained in favor of SRS vs WBRT (median, 6.5 months [95% CI, 5.5-8.0] vs 5.2 months [95% CI, 4.4-6.7]; P = .003) (Figure 2C). In the matched data set evaluating CNS control (eTable 4 in the Supplement), TTCP was improved with WBRT (median, 9.0 months [95% CI, 6.5-17.6] for SRS vs not reached for WBRT; HR, 0.38; 95% CI, 0.26-0.55; P < .001) (Figure 2D), whereas no differences were observed in CNS PFS (median, 4.0 months for SRS vs 3.8 months for WBRT; P = .79) (eFigure 1B in the Supplement).
Among the 524 patients (331 in the SRS cohort, and 193 in the WBRT cohort) with data on both extracranial metastases and extracranial disease control, multivariable analyses controlling for these factors found results similar to those of the overall analyses. Compared with WBRT, SRS was associated with favorable overall survival (HR, 1.94; 95% CI, 1.48-2.53; P < .001) and an inferior TTCP (HR, 0.33; 95% CI, 0.24-0.47; P < .001). Subset analyses by extracranial metastases and extracranial disease control status are displayed in eTables 7 and 8 in the Supplement.
On subset analyses, no significant differences in the association of treatment modality (SRS or WBRT) with overall survival were observed by age, sex, or number of brain metastases. Significant interactions between treatment modality and the covariables of KPS score, brain metastases at diagnosis, and treatment year were found, which suggested inferior outcomes after WBRT among patients with a KPS score of 60 or lower, synchronous brain metastases, and more recent years of treatment (all interaction P < .05) (eFigure 2A in the Supplement). Conversely, no significant interactions were observed between treatment modality and baseline patient characteristics for the end point of TTCP (eFigure 2B in the Supplement).
Discussion
The FIRE-SCLC is a multicenter analysis of outcomes of 710 patients with small cell lung cancer brain metastases treated with first-line SRS. After SRS delivery, encouraging outcomes were observed, with a median overall survival of 8.5 months and TTCP of 8.1 months. The results were particularly impressive among patients with a single brain metastasis, with 11.0 months for median overall survival and 11.7 months for median TTCP. Concerning events that might be attributed to WBRT omission, such as neurological mortality (12.4%) and leptomeningeal progression (10.8%), were uncommon and their rates were not increased over those reported in other series of small cell lung cancer.19,30 Local failures after SRS were rare, with most of CNS progression occurring in the form of new lesions, similar to SRS in other settings.2,3,5,22 Among patients who received salvage therapy for CNS progression, 33.5% received salvage SRS and only 16.1% received salvage WBRT. Both overall survival and TTCP declined with continuous increases in brain metastases. However, similar to the JLGK0901 study,22 the present study observed no significant differences in overall survival or TTCP after SRS between patients with 2 to 4 lesions and those with 5 to 10 lesions.
In addition, we compared the SRS outcomes with individual patient data from a large cohort who received first-line WBRT. The median overall survival of 5.2 months in the WBRT cohort was similar to estimates from other large retrospective studies and prospective data of first-line WBRT.31,32 In this data set, TTCP was improved with WBRT (HR after multivariable adjustment, 0.38), which appears comparable to the CNS control advantages observed with WBRT for other histologies.1 However, similar to WBRT in other settings, the CNS control advantage with WBRT did not translate into an improvement in survival, as the observed overall survival outcomes remained in favor of SRS after adjustment for available prognostic factors. It is important to acknowledge the baseline differences between the SRS and WBRT treatment groups, including an increased number of brain metastases and the inferior KPS scores in the WBRT cohort (Table 3), as well as the observation that the overall survival outcomes became more similar after adjustment for baseline factors (median overall survival of 6.5 vs 5.2 months after propensity score matching). Although these retrospective data should not be used to conclude that overall survival is superior with SRS, the findings of this study suggest that the primary trade-offs associated with SRS without WBRT, including a shorter TTCP, are similar to other settings in which SRS alone is well established by multiple randomized clinical trials.1
Before this analysis, a number of smaller studies have reported outcomes after first-line SRS (eTable 9 in the Supplement).16,17,19,33,34,35,36,37,38 Serizawa et al18 compared first-line SRS in non–small cell lung cancer, where SRS is an established paradigm, with small cell lung cancer (n = 34). No statistically significant differences were observed in any end point between small cell lung cancer and non–small cell lung cancer, and the outcomes in the small cell lung cancer cohort were comparable to results of the present analysis (median overall survival, 9.1 months; 12-month local control, 94.5%; median time to new brain lesions, 6.9 months; and 12-month neurological mortality free survival, 86.5%). In a recent analysis of 293 patients with small cell lung cancer treated with SRS, the median overall survival was 7.5 months for 61 patients who received first-line SRS.33 Using the National Cancer Databases, Robin et al17 reported superior overall survival with SRS compared with WBRT (propensity score–matched overall survival, 10.9 months vs 7.6 months; P < .001). We included 98 patients with small cell lung cancer who participated in the prospective JLGK0901 study,22 and they demonstrated median overall survival (8.6 months) and TTCP (7.2 months) outcomes that were similar to the overall SRS cohort. Given the higher number of patients who received first-line SRS in Japan in this study, it is an important finding that no differences in adjusted overall survival or TTCP were observed by region in this analysis.
Strengths and Limitations
This study has several strengths, including the unprecedented size of the SRS cohort, participation from centers in 6 countries, a contemporary WBRT cohort with individual patient data for comparative analyses, and various analytical methods. These methods included competing risk modeling, semi–competing risk modeling, multivariable adjustment, and propensity score matching to control for multiple prognostic factors.
This study has several limitations as well. Given the retrospective design, all analyses were subject to confounding because of unquantified variables. Systemic therapy was not controlled for in this analysis, which has prognostic implications for both overall survival and CNS disease control in small cell lung cancer.10 To this end, the improved overall survival observed with synchronous brain metastases in this study could represent a surrogate for less systemic therapy exposure and the measurement of overall survival from an earlier point in the disease course. Volumetric assessments of CNS disease burden, cognitive function, and quality of life were unavailable for analysis. Patients in the WBRT cohort were treated at a large academic center in Germany and, although the overall survival was similar in this cohort to other large case series and prospective data,31,32 their outcomes may not be globally generalizable. Surveillance was dependent on institutional practices, and documented follow-up MRIs were more common in the SRS than in the WBRT cohort. The less frequent imaging after WBRT may reflect contemporaneous standards and practice patterns,39 but these differences could have increased the apparent CNS control advantage associated with WBRT. The absence of control data beyond the first CNS progression increases the uncertainty associated with local and distant control estimates. Beyond the number of lesions treated, we did not collect granular data on SRS dose and volume for technical analyses. Although it is reassuring that most patients treated with salvage radiation received salvage SRS rather than WBRT, the extent of CNS disease at recurrence was not characterized. In addition, analyses should be interpreted in the context of multiple comparisons without statistical adjustment, underscoring the importance of confirming the observed associations in future studies.28,29
Conclusions
In this large multicenter analysis, we believe that the outcomes of SRS for small cell lung cancer were encouraging overall and were particularly impressive for patients with a single brain metastasis. In addition, the trade-offs inherent to a first-line SRS approach without WBRT, including a shorter time to CNS progression without an associated decrease in overall survival, appear to be similar to those in other settings in which SRS is already well established. We believe that these data provide a benchmark for SRS outcomes and offer support to first-line SRS as a treatment option in carefully selected patients with small cell lung cancer.
eTable 1. List of Participating Institutions
eTable 2. Univariable and Multivariable Analysis of TTCP in the First-Line SRS Cohort
eTable 3. Cumulative Incidence of Local and Distant CNS Failures After SRS
eTable 4. Propensity Score-Matched Datasets for TTCP and CNS PFS Analyses Comparing SRS and WBRT
eTable 5. Univariable and Multivariable Analysis of CNS PFS in First-Line SRS Cohort
eTable 6. Semi-Competing Risk Analysis
eTable 7. OS Subset Analyses in Patients With Data for Both Extracranial Metastases and Extracranial Disease Control Status
eTable 8. TTCP Subset Analyses in Patients With Data for Both Extracranial Metastases and Extracranial Disease Control Status
eTable 9. Published Series Including First-Line SRS for SCLC Without Prior PCI or WBRT
eFigure 1. CNS Progression-Free Survival (PFS)
eFigure 2. Forest Plots Comparing First-Line SRS and WBRT Within Patient Subsets
eMethods.
References
- 1.Tsao MN, Xu W, Wong RK, et al. . Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2018;1:CD003869. doi: 10.1002/14651858.CD003869.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kocher M, Soffietti R, Abacioglu U, et al. . Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141. doi: 10.1200/JCO.2010.30.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang EL, Wefel JS, Hess KR, et al. . Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044. doi: 10.1016/S1470-2045(09)70263-3 [DOI] [PubMed] [Google Scholar]
- 4.Brown PD, Jaeckle K, Ballman KV, et al. . Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401-409. doi: 10.1001/jama.2016.9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoyama H, Shirato H, Tago M, et al. . Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491. doi: 10.1001/jama.295.21.2483 [DOI] [PubMed] [Google Scholar]
- 6.Soffietti R, Kocher M, Abacioglu UM, et al. . A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65-72. doi: 10.1200/JCO.2011.41.0639 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Central nervous system cancers. Version 3.2019. Accessed November 24, 2019. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
- 8.Brown PD, Ballman KV, Cerhan JH, et al. . Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049-1060. doi: 10.1016/S1470-2045(17)30441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network Small cell lung cancer. Version 2.2020. Accessed November 24, 2019. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- 10.Lukas RV, Gondi V, Kamson DO, Kumthekar P, Salgia R. State-of-the-art considerations in small cell lung cancer brain metastases. Oncotarget. 2017;8(41):71223-71233. doi: 10.18632/oncotarget.19333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn L, Mansfield AS, Szczęsna A, et al. ; IMpower133 Study Group . First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-2229. doi: 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 12.Paz-Ares L, Dvorkin M, Chen Y, et al. ; CASPIAN investigators . Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939. doi: 10.1016/S0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 13.Rusthoven CG. Small cell lung cancer: PCI uncertainty and emerging radiosurgery interest. Int J Radiat Oncol Biol Phys. 2019;103(5):1034-1035. doi: 10.1016/j.ijrobp.2018.12.036 [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Yamanaka T, Seto T, et al. . Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(5):663-671. doi: 10.1016/S1470-2045(17)30230-9 [DOI] [PubMed] [Google Scholar]
- 15.Li J, Brown PD. The diminishing role of whole-brain radiation therapy in the treatment of brain metastases. JAMA Oncol. 2017;3(8):1023-1024. doi: 10.1001/jamaoncol.2016.5411 [DOI] [PubMed] [Google Scholar]
- 16.Faramand A, Niranjan A, Kano H, Flickinger J, Lunsford LD. Primary or salvage stereotactic radiosurgery for brain metastatic small cell lung cancer. J Neurooncol. 2019;144(1):217-225. doi: 10.1007/s11060-019-03224-w [DOI] [PubMed] [Google Scholar]
- 17.Robin TP, Jones BL, Amini A, et al. . Radiosurgery alone is associated with favorable outcomes for brain metastases from small-cell lung cancer. Lung Cancer. 2018;120:88-90. doi: 10.1016/j.lungcan.2018.03.027 [DOI] [PubMed] [Google Scholar]
- 18.Serizawa T, Ono J, Iichi T, et al. . Gamma knife radiosurgery for metastatic brain tumors from lung cancer: a comparison between small cell and non-small cell carcinoma. J Neurosurg. 2002;97(5)(suppl):484-488. doi: 10.3171/jns.2002.97.supplement_5.0484 [DOI] [PubMed] [Google Scholar]
- 19.Yomo S, Hayashi M. Is stereotactic radiosurgery a rational treatment option for brain metastases from small cell lung cancer? A retrospective analysis of 70 consecutive patients. BMC Cancer. 2015;15(1):95. doi: 10.1186/s12885-015-1103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stereotactic radiation in patients with small cell lung cancer and 1-6 brain metastases. ClinicalTrials.gov identifier: NCT03391362. Updated November 19, 2019. Accessed November 24, 2019. https://clinicaltrials.gov/ct2/show/NCT03391362
- 21.Whole brain radiation therapy alone vs. radiosurgery for SCLC patients with 1-10 brain metastases (ENCEPHALON). ClinicalTrials.gov identifier: NCT03297788. Updated January 9, 2020. Accessed November 24, 2019. https://clinicaltrials.gov/ct2/show/NCT03297788
- 22.Yamamoto M, Serizawa T, Shuto T, et al. . Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387-395. doi: 10.1016/S1470-2045(14)70061-0 [DOI] [PubMed] [Google Scholar]
- 23.Barnett GH, Linskey ME, Adler JR, et al. ; American Association of Neurological Surgeons; Congress of Neurological Surgeons Washington Committee Stereotactic Radiosurgery Task Force . Stereotactic radiosurgery–an organized neurosurgery-sanctioned definition. J Neurosurg. 2007;106(1):1-5. doi: 10.3171/jns.2007.106.1.1 [DOI] [PubMed] [Google Scholar]
- 24.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2(3):187-193. doi: 10.1200/JCO.1984.2.3.187 [DOI] [PubMed] [Google Scholar]
- 25.Bernhardt D, Adeberg S, Bozorgmehr F, et al. . Outcome and prognostic factors in patients with brain metastases from small-cell lung cancer treated with whole brain radiotherapy. J Neurooncol. 2017;134(1):205-212. doi: 10.1007/s11060-017-2510-0 [DOI] [PubMed] [Google Scholar]
- 26.Bernhardt D, König L, Aufderstrasse S, et al. . Generation of a new disease-specific prognostic score for patients with brain metastases from small-cell lung cancer treated with whole brain radiotherapy (BMS-Score) and validation of two other indices. Clin Lung Cancer. 2018;19(4):340-345. doi: 10.1016/j.cllc.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 27.Haneuse S, Lee KH. Semi-competing risks data analysis: accounting for death as a competing risk when the outcome of interest is nonterminal. Circ Cardiovasc Qual Outcomes. 2016;9(3):322-331. doi: 10.1161/CIRCOUTCOMES.115.001841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43-46. doi: 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 29.Wasserstein RL, Lazar NA. The ASA statement on P values: context, process, and purpose. Am Stat. 2016;70(2):129-133. doi: 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- 30.Seute T, Leffers P, ten Velde GP, Twijnstra A. Leptomeningeal metastases from small cell lung carcinoma. Cancer. 2005;104(8):1700-1705. doi: 10.1002/cncr.21322 [DOI] [PubMed] [Google Scholar]
- 31.Postmus PE, Haaxma-Reiche H, Gregor A, et al. . Brain-only metastases of small cell lung cancer; efficacy of whole brain radiotherapy. An EORTC phase II study. Radiother Oncol. 1998;46(1):29-32. doi: 10.1016/S0167-8140(97)00149-7 [DOI] [PubMed] [Google Scholar]
- 32.Sperduto PW, Kased N, Roberge D, et al. . Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419-425. doi: 10.1200/JCO.2011.38.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cifarelli CP, Vargo JA, Fang W, et al. . Role of gamma knife radiosurgery in small cell lung cancer: a multi-institutional retrospective study of the International Radiosurgery Research Foundation (IRRF). Neurosurgery. 2019;nyz428. doi: 10.1093/neuros/nyz428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cordeiro D, Xu Z, Shepard M, Sheehan D, Li C, Sheehan J. Gamma knife radiosurgery for brain metastases from small-cell lung cancer: institutional experience over more than a decade and review of the literature. J Radiosurg SBRT. 2019;6(1):35-43. [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang W, Haque W, Verma V, Butler B, Teh BS. Stereotactic radiosurgery for brain metastases from newly diagnosed small cell lung cancer: practice patterns and outcomes. Acta Oncol. 2019;58(4):491-498. doi: 10.1080/0284186X.2018.1562207 [DOI] [PubMed] [Google Scholar]
- 36.Jo KW, Kong DS, Lim DH, Ahn YC, Nam DH, Lee JI. The role of radiosurgery in patients with brain metastasis from small cell lung carcinoma. J Korean Neurosurg Soc. 2011;50(2):99-102. doi: 10.3340/jkns.2011.50.2.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wegner RE, Olson AC, Kondziolka D, Niranjan A, Lundsford LD, Flickinger JC. Stereotactic radiosurgery for patients with brain metastases from small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(3):e21-e27. doi: 10.1016/j.ijrobp.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 38.Yomo S, Hayashi M. Upfront stereotactic radiosurgery in patients with brain metastases from small cell lung cancer: retrospective analysis of 41 patients. Radiat Oncol. 2014;9(1):152. doi: 10.1186/1748-717X-9-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalemkerian GP, Akerley W, Bogner P, et al. ; National Comprehensive Cancer Network . Small cell lung cancer. J Natl Compr Canc Netw. 2013;11(1):78-98. doi: 10.6004/jnccn.2013.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. List of Participating Institutions
eTable 2. Univariable and Multivariable Analysis of TTCP in the First-Line SRS Cohort
eTable 3. Cumulative Incidence of Local and Distant CNS Failures After SRS
eTable 4. Propensity Score-Matched Datasets for TTCP and CNS PFS Analyses Comparing SRS and WBRT
eTable 5. Univariable and Multivariable Analysis of CNS PFS in First-Line SRS Cohort
eTable 6. Semi-Competing Risk Analysis
eTable 7. OS Subset Analyses in Patients With Data for Both Extracranial Metastases and Extracranial Disease Control Status
eTable 8. TTCP Subset Analyses in Patients With Data for Both Extracranial Metastases and Extracranial Disease Control Status
eTable 9. Published Series Including First-Line SRS for SCLC Without Prior PCI or WBRT
eFigure 1. CNS Progression-Free Survival (PFS)
eFigure 2. Forest Plots Comparing First-Line SRS and WBRT Within Patient Subsets
eMethods.


