Abstract
Background: Carbapenem-resistant Enterobacterales and Acinetobacter baumannii are of major concern in terms of infection prevention and control. This study evaluated factors that may increase the frequency of Enterobacterales and A. baumannii with carbapenem resistance (CR) in patients admitted to a German University Hospital for implementation of optimized infection control management.
Methods: A five-year-retrospective epidemiological cohort analysis was conducted on anamnestic risk factors for carrying Enterobacterales and/or A. baumannii with CR in patients who were first tested positive for these species at University Hospital Frankfurt (UHF) between January 2013 and June 2018.
Results: 364 patients were tested positive for Enterobacterales and/or A. baumannii with CR, resulting in n=400 bacterial isolates in total, with Klebsiella pneumoniae being the most frequently detected species (n=146/400; 36.5%; 95% confidence interval: 31.8–41.4). In patients who were tested positive for Enterobacterales and/or A. baumannii with CR, any hospital stay within the previous 12 months was the most frequently reported common factor (n=275/364; 75.5%; 70.8–79.9).
Conclusion: A hospital stay within the previous 12 months, including hospitals in Germany and abroad, is a frequent characteristic of patients who tested positive for Enterobacterales and/or A. baumannii with CR. Upon admission, any previous hospital stay of the given patient within the previous 12 months should be determined. Infection control strategies such as screening measures need to be adapted to these patient groups in hospital settings. In order to reflect the varying determinants in “nosocomial” cases in greater detail, the existing criteria used to characterize “nosocomial detection” of gram-negative bacteria with CR should be reviewed.
Keywords: carbapenem resistance, Enterobacterales, A. baumannii, risk factors, infection control management
Zusammenfassung
Hintergrund: Enterobacterales und/oder Acinetobacter baumannii mit Carbapenem-Resistenz (CR) stellen in Kliniken eine enorme medizinische Herausforderung dar. Die vorliegende Studie untersucht anamnestische Charakteristika von Patienten des Universitätsklinikums Frankfurt am Main (UKF), die zum Risiko einer Trägerschaft von Enterobacterales und/oder A. baumannii mit CR beitragen. Ziel der Untersuchung ist die Etablierung einer zielgerichteten Strategie zur Infektionskontrolle.
Methode: Retrospektive epidemiologischen Kohortenanalyse bei Patienten, die zwischen Januar 2013 und Juni 2018 am UKF positiv auf mindestens eine Spezies Enterobacterales und/oder A. baumannii mit CR getestet wurden.
Ergebnisse: Innerhalb des Untersuchungszeitraums wurden bei 364 Patienten 400 Isolate von Enterobacterales und/oder A. baumannii mit CR nachgewiesen. Hiervon am häufigsten vertreten war Klebsiella pneumoniae (n=146/400; 36,5%; 95%-Konfidenzintervall: 31,8–41,4). Die anamnestische Angabe mindestens eines Krankenhausaufenthalts „innerhalb der letzten 12 Monate“ wurde am häufigsten berichtet (n=275/364; 75,5%; 70,8–79,9).
Zusammenfassung: Da sich ein Krankenhausaufenthalt innerhalb der letzten 12 Monate mit Nachweis von Enterobacterales und/oder A. baumannii mit CR als das am häufigsten berichtete Risikomerkmal herausstellte, sollte das bei Aufnahme eines Patienten sorgfältig abgefragt werden. Krankenhaushygienische Strategien zur Infektionsprävention, z.B. Implementierung von Screeningprotokollen für Patienten mit diesem Risikofaktor, können hilfreich sein und sollten situationsadaptiert eingesetzt werden. Um die derzeit als „nosokomiale Nachweise“ zu bewertenden Fälle richtig darstellen zu können, ist eine Überarbeitung der derzeitigen Definitionskriterien notwendig.
Introduction
The dramatic worldwide spread of multidrug-resistant organisms (MDRO), and that of multidrug-resistant gram-negative organisms with carbapenem-resistance (MDRGN with CR) in particular, is an issue of major concern in terms of epidemiology and infection control [1], [2], [3], [4], [5], [6], [7]. Several factors contributing to MDRGN’s global spread have been determined, such as international travel [8], [9], [10], refugee history [11], [12], [13], [14], medical tourism or medical pre-treatment abroad [11], [14], [15]. Despite the knowledge of such factors and the implementation of strict infection control measures, such as isolation strategies [11], [12], MDRGN remain a major health challenge in hospitals. This suggests that several other factors might additionally contribute to the massive spread of MDRGN with CR. On average, University Hospital Frankfurt, Germany (UHF) deals with around 75 cases of newly detected Enterobacterales and A. baumannii with resistance to carbapenems (CR) every year. This high number of MDRGN with CR might particularly be attributed to the UHF’s direct vicinity to Frankfurt International Airport, whence a relevant number of patients reporting a stay abroad are admitted.
In order to maintain a firm infection control strategy, we evaluated factors that might additionally pose a risk factor of carrying Enterobacterales and/or A. baumannii with CR detected in patients admitted to UHF over a five-year period. Data were regularly obtained in accordance with mandatory reporting stipulated by specific regulations of the federal state of Hesse and Germany [16], [17], [18], [19], [20]. They form the basis of discussion within a local MDRO network (Rhine-Main-Network, Hesse [21]) to build trans-hospital knowledge in the regional infection control departments. This study evaluated patients who were tested positive for Enterobacterales or A. baumannii with CR at UHF for anamnestic risk factors, e.g., being a resident of a nursing home or having a history of hospital stays in Germany or abroad, as well as previous non-medical (tourism) stays abroad.
Materials and methods
Definition of multidrug-resistant gram-negative bacteria with resistance to carbapenems (MDRGN with CR)
MDRGN are defined as “Enterobacterales with extended spectrum beta-lactamase (ESBL)–phenotype as well as Enterobacterales, and Acinetobacter baumannii resistant against piperacillin, any 3rd/4th generation cephalosporin, and fluoroquinolones ± carbapenems”, as previously described [11], [12]. In case of carbapenem-resistance, the addendum “CR” is given.
Infection control surveillance and German infection protection law
In Germany, the infection protection law (Infektionsschutzgesetz; IfSG) determines various aspects of infection control and epidemiological surveillance, and mandatory reporting of certain infections or infectious agents [20]. Additionally, the Federal States of Germany may require further mandatory reporting. Hence, in 2011, the federal state of Hesse made reporting of multidrug-resistant gram-negative species (Enterobacterales, A. baumannii and Pseudomonas spp.) with CR mandatory, regardless of the resistance mechanism or the nature of the patient sample from which the respective species was obtained [16]. In 2013, Pseudomonas aeruginosa was omitted from this regulation [17]. Based on the Hessian experience, the obligation to report Enterobacterales and A. baumannii with CR was introduced across Germany [18], [19], [20]. In Hesse, mandatory MDRGN notification is given by using a standardized questionnaire [18]. Data obtained by the questionnaire encompass information on the patient’s current residency status (e.g., in a nursing home), sojourns outside Germany or hospital stays within the preceding 12 months, i.a. grouping into colonization versus infection and in hospital-acquired versus community-acquired (definitions given below) is also required as part of the notification to the responsible public health authority. Thus, Hesse was the first German federal state to introduce the obligation to report carbapenem-resistant organisms, resulting in long-standing experience in reporting and management of these pathogens in Hesse, especially in UHF.
Screening procedure at UHF
German hospitals need to adhere to an infection control strategy which describes actions necessary to prevent the transmission of harmful organisms during the patients’ hospital stay. This legal obligation is based on the German infection protection law; risk-adapted screening is recommended by KRINKO [7], [20], as mentioned above, and is therefore mandatory for the hospital’s employees. At UHF, this demand is met and documented in the UHF’s infection control strategy, as previously described [11]. Upon admission, screening for MDRGN (Enterobacterales and A. baumannii) includes rectal and throat samples as well as swabs from wounds, if the patient has wounds, as well as tracheal secretion, if patient is intubated.
Colonization versus infection and case definition
Colonization (CO) and infection (INF) were recorded in the questionnaire [18]. CO was entered if Enterobacterales and/or A. baumannii with CR were detected solely in screening samples, e.g., rectal, throat or cutaneous swabs, and the patient was free of any local or systemic infection signs. Following the definition by the hospital infection surveillance system (Krankenhaus-Infektions-Surveillance-System, KISS) of the National Reference Center for Surveillance of Nosocomial Infections, Berlin, Germany (Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen), INF was entered if Enterobacterales or A. baumannii with CR were found in primarily sterile materials (e.g., blood), pus, wounds, and certain infection symptoms (e.g, fever) or associated with typical laboratory results (e.g., leukocytosis, microbiological detection of an infectious agent) as well as corresponding results from imaging procedures (e.g., X-ray, computed tomography, nuclear magnetic resonance tomography, ultrasound) and endoscopic examinations [22]. Further, also following the KISS definition, CO or INF are labelled as “community-acquired” (CA), if the pathogen is detected within the first three days after admittance, with the day of admittance being day one [22]. If the pathogen is detected later than three days after admittance, with the day of admittance being the first day, CO/INF are labelled as “nosocomial” which is commonly meant to be “hospital-acquired” (HA).
Patients and samples
In this retrospective study, n=364 patients were analyzed. These patients were tested positive for the first time for Enterobacterales and/or A. baumannii with CR upon being admitted to UHF between January 1st, 2013 and June 15th, 2018. Patients’ data were obtained from the notification questionnaire [18], as indicated above, as well as from the patients’ digital records.
Detection of MDRGN and molecular resistance analysis
Laboratory testing was performed under strict quality-controlled criteria (laboratory accreditation according to ISO 15189:2007 standards) at the Institute for Medical Microbiology and Infection Control, University Hospital Frankfurt, Germany. Samples were collected using culture swabs as well as Amies collection and transport medium (Hain Lifescience, Nehren, Germany) and streaked onto selective CHROMagarTM ESBL plates (Mast Diagnostica, Paris, France). Identification of presumed MDRGN species and antibiotic susceptibility testing were performed as previously described [11]. Carbapenemase encoding genes were detected via PCR analysis and subsequent sequencing from carbapenem-resistant Enterobacterales, including the bla genes for carbapenemases NDM, VIM, IMP, OXA–48–like and KPC as well as OXA–23, OXA–24 (including subtypes), and OXA–58 and NDM as well as species-specific OXA-51 for A. baumannii [11], [23], [24].
Statistical analysis
The biostatistical data file from the University of Münster, Germany, was used for statistical analyses of the pseudonymized data [25]. 95% confidence intervals (95%CI) were calculated based on binomial distribution; p-values (2-tailed) of p≤0.05 were considered statistically significant.
Results
General characteristics of the study cohort
Between January 1st, 2013 and June 15th, 2018, a total of 364 patients were tested positive for the first time for Enterobacterales and A. baumannii with CR at UHF. N=238/364 were male (65.4%). The mean age was 58.5 years (standard deviation 21.3), with a median of 65 years.
Notifications within the observation period
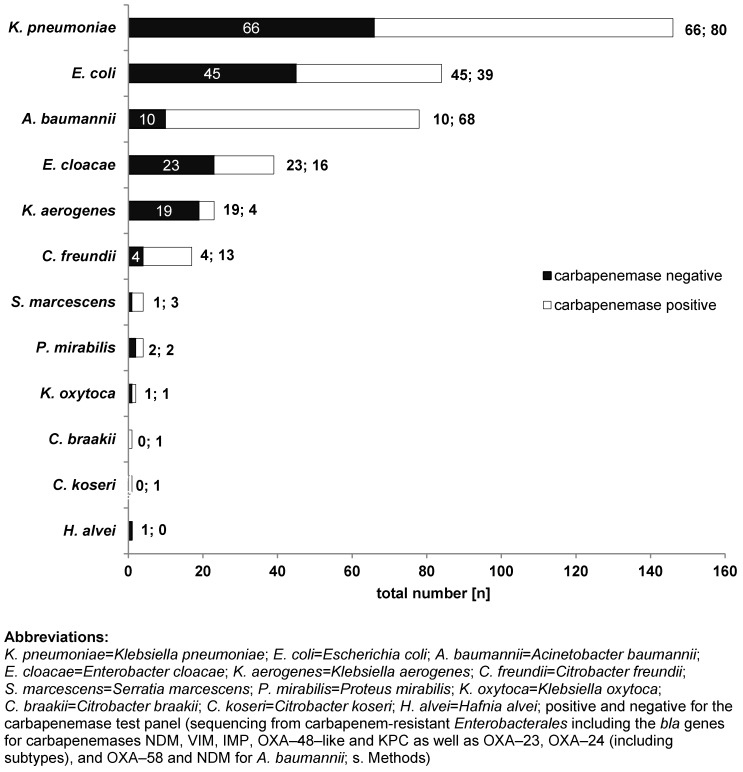
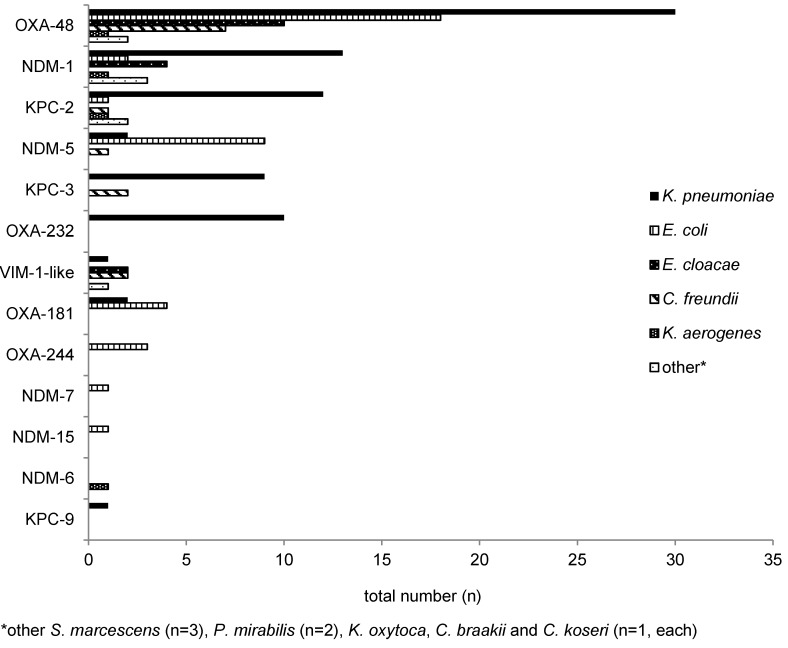
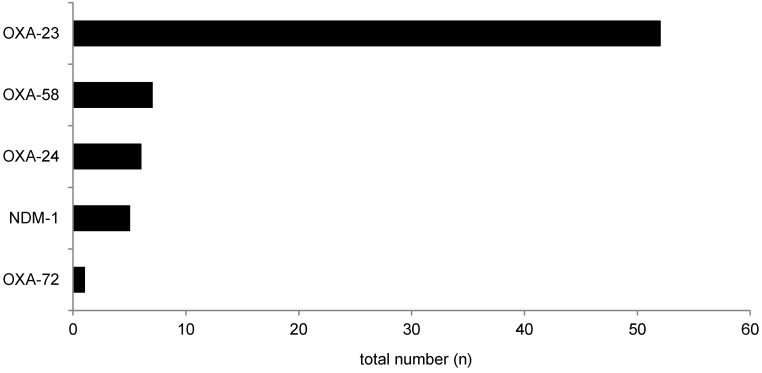
N=28/364 patients were tested positive for more than one species of Enterobacterales and/or A. baumannii with CR, resulting in a total number of n=400 isolates reported to the public health authority in Frankfurt am Main, Germany. Of these, K. pneumoniae with CR was the most frequently detected species, n=146/400 (36.5%), followed by E. coli with CR, n=84/400 (21.0%), and A. baumannii n=78/400 (19.5%) (Figure 1 (Fig. 1)). The types of carbapenemases identified in the study population are given in Figure 2 (Fig. 2) and Figure 3 (Fig. 3). One (n=1) A. baumannii isolate was found positive for three carbapenemases (NDM-1 + OXA-23 + OXA-58) and one (n=1) A. baumannii isolate was tested positive for two carbapenemases (OXA-23 + OXA-58).
Figure 1. Number of Enterobacterales and A. baumannii with CR detected in UHF reported to the Public Health Authority Frankfurt am Main, Germany (n=364; as of January 1st, 2013 and June 15th, 2018).
Figure 2. Carbapenemases detected in Enterobacterales with CR (n=160) during the observation period.
Figure 3. Carbapenemases detected in A. baumannii with CR (n=71).
Case characteristics
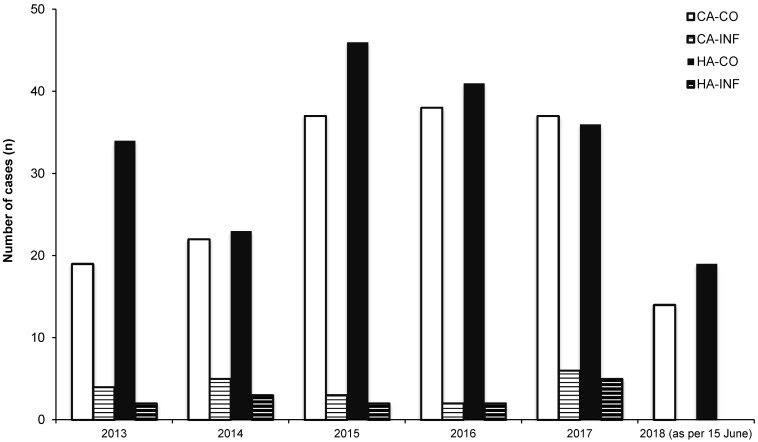
In total, n=187/400 (46.8%) isolates were found to be community-acquired (CA) and n=213/400 (53.3%) were hospital-acquired (HA), based on the definition by KISS ([22]; see methods). Regarding CA isolates, n=167/187 (89.3%) and n=20/187 (10.7%) isolates were obtained from screening material (“colonization”; CA-CO) and from invasive materials (“infection”; CA-INF), respectively. In HA, n=199/213 (93.4%) of the patients were colonized (HA-CO), whereas n=14/213 (6.6%) were infected (HA-INF). Further details are shown in Figure 4 (Fig. 4).
Figure 4. Number of “community acquired and colonized” (HA-CO), CA and infected (CA-INF), hospital-acquired and colonized (HA-CO) as well as HA and infected (HA-INF) cases between January 2013 and June 2018.
Enterobacterales and A. baumannii with CR: anamnestic evaluation
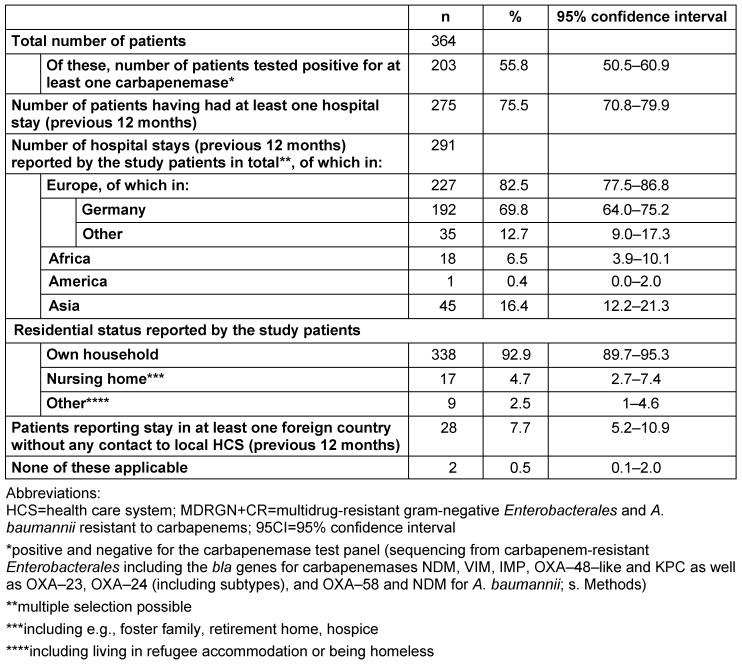
The anamnestic factors found in the history of patients who were tested positive for Enterobacterales and/or A. baumannii with CR are summarized in Table 1 (Tab. 1). A selection of multiple items was possible. In terms of residential status, patients who were tested positive for Enterobacterales and/or A. baumannii with CR most frequently reported “own household” with n=338/364 (92.9%; 89.7–95.3). n=275 patients reported n=291 stays in hospital within the previous 12 months. Of these, n=99 stays were at hospitals abroad and n=192 stays in a German hospital within the previous 12 months. Due to multiple answers, both admittance to a German hospital and a hospital outside of Germany within the previous 12 months was reported by n=16 patients. “Stay in foreign country without any contact to local health care system (HCS) within the previous 12 months” was reported by n=28/364 (7.7%; 5.2–10.9) patients.
Table 1. Anamnestic factors reported by patients who tested positive for Enterobacterales and/or A. baumannii with CR; selection of multiple items was possible.
Discussion
Multidrug-resistant gram-negative bacteria remain a critical issue in terms of infection control. Deeper knowledge of their epidemiology, associated risk factors, and effective infection control strategies is needed in order to prevent the spread of these challenging pathogens. The anamnestic background of patients who were tested positive for Enterobacterales and/or A. baumannii with CR could give valuable insight into the epidemiology of these species and identify markers, in order to develop optimized, risk-adapted infection control management.
In more than a half of the isolates in the present study, at least one carbapenemase (n=228/400; 57.0%) was identified. Of these, n=160/322 (49.7%) and n=68/78 (87.2%) Enterobacterales and/or A. baumannii, respectively, were found to be positive for a carbapenemase of the tested panel. K. pneumoniae with CR was the most frequently detected species, followed by E. coli with CR and A. baumannii with CR (Figure 1 (Fig. 1)). As demonstrated in Figure 2 (Fig. 2) and Figure 3 (Fig. 3), n=13 different carbapenemases were detected in Enterobacterales and five different carbapenemases in A. baumannii isolates, partly in combination with other carbapenemases. Of the carbapenemases identified, OXA-48-like and OXA-23 were most frequently found in Enterobacterales and A. baumannii, respectively. This is congruent with data obtained from the Rhine-Main-area and the German National Reference Laboratory (NRC) for multidrug-resistant gram-negative bacteria [26], [27]. The possibility must be mentioned that isolates which were tested negative for carbapenemase may nevertheless be positive for other carbapenemases which were not included in our laboratory panel. In addition, altered expression of porins or function of efflux pumps can also result in carbapenemase-negative carbapenem resistance [28].
Interestingly, VIM-1, which is the second most frequently detected carbapenemase in Enterobacteriaceae in Germany [27], ranked rather low in our study (n=6 isolates). Concerning KPC-2 and KPC-3 as well as NDM-1 and NDM-5, however, data from the NRC [26] and our study (Figure 2 (Fig. 2) and Figure 3 (Fig. 3)) largely match. The large proportion of MDRGN tested positive for carbapenemases and the occurrence of isolates that were tested positive for more than one carbapenemase particularly emphasizes the hazard potential of these pathogens. Clearly, the appearance of MDRGN expressing several different carbapenemases calls for infection control strategies to prevent the development of a new hospital health threat.
Within the study’s observation period, n=400 isolates of MDRGN with CR were reported, which is an estimated number of ca. n=75 cases annually at UHF. In comparison, data on Enterobacterales and A. baumannii with CR reported by all Frankfurt hospitals to the municipal public health department of Frankfurt am Main, Hesse, Germany show a total number of n=559 notifications for Enterobacterales and A. baumannii with CR between April 2012 and December 2015 [27], which is an estimated 150 cases annually. This highlights the glaringly high number of Enterobacterales and A. baumannii with CR UHF has to deal with. With regard to the patient population at university hospitals, this high number of patients carrying MDRGN with CR is not surprising. For example, distinct and harmonized screening procedures for MDRO, critically ill patients suffering from complex diseases with a long history of pre-treatment in other hospitals before being transferred to our university hospital, and patients admitted after pre-treatment abroad may greatly contribute to a high prevalence of MDRGN with CR at any university hospital. In turn, the number of HA-INF within the observation period was low, n=14/214 (Figure 4 (Fig. 4)), indicating effective infection control management.
Regarding the patients’ residential status, the anamnestic status “own household” should be interpreted carefully. Although people living in their own household may generally be healthier compared to people living in a nursing home, who may have a higher risk of carrying health-care-associated MDRGN with CR, the former groups are suggested to have travelled more, for instance. This in turn might be associated with travel-associated factors, e.g., a hospital stay abroad, to be at higher risk of testing positive for MDRGN with CR. Regarding our data, 92.9% (89.7–95.3) of the patients who were tested positive for MDRGN with CR reported living in their “own household” compared to a significantly lower percentage, 4.7% (2.7–7.4), reporting “nursing home” as their residential status. However, the number of patients who were tested negative for MDRGN with CR and reported living in their own household versus a nursing home has not been evaluated. Thus, because these data were not available in our study, a reliable conclusion about this aspect cannot be given.
The study patients who were tested positive for Enterobacterales and/or A. baumannii with CR reported n=275 stays in a hospital within the previous 12 months, indicating that a previous hospitalization is strongly associated with carrying Enterobacterales and/or A. baumannii with CR, which has also been suggested previously [11], [27], [29], [30], [31]. Of this patient group, the majority of hospital stays (n=192/275; 69.8%; 64.0–75.2) were in a German hospital within the previous 12 months, indicating a key characteristic among patients carrying Enterobacterales and/or A. baumannii with CR. To support this conclusion, however, data on the basic population of all patients being admitted to UHF reporting a stay in a German hospital within the previous 12 months would be necessary, especially in order to compare this to any hospital stay abroad (n=99/275; 36.0%; 30.3–42.0). The average number of patients who are directly transferred from any German hospital to UHF amounts to at least 2,000 per anno. This number, however, might only be a fraction of the total patient number reporting a stay in a German hospital within the previous 12 months; an epidemiological conclusion can only be drawn to a limited extent.
Interestingly, 7.7% (5.2–10.9) of the patients who were tested positive for Enterobacterales and/or A. baumannii with CR reported a “stay in a foreign country without any contact to local HCS”, which itself has also been identified as a risk factor for carrying MDRGN, [9], [10], [11], [14], [15], [32], [33], [34], [35], [36], [37]. In order to investigate this patient group, however, data on the countries they visited as well as their duration of stay in the respective countries would be needed. In an ongoing project (different patient cohort, observation period January 2013–January 2017), n=23 patients were identified reporting a stay abroad in the UN region Southern Asia without any contact to local health care systems. Of these, India (n=15) and Pakistan (n=3) were the most frequently reported countries visited. Of these, n=17/23 patients (73.9%; 51.6–89.8) were tested positive for MDRGN. Three patients were found positive for n=3 MDRGN + CR isolates in total (with n=1 isolate in each patient), with all of them reporting a stay in India. The types of carbapenemases detected in these isolates were NDM-5 and OXA-181 (n=1 each); in one isolate, no carbapenemase was detected (Steinmann et al., unpublished data).
Furthermore, 0.5% (0.1–2.0) of the patients who were tested positive for Enterobacterales and/or A. baumannii with CR did not report any of the listed characteristics (Table 1 (Tab. 1)). Based on this finding, this cohort should be evaluated in more detail in the future for other potential risk factors. For instance, because aquatic environments and seafood have been shown to be potentially contaminated with fecal indicators, such as carbapenem-resistant bacteria [38], [39], [40], outdoor activities such as rowing, canoeing, diving, snorkeling or consumption of seafood might be risk factors for acquiring colonization or infections by MDRGN. This was reported during the Olympic Games in Rio de Janeiro in 2016 [41], [42], [43].
Considering the high number of reported stays in hospital within the previous 12 months of patients who were tested positive for Enterobacterales and/or A. baumannii with CR, this factor should be a standard item in the patient’s anamnesis and is suggested to be included future considerations on infection control strategies in hospitals.
Clearly, our study has two major limitations. Since the analysis was based on cases for which notification to the Public Health Agency is legally required, the comparison to a control group is nearly impossible. Theoretically, such a control group should include individuals with the risk factors mentioned above but who were tested negative for Enterobacterales and/or A. baumannii with CR. These combinations, however, are not systemically recorded for patients at UHF, which in turn restricts the use of statistical tests for significance. Furthermore, the data on anamnestic risk factors obtained by the questionnaire are limited only to previous hospital stays, residential status and country of origin or pre-treatment. These factors are essential to evaluate possible risk factors, but seem to be insufficient in terms of discriminatory power. The official questionnaire would profit from a revision with the intent to find additional, more highly discriminatory questions to identify risk patients carrying MDRGN with CR. For example, additional questions could address the patient’s leisure behavior (e.g., water sports, see above) or profession (e.g., persons with professional contact to waste water). In addition, we found n=99 patients who reported hospitalization abroad within the previous 12 months. Due to language barriers, this group might be misidentified by our questionnaire (which is presented in German).
In order to facilitate the distinction between “community-acquired” and “hospital-acquired”, KISS recommends determination of the period since patient’s admission to the hospital and set the decisive criterion to three days. Thus, “nosocomial” encompasses cases not detected on admittance, either because
they were not screened at that time, or
were screened on admittance, but the (low) presence of carbapenem-resistant organisms (CRO) was “masked” by the large amount of other bacteria in the intestinal microbiota and were only unmasked with inception of antibiotic therapy, or
CRO were actually generated during antibiotic therapy or, finally
CRO were transmitted during a hospital stay.
It is important to realize that only the last case 4 would be sufficient to be influenced by infection control measures. Furthermore, in case of the occurrence of a “resistance plasmid transfer” [44], the label “nosocomial” can also be highly problematic, as the transfer of a resistance plasmid from one species to another (e.g., from an E. coli with CR into a carbapenem-susceptible strain of K. pneumoniae) might result in the initial detection of a K. pneumoniae with CR [44], for instance, which might be misinterpreted as a new nosocomial acquisition. It has been previously shown that resistant strains which are present in the gastrointestinal tract are not detectable via conventional microbiological methods due to their low number. During antimicrobial therapy, these bacteria will be selected, bloom and colonize the intestine [45]. In cases of colonizing organisms with possibly lower screening sensitivity, the current scope of this definition for surveillance according to KISS and Centers for Disease Control and Prevention (CDC) does not completely match the complex coherences in terms of epidemiology, infection control, microbiological and public health. The criteria for using the category “nosocomial” should therefore be reviewed in order to adequately address these complex circumstances.
Conclusions
Enterobacterales and A. baumannii with carbapenem resistance are a major global threat in terms of infection prevention and public health. Regarding the cohort described here, hospital stay within the previous 12 months, including hospitalization abroad as well as in Germany, was the most frequently reported anamnestic factor (75.5% patients reported at least one hospital stay within the previous 12 months). Hospital stay within the previous 12 months therefore is a key anamnestic predictor for carrying Enterobacterales and/or A. baumannii with CR. Patients should be regularly asked about this anamnestic factor on the day of admission. In order to improve infection control efforts, patients reporting any hospital stay within the previous 12 months should be screened, e.g. by rectal swabs, for Enterobacterales and A. baumannii upon admission and pre-emptive isolation should be considered. With regard to MDRGN + CR, the criteria characterizing “nosocomial” need to be reviewed.
Notes
Competing interests
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Ethics Board of the University Hospital Frankfurt, Germany (votum # E151/17).
References
- 1.Logan LK, Weinstein RA. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J Infect Dis. 2017 Feb;215(suppl_1):S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordmann P. Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Med Mal Infect. 2014 Feb;44(2):51–56. doi: 10.1016/j.medmal.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Satilmis L, Vanhems P, Bénet T. Outbreaks of Vancomycin-Resistant Enterococci in Hospital Settings: A Systematic Review and Calculation of the Basic Reproductive Number. Infect Control Hosp Epidemiol. 2016 Mar;37(3):289–294. doi: 10.1017/ice.2015.301. [DOI] [PubMed] [Google Scholar]
- 4.Gastmeier P, Schröder C, Behnke M, Meyer E, Geffers C. Dramatic increase in vancomycin-resistant enterococci in Germany. J Antimicrob Chemother. 2014 Jun;69(6):1660–1664. doi: 10.1093/jac/dku035. [DOI] [PubMed] [Google Scholar]
- 5.Maechler F, Geffers C, Schwab F, Peña Diaz LA, Behnke M, Gastmeier P. Entwicklung der Resistenzsituation in Deutschland: Wo stehen wir wirklich. [Development of antimicrobial resistance in Germany: What is the current situation?]. Med Klin Intensivmed Notfmed. 2017 Apr;112(3):186–191. doi: 10.1007/s00063-017-0272-2. (Ger). [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011 Jul;53(1):60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 7.Kommission für Krankenhaushygiene und Infektionsprävention beim Robert-Koch Institut (KRINKO) Hygienemaßnahmen bei Infektionen oder Besiedlung mit multiresistenten gramnegativen Stäbchen. Bundesgesundheitsbl. 2012 Oct;55(10):1311–1354. doi: 10.1007/s00103-012-1549-5. [DOI] [PubMed] [Google Scholar]
- 8.Arcilla MS, van Hattem JM, Haverkate MR, Bootsma MCJ, van Genderen PJJ, Goorhuis A, Grobusch MP, Lashof AMO, Molhoek N, Schultsz C, Stobberingh EE, Verbrugh HA, de Jong MD, Melles DC, Penders J. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017 Jan;17(1):78–85. doi: 10.1016/S1473-3099(16)30319-X. [DOI] [PubMed] [Google Scholar]
- 9.Lübbert C, Straube L, Stein C, Makarewicz O, Schubert S, Mössner J, Pletz MW, Rodloff AC. Colonization with extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacteriaceae in international travelers returning to Germany. Int J Med Microbiol. 2015 Jan;305(1):148–156. doi: 10.1016/j.ijmm.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Tängdén T, Cars O, Melhus A, Löwdin E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum beta-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother. 2010 Sep;54(9):3564–3568. doi: 10.1128/AAC.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinheimer C, Kempf VA, Jozsa K, Wichelhaus TA, Hogardt M, O'Rourke F, Brandt C. Prevalence of multidrug-resistant organisms in refugee patients, medical tourists and domestic patients admitted to a German university hospital. BMC Infect Dis. 2017 Jan;17(1):17. doi: 10.1186/s12879-016-2105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinheimer C, Kempf VA, Göttig S, Hogardt M, Wichelhaus TA, O'Rourke F, Brandt C. Multidrug-resistant organisms detected in refugee patients admitted to a University Hospital, Germany June-December 2015. Euro Surveill. 2016;21(2) doi: 10.2807/1560-7917.ES.2016.21.2.30110. [DOI] [PubMed] [Google Scholar]
- 13.Heudorf U, Albert-Braun S, Hunfeld KP, Birne FU, Schulze J, Strobel K, Petscheleit K, Kempf VA, Brandt C. Multidrug-resistant organisms in refugees: prevalences and impact on infection control in hospitals. GMS Hyg Infect Control. 2016;11:Doc16. doi: 10.3205/dgkh000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josseaume J, Verner L, Brady WJ, Duchateau FX. Multidrug-resistant bacteria among patients treated in foreign hospitals: management considerations during medical repatriation. J Travel Med. 2013 Jan-Feb;20(1):22–28. doi: 10.1111/j.1708-8305.2012.00668.x. [DOI] [PubMed] [Google Scholar]
- 15.Lohr B, Pfeifer Y, Heudorf U, Rangger C, Norris DE, Hunfeld KP. High Prevalence of Multidrug-Resistant Bacteria in Libyan War Casualties Admitted to a Tertiary Care Hospital, Germany. Microb Drug Resist. 2018 Jun;24(5):578–584. doi: 10.1089/mdr.2017.0141. [DOI] [PubMed] [Google Scholar]
- 16.Hessisches Sozialministerium. Gesetz- und Verordnungsblatt für das Land Hessen, Teil I, 16.12.2011; S772. 2011. [accessed 30.06.2019]. Verordnung über die Ausdehnung der Meldepflicht nach dem Infektionsschutzgesetz (IfSGMeldeVO) vom 29. November 2011. Available from: http://starweb.hessen.de/cache/GVBL/2011/00025.pdf. [Google Scholar]
- 17.Hessisches Sozialministerium. Ausführungserlass zur Verordnung über die Ausdehnung der Meldepflicht nach dem Infektionsschutzgesetz (IfSGMeldeVO) vom 29. November 2011: „Erweiterung der Meldepflichten gramnegativer Erreger mit erworbener Carbapenemresistenz“; per 8 April 2013. Apr 8, 2013. [Google Scholar]
- 18.Hauri AM, Kaase M, Hunfeld KP, Heinmüller P, Imirzalioglu C, Wichelhaus TA, Heudorf U, Bremer J, Wirtz A. Meldepflicht für Carbapenem-resistente gramnegative Erreger: eine Public Health-Priorität? [Notification requirement for carbapenem-resistant organisms: A public health priority?]. Hyg Med. 2015;40(1/2):26–35. (Ger). [Google Scholar]
- 19.Verordnung zur Anpassung der Meldepflichten nach dem Infektionsschutzgesetz an die epidemische Lage. Bundesgesetzblatt 2016, Teil I, Nr. 13, 31.03.2016, S.515. [accessed 30.06.2019]. Available from: https://www.bgbl.de/xaver/bgbl/start.xav#__bgbl__%2F%2F*%5B%40attr_id%3D%27bgbl116s0515.pdf%27%5D__1561927173301.
- 20.German Infection Protection Law: Infektionsschutzgesetz vom 20. Juli 2000 (BGBl. I S. 1045), zuletzt geändert durch Artikel 14b v. 6. Mai 2019 (BGBl. I S. 646) [accessed 17.07.2019].
- 21.MRE-Netz Rhein-Main. Gemeinsam gegen antibiotikaresistente Keime MRE-Netz Rhein-Main. [accessed 30.06.2019]. Available from: http://www.mre-rhein-main.de/ [accessed 30.06.2019] [Google Scholar]
- 22.Robert Koch-Institut. Definitionen nosokomialer Infektionen für die Surveillance im Krankenhaus-Infektions-Surveillance-System (KISS-Definitionen) 2016. [accessed 30.06.2019]. [DOI] [Google Scholar]
- 23.Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JD. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J Clin Microbiol. 2012 Dec;50(12):3877–3880. doi: 10.1128/JCM.02117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010 Feb;65(2):233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 25.University of Münster. Konfidenzintervall für binominalverteilte Anteilswerte. https: //www. [accessed 30.06.2019]. Available from: https://www.medizin.uni-muenster.de/fileadmin/einrichtung/imib/lehre/skripte/biomathe/bio/konf1.html (accessed 30 June 2019) [Google Scholar]
- 26.Robert-Koch-Institut. Bericht des Nationalen Referenzzentrums für gramnegative Krankenhauserreger, 2018. Epidemiologisches Bulletin. 2019 Aug 1;(31) [Google Scholar]
- 27.Heudorf U, Büttner B, Hauri AM, Heinmüller P, Hunfeld KP, Kaase M, Kleinkauf N, Albert-Braun S, Tessmann R, Kempf VA. Carbapenem-resistant Gram-negative bacteria - analysis of the data obtained through a mandatory reporting system in the Rhine-Main region, Germany, 2012-2015. GMS Hyg Infect Control. 2016;11:Doc10. doi: 10.3205/dgkh000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández L, Hancock RE. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev. 2012 Oct;25(4):661–681. doi: 10.1128/CMR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardoso T, Ribeiro O, Aragão IC, Costa-Pereira A, Sarmento AE. Additional risk factors for infection by multidrug-resistant pathogens in healthcare-associated infection: a large cohort study. BMC Infect Dis. 2012 Dec;12:375. doi: 10.1186/1471-2334-12-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu P, Li X, Luo M, Xu X, Su K, Chen S, Qing Y, Li Y, Qiu J. Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Infection: A Meta-Analysis. Microb Drug Resist. 2018 Mar;24(2):190–198. doi: 10.1089/mdr.2017.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Loon K, Voor In 't Holt AF, Vos MC. A Systematic Review and Meta-analyses of the Clinical Epidemiology of Carbapenem-Resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2018 Jan;62(1):e01730–e01717. doi: 10.1128/AAC.01730-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaspar T, Schweiger A, Droz S, Marschall J. Colonization with resistant microorganisms in patients transferred from abroad: who needs to be screened? Antimicrob Resist Infect Control. 2015;4:31. doi: 10.1186/s13756-015-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray CK, Yun HC, Griffith ME, Thompson B, Crouch HK, Monson LS, Aldous WK, Mende K, Hospenthal DR. Recovery of multidrug-resistant bacteria from combat personnel evacuated from Iraq and Afghanistan at a single military treatment facility. Mil Med. 2009 Jun;174(6):598–604. doi: 10.7205/milmed-d-03-8008. [DOI] [PubMed] [Google Scholar]
- 34.Izdebski R, Bojarska K, Baraniak A, Literacka E, Herda M, Żabicka D, Guzek A, Półgrabia M, Hryniewicz W, Gniadkowski M. NDM-1- or OXA-48-producing Enterobacteriaceae colonising Polish tourists following a terrorist attack in Tunis, March 2015. Euro Surveill. 2015 Jun;20(23):pii=21150. doi: 10.2807/1560-7917.es2015.20.23.21150. [DOI] [PubMed] [Google Scholar]
- 35.Armand-Lefèvre L, Andremont A, Ruppé E. Travel and acquisition of multidrug-resistant Enterobacteriaceae. Med Mal Infect. 2018 Oct;48(7):431–441. doi: 10.1016/j.medmal.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Kuenzli E, Jaeger VK, Frei R, Neumayr A, DeCrom S, Haller S, Blum J, Widmer AF, Furrer H, Battegay M, Endimiani A, Hatz C. High colonization rates of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli in Swiss travellers to South Asia- a prospective observational multicentre cohort study looking at epidemiology, microbiology and risk factors. BMC Infect Dis. 2014 Oct;14:528. doi: 10.1186/1471-2334-14-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruppé E, Andremont A, Armand-Lefèvre L. Digestive tract colonization by multidrug-resistant Enterobacteriaceae in travellers: An update. Travel Med Infect Dis. 2018 Jan-Feb;21:28–35. doi: 10.1016/j.tmaid.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Müller H, Sib E, Gajdiss M, Klanke U, Lenz-Plet F, Barabasch V, Albert C, Schallenberg A, Timm C, Zacharias N, Schmithausen RM, Engelhart S, Exner M, Parcina M, Schreiber C, Bierbaum G. Dissemination of multi-resistant Gram-negative bacteria into German wastewater and surface waters. FEMS Microbiol Ecol. 2018 May;94(5):fiy057. doi: 10.1093/femsec/fiy057. [DOI] [PubMed] [Google Scholar]
- 39.Zarfel G, Lipp M, Gürtl E, Folli B, Baumert R, Kittinger C. Troubled water under the bridge: Screening of River Mur water reveals dominance of CTX-M harboring Escherichia coli and for the first time an environmental VIM-1 producer in Austria. Sci Total Environ. 2017 Sep;593-594:399–405. doi: 10.1016/j.scitotenv.2017.03.138. [DOI] [PubMed] [Google Scholar]
- 40.Roschanski N, Guenther S, Vu TTT, Fischer J, Semmler T, Huehn S, Alter T, Roesler U. VIM-1 carbapenemase-producing isolated from retail seafood, Germany 2016. Euro Surveill. 2017 Oct;22(43) doi: 10.2807/1560-7917.ES.2017.22.43.17-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fistarol GO, Coutinho FH, Moreira AP, Venas T, Cánovas A, de Paula SE Jr, Coutinho R, de Moura RL, Valentin JL, Tenenbaum DR, Paranhos R, do Valle Rde A, Vicente AC, Amado Filho GM, Pereira RC, Kruger R, Rezende CE, Thompson CC, Salomon PS, Thompson FL. Environmental and Sanitary Conditions of Guanabara Bay, Rio de Janeiro. Front Microbiol. 2015;6:1232. doi: 10.3389/fmicb.2015.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobs A. Keep Your Mouth Closed: Aquatic Olympians Face a Toxic Stew in Rio. The New York Times; Jul 2, 2016. [accessed 03.07.2019]. Available from: https://www.nytimes.com/2016/07/27/world/americas/brazil-rio-water-olympics.html. [Google Scholar]
- 43.Spiegel Wissenschaft. Olympia in Rio: Tausende tote Fische am Segelrevier. Jan 15, 2016. [accessed 03.07.2019]. Available from: https://www.spiegel.de/wissenschaft/natur/olympia-segelrevier-von-rio-tausende-tote-fische-a-1072197.html. [Google Scholar]
- 44.Göttig S, Gruber TM, Stecher B, Wichelhaus TA, Kempf VA. In vivo horizontal gene transfer of the carbapenemase OXA-48 during a nosocomial outbreak. Clin Infect Dis. 2015 Jun;60(12):1808–1815. doi: 10.1093/cid/civ191. [DOI] [PubMed] [Google Scholar]
- 45.Schjørring S, Struve C, Krogfelt KA. Transfer of antimicrobial resistance plasmids from Klebsiella pneumoniae to Escherichia coli in the mouse intestine. J Antimicrob Chemother. 2008 Nov;62(5):1086–1093. doi: 10.1093/jac/dkn323. [DOI] [PMC free article] [PubMed] [Google Scholar]