Abstract
Aims: Healthcare-associated infections linked to contaminated textiles are rare but underline their potential role as a source for transmission. The aim of the review was to summarize the experimental evidence on the survival and persistence of the different types of nosocomial pathogens on textiles.
Methods: A literature search was performed on MedLine. Original data on the survival of bacteria, mycobacteria, and fungi and persistence of viruses on textiles were evaluated.
Results: The survival of bacteria at room temperature was the longest on polyester (up to 206 days), whereas it was up to 90 days for some species on cotton and mixed fibers. Only low inocula of 100 CFU were found on all types of textiles with a short survival time of ≤3 days. Most bacterial species survived better at elevated air humidity. The infectivity of viruses on textiles is lost much faster at room temperature, typically within 2–4 weeks.
Conclusions: Contaminated textiles or fabrics may be a source of transmission for weeks. The presence of pathogens on the coats of healthcare workers is associated with the presence of pathogens on their hands, demonstrating the relevance of textile contamination in patient care.
Keywords: survival, pathogens, textiles, fabrics
Zusammenfassung
Zielsetzung: Nosokomiale Infektionen, die von kontaminierten Textilien ausgehen, sind selten, zeigen aber dennoch ihr Potenzial als Quelle einer Übertragung. Ziel der Übersichtsarbeit war es, experimentell gewonnene Daten zum Überleben bzw. zur Persistenz verschiedener nosokomialer Pathogene auf Textilien zusammenfassend darzustellen.
Methode: Eine Literaturrecherche wurde auf MedLine durchgeführt. Originaldaten zum Überleben von Bakterien, Mykobakterien und Pilzen sowie zur Persistenz von Viren auf Textilien wurden ausgewertet.
Ergebnisse: Das Überleben von Bakterien bei Raumtemperatur war am längsten auf Polyester (bis zu 206 Tage), wohingegen es auf Baumwollen bzw. gemischten Fasern bis zu 90 Tage betrug. Nur kleine Inokula von 100 KBE konnten auf allen Textilien über maximal 3 Tage überleben. Die Mehrzahl der Bakterienspezies überlebte bei höherer Luftfeuchtigkeit besser. Die Infektiosität der Viren ging auf Textilien bei Raumtemperatur schneller verloren, meist innerhalb von 2–4 Wochen.
Fazit: Kontaminierte Textilien können über Wochen eine Quelle für Übertragungen darstellen. Der Nachweis von Pathogenen auf den Kitteln der Mitarbeiter war mit dem Nachweis der Pathogene auf ihren Händen assoziiert. Auch darin zeigt sich die Bedeutung der Kontamination von Textilien in der Patientenversorgung.
Introduction
Healthcare-associated infections linked to contaminated textiles are rare, but play a role as a potential source of transmission. One example is the spread of group A streptococcus infections. An outbreak on a geriatric medical ward was explained by the presence of one healthcare worker (HCW) on the ward who was perineal carrier. Contamination of a fabric-upholstered chair used by the HCW in an office adjacent to the ward was also detected and was suspected to have enhanced the transmission to other HCWs [1]. Another example is an outbreak of meropenem-resistent A. baumannii on an intensive care unit. The major source appeared to be the curtains surrounding the patient’ beds [2]. Feather pillows have been described as an unexpected source of Acinetobacter spp., potentially causing outbreaks [3]. Outbreaks caused by bacterial spores on linen have also been reported, e.g., resulting in bacteraemia [4], [5]. Work garments have also been described to be contaminated with various types of microorganisms. The cuffs of long-sleeved coats frequently contact patients or environmental surfaces [6]. Soiled linen was also described as the source of tinea corporis infections in two HCWs who had only indirect contact to a patient infected with T. tonsurans [7]. The survival of nosocomial pathogens on inanimate surfaces has been well described [8]. But the persistence of pathogens on different types of textiles has not been reviewed. The purpose of this review was therefore to summarize the experimental evidence on the survival and persistence of the different types of nosocomial pathogens on textiles.
Methods
A MedLine search was performed on the 29th and 31st of May, 2019. The following terms were used: cotton bacteria survival (361 hits), cotton virus survival (155 hits), cotton virus persistence (39 hits), cotton yeast survival (16 hits), cotton fungus survival (267 hits), cotton mycobacterium survival (19 hits), polyester bacteria survival (327 hits), polyester virus survival (57 hits), polyester virus persistence (8 hits), polyester yeast survival (13 hits), polyester fungus survival (174 hits), polyester mycobacterium survival (13 hits), wool bacteria survival (46 hits), wool virus survival (26 hits), wool virus persistence (8 hits), wool yeast survival (5 hits), wool fungus survival (31 hits), wool mycobacterium survival (5 hits), silk bacteria survival (51 hits), silk virus survival (14 hits), silk virus persistence (0 hits), silk yeast survival (6 hits), silk fungus survival (32 hits), and silk mycobacterium survival (0 hits). Publications were included and results were extracted from them when they provided original data on the survival or duration of persistence of bacteria, mycobacteria, fungi or viruses on textiles. Articles were excluded when they did not provide any original data on survival or persistence. Reviews were also excluded, but screened for any information within the scope of the review.
Results
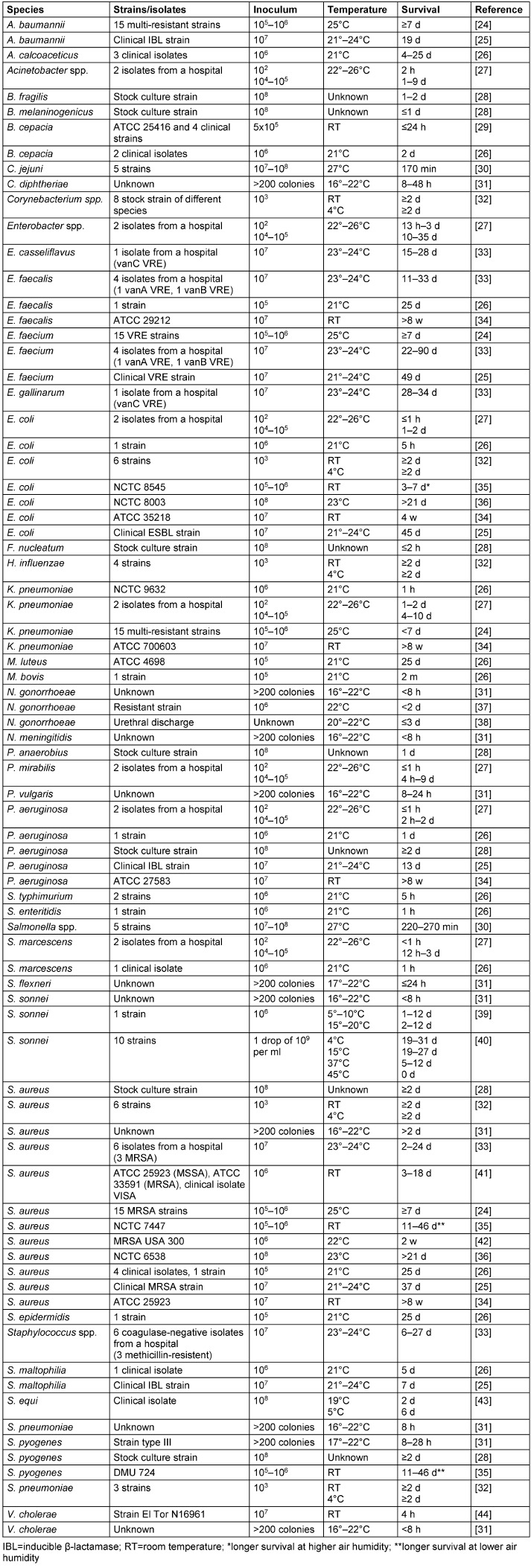
Bacteria
Cotton
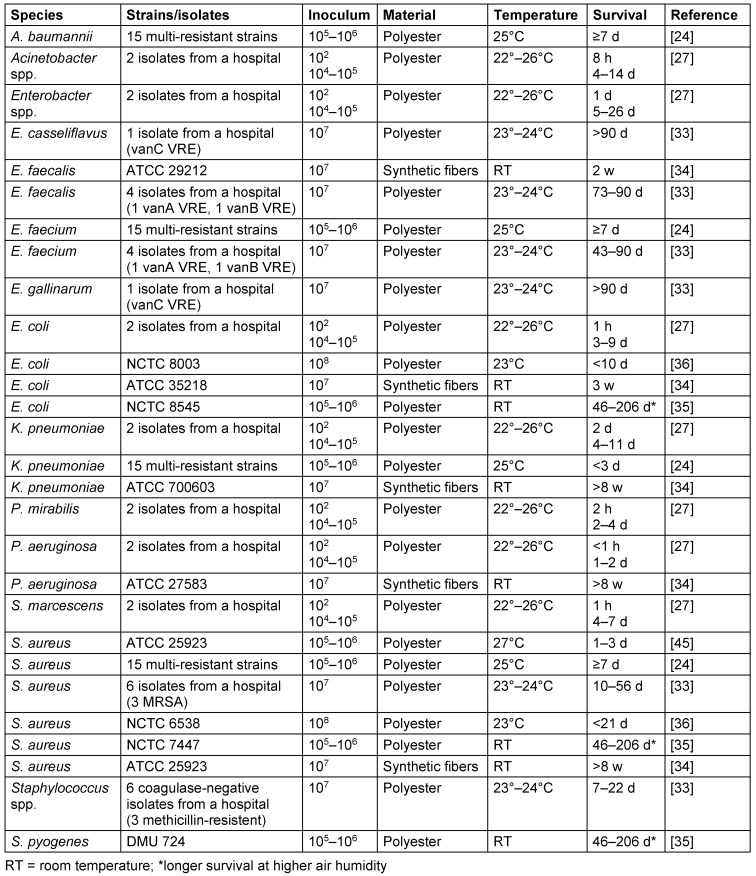
On cotton, many bacterial species are able to survive at room temperature for long periods of time, such as Enterococcus spp. (up to 90 d), P. aeruginosa (up to 8 w), S. aureus (up to 8 w), K. pneumoniae (up to 8 w), S. pyogenes (up to 46 d), E. coli (up to 45 d), Enterobacter spp. (up to 35 d), S. sonnei (up to 27 d), coagulase-negative Staphylococcus spp. (up to 27 d), Acinetobacter spp. (up to 25 d), P. mirabilis (up to 9 d) and S. maltophilia (up to 7 d). Other species at a high initial cell count, however, survive only for short periods of time at room temperature, e.g., N. gonorrhoeae and S. marcescens (both up to 3 d), B. fragilis, B. cepacia and C. diphtheriae (all up to 2 d), P. vulgaris (up to 1 d), V. cholerae (up to 8 h), Salmonella spp. (up to 5 h), C. jejuni (up to 3 h) and F. nucleatum (up to 2 h). At lower temperatures, the survival may be longer, as shown for S. sonnei and S. equi. A low inoculum of approximately 100 CFU was found for many species with only a short survival period of 2 h (Acinetobacter spp.) or ≤1 h (E. coli, P. mirabilis, P. aeruginosa and S. marcescens). Only Enterobacter spp. was able to survive much longer (up to 3 d) when inoculated with only 100 CFU. M. bovis survived on cotton for 2 m. Higher air humidity was associated with longer survival of E. coli, whereas lower air humidity enhanced the survival of S. aureus and S. pyogenes (Table 1 (Tab. 1)).
Table 1. Survival of bacteria on cotton.
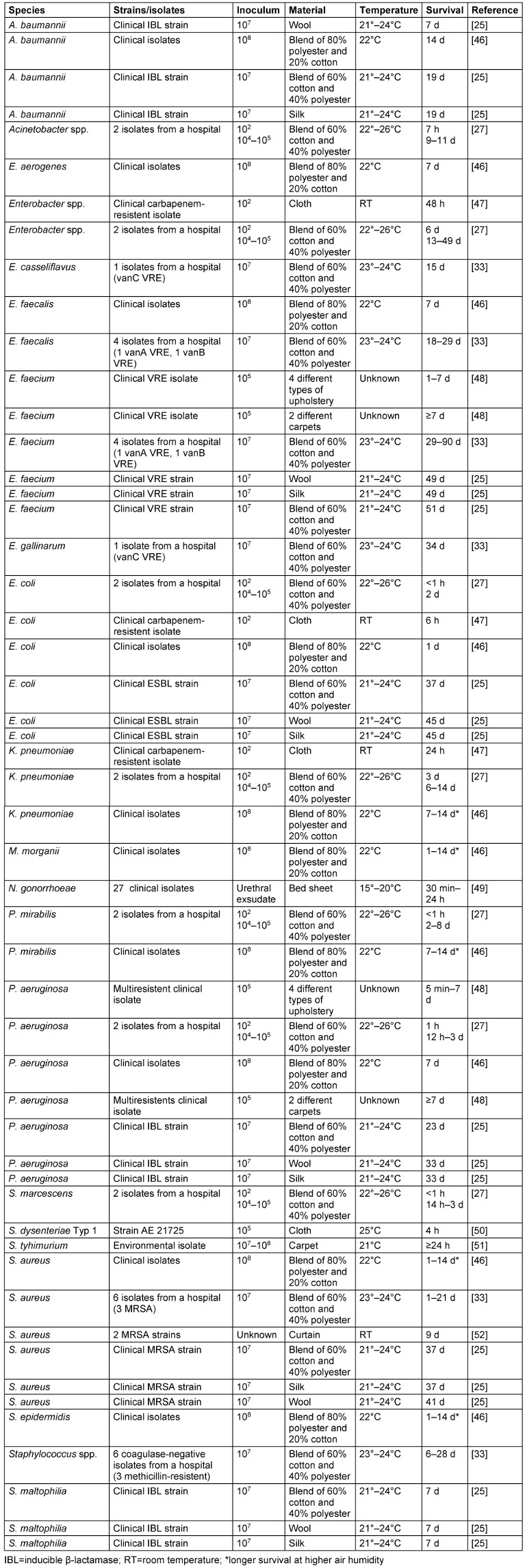
Synthetic fibers
On synthetic fibers such as polyester, the survival times of high inocula at room temperature ranged from up to 206 d (E. coli, S. aureus, S. pyogenes), 90 d (Enterococcus spp.), 56 d (K. pneumoniae, P. aeruginosa), 26 d (Enterobacter spp.), 14 d (Acinetobacter spp.) to 7 d (S. marcescens). A low inoculum of approximately 100 CFU was found for many species with only a short survival period of 2 d (K. pneumoniae), 1 d (Enterobacter spp.), 8 h (Acinetobacter spp.), 2 h (P. mirabilis) or ≤1 h (E. coli, P. aeruginosa, S. marcescens). Higher air humidity favored longer survival of E. coli, S. aureus and S. pyogenes (Table 2 (Tab. 2)).
Table 2. Survival of bacteria on synthetic fibers.
Mixed and other fibers
High inocula applied to mixed and other fibers were able to survive at room temperature for up to 90 d (Enterococcus spp.), 49 d (Enterobacter spp.), 45 d (E. coli), 41 d (S. aureus), 33 d (P. aeruginosa), 28 d (coagulase-negative Staphylococcus spp.), 19 d (Acinetobacter spp.), 14 d (K. pneumoniae, M. morganii, P. mirabilis), and 7 d (S. maltophilia). Short survival times were found with S. typhimurium (≥1 d), N. gonorrhoeae (up to 1 d), and S. dysenteriae (4 h). Low inocula of 100 CFU were often associated with shorter survival times, e.g., in K. pneumoniae (1–3 d), Acinetobacter spp. (7 h), E. coli (≤6 h) or P. mirabilis, P. aeruginosa and S. marcescens (≤1 h). Longer survival was associated with higher air humidity in K. pneumoniae, M. morganii, P. mirabilis, S. aureus and S. epidermidis (Table 3 (Tab. 3)).
Table 3. Survival of bacteria on mixed and other fibers.
Fungi
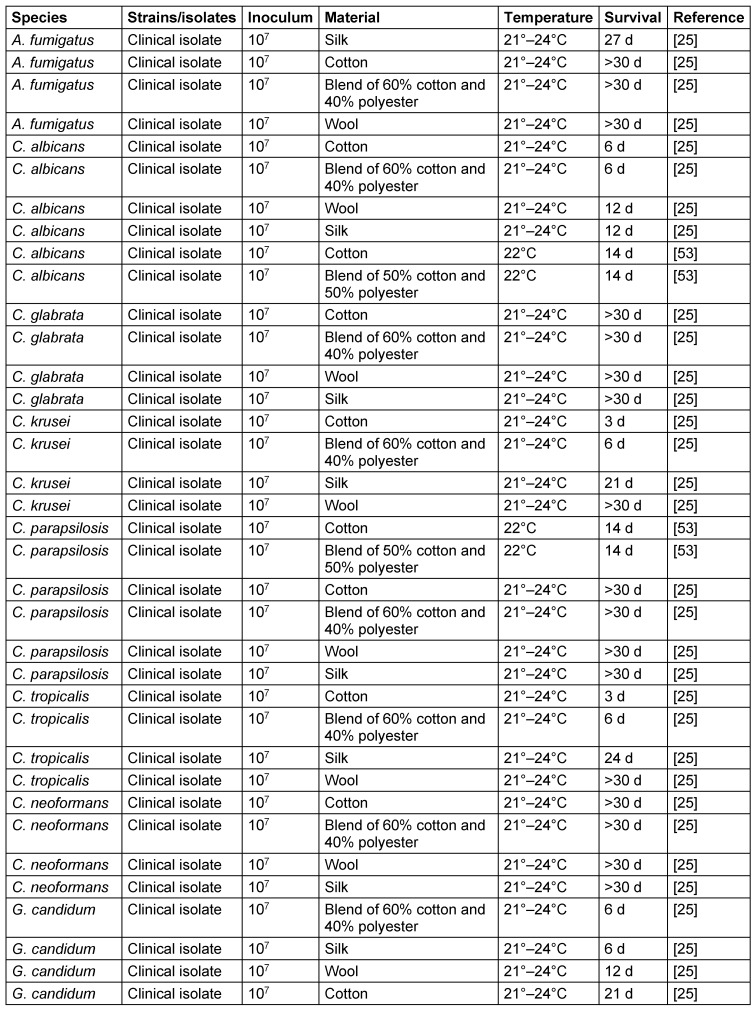
Most fungal species applied as high inocula were able to survive at room temperature on various types of fibers for 30 d or more (A. fumigatus, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. neoformans), 21 d (G. candidum), or 14 d (C. albicans). The differences between the fiber materials were variable. Four fungal species survived better on cotton or wool, three species on the blended fiber, and two on silk (Table 4 (Tab. 4)).
Table 4. Survival of fungi on different types of fibers.
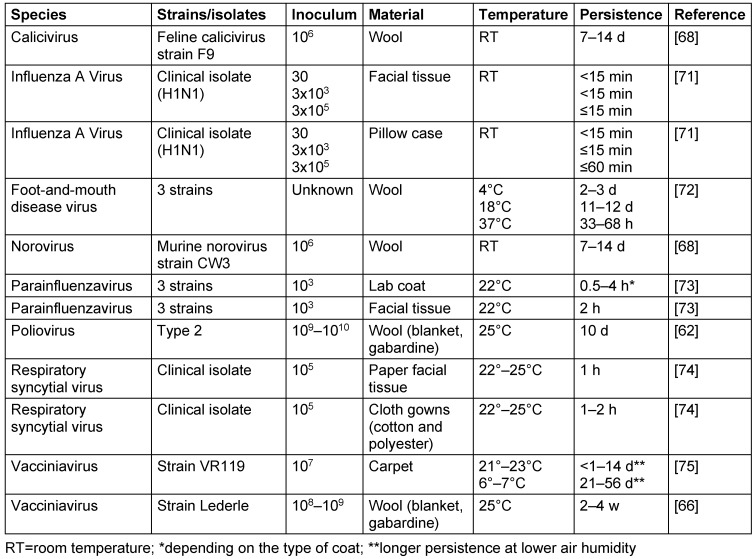
Viruses
Cotton
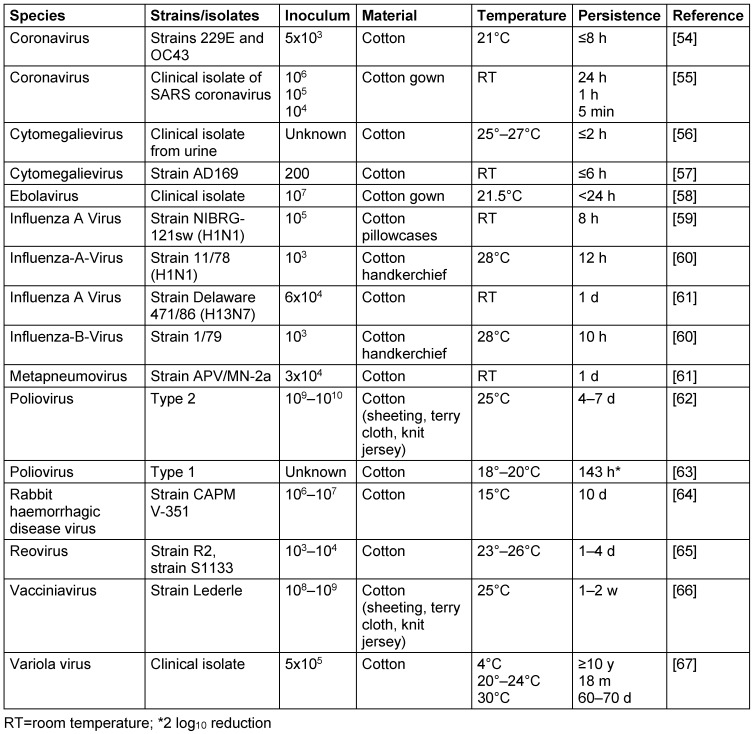
At room temperature, some viruses persisted for long periods of time, such as the variola virus (18 m), vacciniavirus (up to 2 w), rabbit haemorrhagic disease virus (up to 10 d) or poliovirus (up to 7 d). The majority of viruses lose their infectivity at room temperature on cotton within 1 d (coronavirus, cytomegalievirus, ebolavirus, influenza A virus, influenza B virus, metapneumovirus). Low inocula have a substantially shorter persistence, as demonstrated with the coronavirus. The variola virus remained infectious for more than 10 years at 4°C (Table 5 (Tab. 5)).
Table 5. Persistence of viruses on cotton.
Synthetic fibers
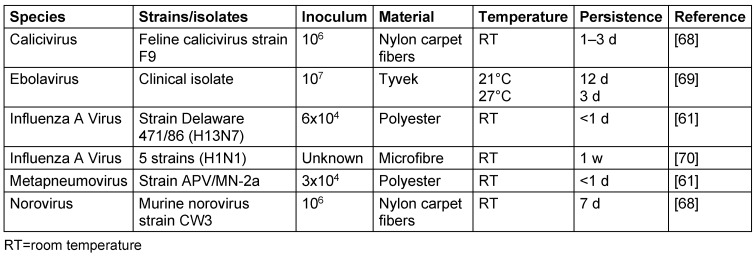
On synthetic fibers, some viruses kept their infectivity at room temperature for 12 d (ebolavirus), up to 7 d (influenza A virus, norovirus), or up to 3 d (calicivirus). The metapneumovirus could only persist for less than 1 d (Table 6 (Tab. 6)).
Table 6. Persistence of viruses on synthetic fibers.
Mixed and other fibers
On the different types of fibers, viruses kept their infectivity at room temperature for up to 28 d (vacciniavirus), 14 d (calicivirus, norovirus), 12 d (foot-and-mouth disease virus) and 10 d (poliovirus). The influenza A virus, however, persisted only for up to 1 h. A low temperature enabled the vacciniavirus to persist longer, whereas the foot-and-mouth disease virus lost its infectivity sooner (Table 7 (Tab. 7)).
Table 7. Persistence of viruses on mixed and other fibers.
Discussion
The compilation of data shows that the survival of bacteria at room temperature was the longest on polyester (up to 206 d), whereas it was 90 d for some species on cotton and mixed fibers. Only low inocula of 100 CFU were found on all types of textiles with a short survival time of ≤3 d. Most bacterial species survived better at elevated air humidity. The infectivity of viruses on textiles is lost much faster at room temperature, typically within 2–4 w. These data show that contaminated textiles may well serve as a source of transmission, provided the inoculum is high enough. Elevated air humidity is an advantage for survival of bacteria.
These data may have implications for the washing intervals of clothes worn at work. The duration of wear has an impact on the overall microbial load. It has been shown that the bacterial contamination of nurses’ coats is significantly higher after the second shift than after the first [9]. The change intervals in clinical practice may not reflect the real risk of contaminated clothes. In France, doctors changed their coats on average every 20 days [10]. Contaminated clothes may also have an impact on the contamination of the HCWs’ hands and vice versa. The presence of pathogens on coats is associated with the presence of pathogens on the hands of HCWs, whence they were probably originally transferred to the coats. Nevertheless, this still suggests that a contaminated coat can serve as a reservoir for contamination of the HCWs’ hands [11]. It has therefore been proposed that doctors leave their arms bare below the elbows, hang up their coat before patient contact, and launder their coat daily [12].
The impregnation of textiles with antimicrobial agents such as silver compounds, triclosan or copper has also been discussed to reduce their contamination in healthcare [13]. Copper-impregnated textiles can reduce multi-resistant bacterial species within 1 h [14]. Among chronic ventilator-dependent patients, a significant reduction of healthcare infections indicators, such as antibiotic treatment initiation events, fever days and antibiotic usage, was described when the HCWs wore copper-oxide impregnated textiles instead of regular hospital textiles [15]. Copper-impregnated linens were even described to reduce healthcare-associated C. difficile infections [16]. Despite all the encouraging results, the permanent exposure of nosocomial pathogens to a biocidal agent is likely to enhance tolerance to this agent [17]. A. baumannii, for example, has been described to become resistant to copper, also by exposure to subinhibitory concentrations of copper [18]. The increased tolerance may well be explained by copper efflux systems [19]. Certain P. aeruginosa isolates have also been found to possess copper tolerance [20]. Items with permanent biocidal impregnation should therefore be regarded with great caution, because it seems to be a matter of time before nosocomial pathogens develop a tolerance to them, possibly even a cross-tolerance to other biocidal agents or antibiotics [21], [22], [23].
Conclusions
Contaminated textiles or fabrics may be a source of transmission for weeks. The presence of pathogens on the coats of healthcare workers is associated with the presence of pathogens on their hands, demonstrating the relevance of textile contamination in patient care.
Notes
Competing interests
The author declares that he has no competing interests.
References
- 1.Mahida N, Prescott K, Yates C, Spencer F, Weston V, Boswell T. Outbreak of invasive group A streptococcus: investigations using agar settle plates detect perineal shedding from a healthcare worker. J Hosp Infect. 2018 Dec;100(4):e209–e215. doi: 10.1016/j.jhin.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Das I, Lambert P, Hill D, Noy M, Bion J, Elliott T. Carbapenem-resistant Acinetobacter and role of curtains in an outbreak in intensive care units. J Hosp Infect. 2002 Feb;50(2):110–114. doi: 10.1053/jhin.2001.1127. [DOI] [PubMed] [Google Scholar]
- 3.Weernink A, Severin WP, Tjernberg I, Dijkshoorn L. Pillows, an unexpected source of Acinetobacter. J Hosp Infect. 1995;29(3):189–199. doi: 10.1016/0195-6701(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 4.Cheng VCC, Chen JHK, Leung SSM, So SYC, Wong SC, Wong SCY, Tse H, Yuen KY. Seasonal Outbreak of Bacillus Bacteremia Associated With Contaminated Linen in Hong Kong. Clin Infect Dis. 2017 May;64(suppl_2):S91–S97. doi: 10.1093/cid/cix044. [DOI] [PubMed] [Google Scholar]
- 5.Hosein IK, Hoffman PN, Ellam S, Asseez TM, Fakokunde A, Silles J, Devereux E, Kaur D, Bosanquet J. Summertime Bacillus cereus colonization of hospital newborns traced to contaminated, laundered linen. J Hosp Infect. 2013 Oct;85(2):149–154. doi: 10.1016/j.jhin.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 6.John AR, Alhmidi H, Gonzalez-Orta MI, Cadnum JL, Donskey CJ. A Randomized Trial to Determine Whether Wearing Short-Sleeved White Coats Reduces the Risk for Pathogen Transmission. Infect Control Hosp Epidemiol. 2018 Feb;39(2):233–234. doi: 10.1017/ice.2017.264. [DOI] [PubMed] [Google Scholar]
- 7.Arnow PM, Houchins SG, Pugliese G. An outbreak of tinea corporis in hospital personnel caused by a patient with Trichophyton tonsurans infection. Pediatr Infect Dis J. 1991 May;10(5):355–359. doi: 10.1097/00006454-199105000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006 Aug;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta P, Bairagi N, Priyadarshini R, Singh A, Chauhan D, Gupta D. Bacterial contamination of nurses' white coats after first and second shift. Am J Infect Control. 2017 Jan;45(1):86–88. doi: 10.1016/j.ajic.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Gouraud D, Dumont R, Asehnoune K, Lejus C. White coats: how long should doctors wear them? Ann Fr Anesth Reanim. 2014 Jan;33(1):e23–e25. doi: 10.1016/j.annfar.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Munoz-Price LS, Arheart KL, Mills JP, Cleary T, Depascale D, Jimenez A, Fajardo-Aquino Y, Coro G, Birnbach DJ, Lubarsky DA. Associations between bacterial contamination of health care workers' hands and contamination of white coats and scrubs. Am J Infect Control. 2012 Nov;40(9):e245–e248. doi: 10.1016/j.ajic.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Kuehn BM. Time to hang up the white coat? Epidemiologists suggest ways to prevent clothing from spreading infection. JAMA. 2014 Feb;311(8):786–787. doi: 10.1001/jama.2014.794. [DOI] [PubMed] [Google Scholar]
- 13.Kramer A, Guggenbichler P, Heldt P, Jünger M, Ladwig A, Thierbach H, Weber U, Daeschlein G. Hygienic relevance and risk assessment of antimicrobial-impregnated textiles. Curr Probl Dermatol. 2006;33:78–109. doi: 10.1159/000093938. [DOI] [PubMed] [Google Scholar]
- 14.Irene G, Georgios P, Ioannis C, Anastasios T, Diamantis P, Marianthi C, Philippe W, Maria S. Copper-coated textiles: armor against MDR nosocomial pathogens. Diagn Microbiol Infect Dis. 2016 Jun;85(2):205–209. doi: 10.1016/j.diagmicrobio.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Marcus EL, Yosef H, Borkow G, Caine Y, Sasson A, Moses AE. Reduction of health care-associated infection indicators by copper oxide-impregnated textiles: Crossover, double-blind controlled study in chronic ventilator-dependent patients. Am J Infect Control. 2017 Apr;45(4):401–403. doi: 10.1016/j.ajic.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Butler JP. Effect of copper-impregnated composite bed linens and patient gowns on healthcare-associated infection rates in six hospitals. J Hosp Infect. 2018 Nov;100(3):e130–e134. doi: 10.1016/j.jhin.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Kampf G. Antiseptic Stewardship: Biocide Resistance and Clinical Implications. Cham: Springer International Publishing; 2018. [DOI] [Google Scholar]
- 18.Williams CL, Neu HM, Gilbreath JJ, Michel SL, Zurawski DV, Merrell DS. Copper Resistance of the Emerging Pathogen Acinetobacter baumannii. Appl Environ Microbiol. 2016 Oct;82(20):6174–6188. doi: 10.1128/AEM.01813-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alquethamy SF, Khorvash M, Pederick VG, Whittall JJ, Paton JC, Paulsen IT, Hassan KA, McDevitt CA, Eijkelkamp BA. The Role of the CopA Copper Efflux System in Virulence. Int J Mol Sci. 2019 Jan;20(3) doi: 10.3390/ijms20030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeanvoine A, Meunier A, Puja H, Bertrand X, Valot B, Hocquet D. Contamination of a hospital plumbing system by persister cells of a copper-tolerant high-risk clone of Pseudomonas aeruginosa. Water Res. 2019 Jun;157:579–586. doi: 10.1016/j.watres.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Kampf G. Challenging biocide tolerance with antiseptic stewardship. J Hosp Infect. 2018 Nov;100(3):e37–e39. doi: 10.1016/j.jhin.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Kampf G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics (Basel) 2018 Dec;7(4) doi: 10.3390/antibiotics7040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kampf G. Antibiotic ResistanceCan Be Enhanced in Gram-Positive Species by Some Biocidal Agents Used for Disinfection. Antibiotics (Basel) 2019 Feb;8(1) doi: 10.3390/antibiotics8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanczvikkel A, Tóth Á. Quantitative study about the role of environmental conditions in the survival capability of multidrug-resistant bacteria. J Infect Public Health 2018 Nov -;11(6):801–806. doi: 10.1016/j.jiph.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Koca O, Altoparlak U, Ayyildiz A, Kaynar H. Persistence of nosocomial pathogens on various fabrics. Eurasian J Med. 2012 Apr;44(1):28–31. doi: 10.5152/eajm.2012.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirai Y. Survival of bacteria under dry conditions; from a viewpoint of nosocomial infection. J Hosp Infect. 1991 Nov;19(3):191–200. doi: 10.1016/0195-6701(91)90223-u. [DOI] [PubMed] [Google Scholar]
- 27.Neely AN. A survey of gram-negative bacteria survival on hospital fabrics and plastics. J Burn Care Rehabil. 2000 Nov-Dec;21(6):523–527. doi: 10.1097/00004630-200021060-00009. [DOI] [PubMed] [Google Scholar]
- 28.Yrios JW, Balish E, Helstad A, Field C, Inhorn S. Survival of anaerobic and aerobic bacteria on cotton swabs in three transport systems. J Clin Microbiol. 1975 Feb;1(2):196–200. doi: 10.1128/jcm.1.2.196-200.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drabick JA, Gracely EJ, Heidecker GJ, LiPuma JJ. Survival of Burkholderia cepacia on environmental surfaces. J Hosp Infect. 1996 Apr;32(4):267–276. doi: 10.1016/s0195-6701(96)90037-7. [DOI] [PubMed] [Google Scholar]
- 30.De Cesare A, Sheldon BW, Smith KS, Jaykus LA. Survival and persistence of Campylobacter and Salmonella species under various organic loads on food contact surfaces. J Food Prot. 2003 Sep;66(9):1587–1594. doi: 10.4315/0362-028x-66.9.1587. [DOI] [PubMed] [Google Scholar]
- 31.Rubbo SD, Benjamin M. Some observations on survival of pathogenic bacteria on cotton-wool swabs; development of a new type of swab. Br Med J. 1951 May;1(4713):983–987. doi: 10.1136/bmj.1.4713.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross PW, Lough H. Survival of upper respiratory tract bacteria on cotton-wool swabs. J Clin Pathol. 1978 May;31(5):430–433. doi: 10.1136/jcp.31.5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol. 2000 Feb;38(2):724–726. doi: 10.1128/jcm.38.2.724-726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esteves DC, Pereira VC, Souza JM, Keller R, Simões RD, Winkelstroter Eller LK, Rodrigues MV. Influence of biological fluids in bacterial viability on different hospital surfaces and fomites. Am J Infect Control. 2016 Mar;44(3):311–314. doi: 10.1016/j.ajic.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins RO, Sherburn RE. Growth and survival of bacteria implicated in sudden infant death syndrome on cot mattress materials. J Appl Microbiol. 2005;99(3):573–579. doi: 10.1111/j.1365-2672.2005.02620.x. [DOI] [PubMed] [Google Scholar]
- 36.Riley K, Williams J, Owen L, Shen J, Davies A, Laird K. The effect of low-temperature laundering and detergents on the survival of Escherichia coli and Staphylococcus aureus on textiles used in healthcare uniforms. J Appl Microbiol. 2017 Jul;123(1):280–286. doi: 10.1111/jam.13485. [DOI] [PubMed] [Google Scholar]
- 37.Elmros T. Survival of Neisseria gonorrhoeae on surfaces. Acta Derm Venereol. 1977;57(2):177–180. [PubMed] [Google Scholar]
- 38.Srivastava AC. Survival of gonococci in urethral secretions with reference to the nonsexual transmission of gonococcal infection. J Med Microbiol. 1980 Nov;13(4):593–596. doi: 10.1099/00222615-13-4-593. [DOI] [PubMed] [Google Scholar]
- 39.Spicer CC. The survival of Shigella sonnei on cotton threads. J Hyg (Lond) 1959 Jun;57(2):210–215. doi: 10.1017/s0022172400020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura M. The survival of Shigella sonnei on cotton, glass, wood, paper, and metal at various temperatures. J Hyg (Lond) 1962 Mar;60:35–39. doi: 10.1017/s0022172400039280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarpellon MN, Gales AC, Sasaki AL, Selhorst GJ, Menegucci TC, Cardoso CL, Garcia LB, Tognim MC. Survival of vancomycin-intermediate Staphylococcus aureus on hospital surfaces. J Hosp Infect. 2015 Aug;90(4):347–350. doi: 10.1016/j.jhin.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Desai R, Pannaraj PS, Agopian J, Sugar CA, Liu GY, Miller LG. Survival and transmission of community-associated methicillin-resistant Staphylococcus aureus from fomites. Am J Infect Control. 2011 Apr;39(3):219–225. doi: 10.1016/j.ajic.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Durham AE, Hall YS, Kulp L, Underwood C. A study of the environmental survival of Streptococcus equi subspecies equi. Equine Vet J. 2018 Nov;50(6):861–864. doi: 10.1111/evj.12840. [DOI] [PubMed] [Google Scholar]
- 44.Farhana I, Hossain ZZ, Tulsiani SM, Jensen PK, Begum A. Survival of Vibrio cholerae O1 on fomites. World J Microbiol Biotechnol. 2016 Sep;32(9):146. doi: 10.1007/s11274-016-2100-x. [DOI] [PubMed] [Google Scholar]
- 45.Cuesta A, Nastri N, Bernat M, Brusca M, Turcot L, Nastri M, Rosa AC. Survival of Staphylococcus aureus on fomites. Acta Odontol Latinoam. 2008;21(2):141–146. [PubMed] [Google Scholar]
- 46.López-Gigosos R, Mariscal A, Gutierrez-Bedmar M, Mariscal-Lopez E, Fernández-Crehuet J. Persistence of nosocomial bacteria on 2 biocidal fabrics based on silver under conditions of high relative humidity. Am J Infect Control. 2014 Aug;42(8):879–884. doi: 10.1016/j.ajic.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Weber DJ, Rutala WA, Kanamori H, Gergen MF, Sickbert-Bennett EE. Carbapenem-resistant Enterobacteriaceae: frequency of hospital room contamination and survival on various inoculated surfaces. Infect Control Hosp Epidemiol. 2015 May;36(5):590–593. doi: 10.1017/ice.2015.17. [DOI] [PubMed] [Google Scholar]
- 48.Lankford MG, Collins S, Youngberg L, Rooney DM, Warren JR, Noskin GA. Assessment of materials commonly utilized in health care: implications for bacterial survival and transmission. Am J Infect Control. 2006 Jun;34(5):258–263. doi: 10.1016/j.ajic.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Pérez JL, Gómez E, Sauca G. Survival of gonococci from urethral discharge on fomites. Eur J Clin Microbiol Infect Dis. 1990 Jan;9(1):54–55. doi: 10.1007/bf01969538. [DOI] [PubMed] [Google Scholar]
- 50.Islam MS, Hossain MA, Khan SI, Khan MN, Sack RB, Albert MJ, Huq A, Colwell RR. Survival of Shigella dysenteriae type 1 on fomites. J Health Popul Nutr. 2001 Sep;19(3):177–182. [PubMed] [Google Scholar]
- 51.Dawson P, Han I, Cox M, Black C, Simmons L. Residence time and food contact time effects on transfer of Salmonella Typhimurium from tile, wood and carpet: testing the five-second rule. J Appl Microbiol. 2007 Apr;102(4):945–953. doi: 10.1111/j.1365-2672.2006.03171.x. [DOI] [PubMed] [Google Scholar]
- 52.Huang R, Mehta S, Weed D, Price CS. Methicillin-resistant Staphylococcus aureus survival on hospital fomites. Infect Control Hosp Epidemiol. 2006 Nov;27(11):1267–1269. doi: 10.1086/507965. [DOI] [PubMed] [Google Scholar]
- 53.Traoré O, Springthorpe VS, Sattar SA. A quantitative study of the survival of two species of Candida on porous and non-porous environmental surfaces and hands. J Appl Microbiol. 2002;92(3):549–555. doi: 10.1046/j.1365-2672.2002.01560.x. [DOI] [PubMed] [Google Scholar]
- 54.Sizun J, Yu MW, Talbot PJ. Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: a possible source ofhospital-acquired infections. J Hosp Infect. 2000 Sep;46(1):55–60. doi: 10.1053/jhin.2000.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai MY, Cheng PK, Lim WW. Survival of severe acute respiratory syndrome coronavirus. Clin Infect Dis. 2005 Oct;41(7):e67–e71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faix RG. Survival of cytomegalovirus on environmental surfaces. J Pediatr. 1985 Apr;106(4):649–652. doi: 10.1016/s0022-3476(85)80096-2. [DOI] [PubMed] [Google Scholar]
- 57.Stowell JD, Forlin-Passoni D, Din E, Radford K, Brown D, White A, Bate SL, Dollard SC, Bialek SR, Cannon MJ, Schmid DS. Cytomegalovirus survival on common environmental surfaces: opportunities for viral transmission. J Infect Dis. 2012 Jan;205(2):211–214. doi: 10.1093/infdis/jir722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cook BW, Cutts TA, Nikiforuk AM, Poliquin PG, Court DA, Strong JE, Theriault SS. Evaluating environmental persistence and disinfection of the Ebola virus Makona variant. Viruses. 2015 Apr;7(4):1975–1986. doi: 10.3390/v7041975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oxford J, Berezin EN, Courvalin P, Dwyer DE, Exner M, Jana LA, Kaku M, Lee C, Letlape K, Low DE, Madani TA, Rubino JR, Saini N, Schoub BD, Signorelli C, Tierno PM, Zhong X. The survival of influenza A(H1N1)pdm09 virus on 4 household surfaces. Am J Infect Control. 2014 Apr;42(4):423–425. doi: 10.1016/j.ajic.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 60.Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, Balfour HH., Jr Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982 Jul;146(1):47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 61.Tiwari A, Patnayak DP, Chander Y, Parsad M, Goyal SM. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 2006 Jun;50(2):284–287. doi: 10.1637/7453-101205R.1. [DOI] [PubMed] [Google Scholar]
- 62.Dixon GJ, Sidwell RW, McNeil E. Quantitative studies on fabrics as disseminators of viruses. II. Persistence of poliomyelitis virus on cotton and wool fabrics. Appl Microbiol. 1966 Mar;14(2):183–188. doi: 10.1128/am.14.2.183-188.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamrakar SB, Henley J, Gurian PL, Gerba CP, Mitchell J, Enger K, Rose JB. Persistence analysis of poliovirus on three different types of fomites. J Appl Microbiol. 2017 Feb;122(2):522–530. doi: 10.1111/jam.13299. [DOI] [PubMed] [Google Scholar]
- 64.Henning J, Meers J, Davies PR, Morris RS. Survival of rabbit haemorrhagic disease virus (RHDV) in the environment. Epidemiol Infect. 2005 Aug;133(4):719–730. doi: 10.1017/s0950268805003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Savage CE, Jones RC. The survival of avian reoviruses on materials associated with the poultry house environment. Avian Pathol. 2003 Aug;32(4):419–425. [PubMed] [Google Scholar]
- 66.Sidwell RW, Dixon GJ, McNeil E. Quantitative studies on fabrics as disseminators of viruses. I. Persistence of vaccinia virus on cotton and wool fabrics. Appl Microbiol. 1966 Jan;14(1):55–59. doi: 10.1128/am.14.1.55-59.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maccallum FO, McDonald JR. Survival of variola virus in raw cotton. Bull World Health Organ. 1957;16(2):247–254. [PMC free article] [PubMed] [Google Scholar]
- 68.Buckley D, Fraser A, Huang G, Jiang X. Recovery Optimization and Survival of the Human Norovirus Surrogates Feline Calicivirus and Murine Norovirus on Carpet. Appl Environ Microbiol. 2017 Nov;83(22) doi: 10.1128/AEM.01336-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer R, Judson S, Miazgowicz K, Bushmaker T, Prescott J, Munster VJ. Ebola Virus Stability on Surfaces and in Fluids in Simulated Outbreak Environments. Emerging Infect Dis. 2015 Jul;21(7):1243–1246. doi: 10.3201/eid2107.150253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson KA, Bennett AM. Persistence of influenza on surfaces. J Hosp Infect. 2017 Feb;95(2):194–199. doi: 10.1016/j.jhin.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Mukherjee DV, Cohen B, Bovino ME, Desai S, Whittier S, Larson EL. Survival of influenza virus on hands and fomites in community and laboratory settings. Am J Infect Control. 2012 Sep;40(7):590–594. doi: 10.1016/j.ajic.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 72.McColl KA, Westbury HA, Kitching RP, Lewis VM. The persistence of foot-and-mouth disease virus on wool. Aust Vet J. 1995 Aug;72(8):286–292. doi: 10.1111/j.1751-0813.1995.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 73.Brady MT, Evans J, Cuartas J. Survival and disinfection of parainfluenza viruses on environmental surfaces. Am J Infect Control. 1990 Feb;18(1):18–23. doi: 10.1016/0196-6553(90)90206-8. [DOI] [PubMed] [Google Scholar]
- 74.Hall CB, Douglas RG, Jr, Geiman JM. Possible transmission by fomites of respiratory syncytial virus. J Infect Dis. 1980 Jan;141(1):98–102. doi: 10.1093/infdis/141.1.98. [DOI] [PubMed] [Google Scholar]
- 75.Wood JP, Choi YW, Wendling MQ, Rogers JV, Chappie DJ. Environmental persistence of vaccinia virus on materials. Lett Appl Microbiol. 2013 Nov;57(5):399–404. doi: 10.1111/lam.12126. [DOI] [PubMed] [Google Scholar]