Abstract
Aims
Data on patient characteristics, prevalence, and outcomes of atrial fibrillation (AF) patients without traditional risk factors, often labelled ‘lone AF’, are sparse.
Methods and results
The RE-LY AF registry included 15 400 individuals who presented to emergency departments with AF in 47 countries. This analysis focused on patients without traditional risk factors, including age ≥60 years, hypertension, coronary artery disease, heart failure, left ventricular hypertrophy, congenital heart disease, pulmonary disease, valve heart disease, hyperthyroidism, and prior cardiac surgery. Patients without traditional risk factors were compared with age- and region-matched controls with traditional risk factors (1:3 fashion). In 796 (5%) patients, no traditional risk factors were present. However, 98% (779/796) had less-established or borderline risk factors, including borderline hypertension (130–140/80–90 mmHg; 47%), chronic kidney disease (eGFR < 60 mL/min; 57%), obesity (body mass index > 30; 19%), diabetes (5%), excessive alcohol intake (>14 units/week; 4%), and smoking (25%). Compared with patients with traditional risk factors (n = 2388), patients without traditional risk factors were more often men (74% vs. 59%, P < 0.001) had paroxysmal AF (55% vs. 37%, P < 0.001) and less AF persistence after 1 year (21% vs. 49%, P < 0.001). Furthermore, 1-year stroke occurrence rate (0.6% vs. 2.0%, P = 0.013) and heart failure hospitalizations (0.9% vs. 12.5%, P < 0.001) were lower. However, risk of AF-related re-hospitalization was similar (18% vs. 21%, P = 0.09).
Conclusion
Almost all patients without traditionally defined AF risk factors have less-established or borderline risk factors. These patients have a favourable 1-year prognosis, but risk of AF-related re-hospitalization remains high. Greater emphasis should be placed on recognition and management of less-established or borderline risk factors.
Keywords: Substrate, Less-established risk factors, Borderline risk factors, Atrial fibrillation hospitalization, Lone atrial fibrillation, Registry
What’s new?
In this sub-analysis of the RE-LY AF registry, we show that almost all patients presenting to the emergency department without traditionally defined atrial fibrillation (AF) risk factors have less-established or borderline risk factors upon closer examination.
These patients without traditional risk factors have seemingly less severe AF with predominantly paroxysmal episodes, less AF persistence, and a low 1-year risk of death, stroke, and heart failure hospitalizations. Nevertheless, their risk of AF-related re-hospitalization is high.
Almost all patients without traditional AF risk factors have other risk factors that require treatment or careful follow-up. Ultimately, this may facilitate the maintenance of sinus rhythm and improve cardiovascular outcomes.
Introduction
Sixty-five years ago, atrial fibrillation (AF) in the absence of heart disease was coined ‘lone AF’.1 However, that concept has come under scrutiny2 as our knowledge of risk factors and their importance is evolving.2–4 Over the last decade, a re-evaluation of traditional frameworks for understanding and managing of AF occurred, and focus has shifted towards optimal treatment of underlying conditions and risk factors. This includes less-established and borderline risk factors such as obesity, diabetes, sleep apnoea, borderline hypertension, chronic kidney disease, smoking, and excessive alcohol intake.2,5 Furthermore, many thresholds for detecting and defining comorbid conditions have changed, making some conditions such as hypertension more prevalent.2,4 Due to this improved ascertainment of underlying cardiovascular diseases and risk factors, the reported proportion of seemingly ‘lone AF’ decreased over the years from ∼30% to 3%.2,6,7 Therefore, it has been recommended that use of the term ‘lone AF’ should be avoided.2 Nevertheless, it still remains in use today.
Our current understanding of outcomes in AF patients previously thought to have ‘lone AF’ is largely confined to patients from North America and Europe.3,6,8,9 This is a major limitation, as we know that important regional variation exists among the global population of individuals with AF.10–12 The current analysis aimed to examine patient characteristics, prevalence of less-established or borderline risk factors, and outcome in patients without traditional risk factors from different geographic regions using data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) AF registry10,11
Methods
RE-LY AF registry
The methods of the RE-LY AF registry have been described previously.10,11 Patients from 164 sites in 47 countries, representing all inhabited continents, who presented to an emergency department or equivalent acute-care setting with AF or atrial flutter (AFL), were included in this prospective registry. The atrial rhythm disturbance could be either the primary reason for their visit or a secondary diagnosis. Although patients were not consecutive, study sites were encouraged to enrol patients as rapidly as possible to minimize bias. All patients gave written informed consent for study participation.
Study population
Between 24 December 2007 and 21 October 2011, 15 400 patients were enrolled, of whom 97.7% had AF and the rest had AFL. The present analysis excluded all patients with traditional AF risk factors including advancing age (≥60 years), myocardial infarction, coronary artery disease, congenital heart disease, heart failure, left ventricular hypertrophy or systolic dysfunction, hypertension, rheumatic heart disease, significant valvular heart disease [defined as moderate to severe (Grade 3) or severe (Grade 4)], pulmonary disease including emphysema and chronic obstructive pulmonary disease, stroke or transient ischaemic attack, hyperthyroidism, or recent cardiac surgery. These ‘traditional’ risk factors are the ones used in the Olmsted Country and Framingham cohorts (Figure 1),3,9 whose absence used to define ‘lone AF’ The non-traditional risk factors, and the terminology ‘ less-established and borderline’ are in line with the 2014 ‘Lone AF does it exist’ paper by Wyse et al.2
Figure 1.
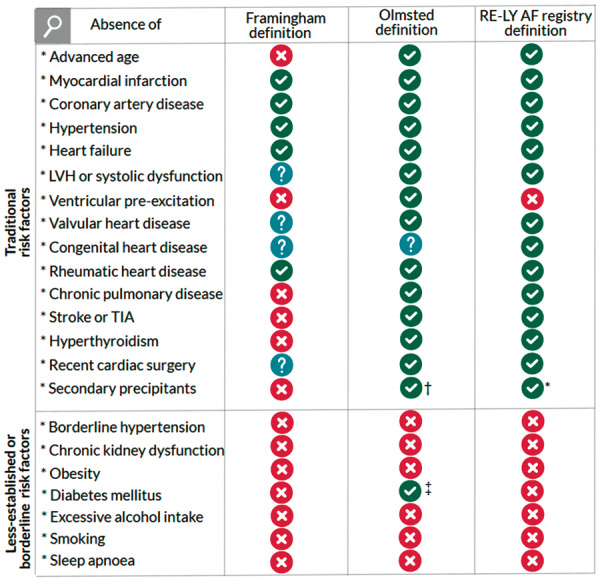
Traditional risk factors. The columns show the traditional risk factors used in the Framingham, Olmsted, and RE-LY cohorts.3,6,9aSecondary precipitants for AF were excluded, including acute coronary syndrome or arrest, pericarditis or pericardial effusion (in our cohort mainly caused by tuberculosis and HIV), myocarditis, pulmonary oedema, cerebrovascular vascular accident, aortic dissection, ICD shock, or heart failure. bPatients with AF related to surgery, trauma, or acute medical illness were excluded. cInsulin dependent diabetes mellitus. AF, atrial fibrillation; BMI, body mass index; eGFR, estimated glomerular filtration rate; LVH, left ventricular hypertrophy; TIA, transient ischaemic attack.
Patients with missing variables (n = 10) or patients with secondary precipitants for AF including acute coronary syndrome, acute pericardial disease, heart failure, infection, or other acute cerebral-, pulmonary-, or rheumatic disease were excluded from the current analysis (Figure 2 and Supplementary material online, Table S1).
Figure 2.
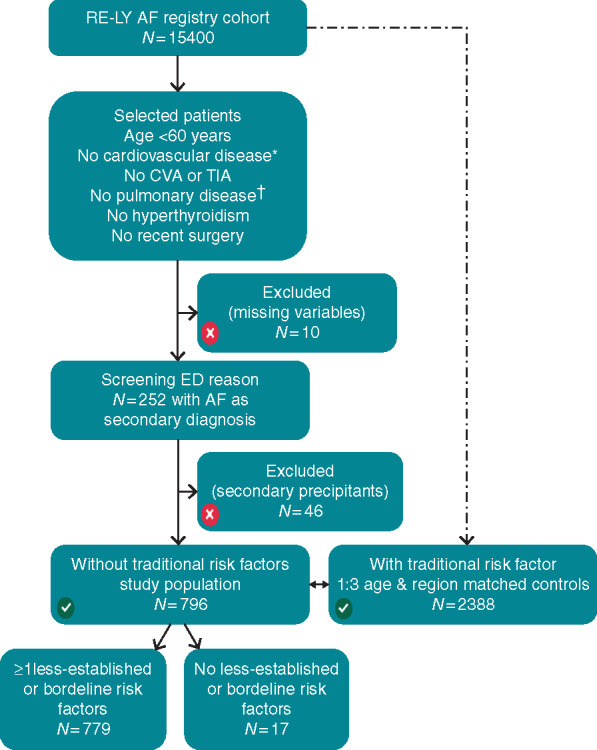
A flowchart. aNo myocardial infarction, coronary artery disease, congenital heart disease, heart failure, left ventricular hypertrophy or systolic dysfunction, hypertension, rheumatic heart disease, or significant valvular disease. bDefined as emphysema or chronic obstructive pulmonary disease. AF, atrial fibrillation; CVA, cerebrovascular accident; ED, emergency department; TIA, transient ischaemic attack.
We studied the following less-established or borderline risk factors: borderline hypertension [relative risk (RR) 130–140/80–90 mmHg], chronic kidney disease (eGFR < 60 mL/min), obesity [body mass index (BMI) >30], diabetes (oral glucose-lowering drugs and/or insulin), excessive alcohol intake (>14 units/week), smoking, and sleep apnoea (Figure 1).2 Our aim was to examine patient characteristics, study prevalence of less-established or borderline risk factors, and assess outcome. We compared patients without traditional risk factors to age- and region-matched controls with traditional risk factors (1:3 fashion) from the RE-LY AF registry.10,11 Additionally, regional comparisons were performed to provide a global overview of region-specific differences.
Follow-up
Patients were assessed 1 year after attending the emergency department. The visit occurred either in-person or consisted of a telephone call. The validated questionnaire for the verification of stroke-free status was administered to all patients. Additional required information was collected from medical records and contact with treating physicians. Clinical data were collected on the endpoints death, stroke, major bleeding, and systemic embolism, as well as admission to hospital for heart failure, myocardial infarction, AF, or AFL. Data were collected on treatment of AF during follow-up including cardioversion, ablation, and rate and rhythm control therapy.
Statistical analysis
Baseline characteristics of patients without traditional risk factors and 1:3 matched subset of patients with traditional risk factors are shown for both groups overall and the different regions. Patients from North America, Western Europe, and Australia were used as the reference population for comparison with patients from South America, Eastern Europe, the Middle East and Mediterranean crescent (including North Africa and Turkey), Sub-Saharan Africa, India, China, and Southeast Asia (participating countries by region were previously published).10 Data are presented as mean (standard deviation) and median (interquartile range) for continuous variables and frequency (%) for categorical variables. Differences between patients were evaluated by the Student’s t-test and the Mann–Whitney U test, depending on normality of the data. The χ2 and Fisher’s exact test were used for comparison of categorical variables. Comparisons between the regions, with North America, Western Europe, and Australia as the reference group, were performed using an analysis of variance or Kruskal–Wallis test for continuous variables and using Pearson χ2 test or Fisher’s exact test for categorical variables. Outcomes were compared using logistic regression models with RR and 95% confidence interval (CI) reported. Models were subsequently adjusted for sex, chronic kidney disease, diabetes mellitus, and anticoagulation/antiplatelet therapy, including warfarin, vitamin K antagonist, or aspirin. The two-sided significance level was set at 0.005 to adjust for multiple comparisons. All statistical analyses were performed using SAS 9.4 for UNIX (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Of the 15 400 patients enrolled in the RE-LY AF registry, 796 (5%) did not have traditionally defined risk factors (Figure 2). Prevalence differed between the regions: ranging from 2% in Eastern Europe to 15% in the Middle East. Baseline characteristics are listed in Table 1 for patients without and with traditional risk factors. Average age of patients without traditional risk factors was 45.7 ± 10.1 years, and 74% were men. Compared with patients with traditional risk factors, patients without traditional risk factors were taller, weighed more, were more likely to be men, and had slightly better kidney function (Table 1).
Table 1.
Baseline characteristics in patients without and with traditional risk factors
| Overall | North America, Western Europe, and Australia | South America | Eastern Europe | Middle East | Africa | India | China | Southeast Asia | P-valuea | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AF without traditional risk factors (%) | 5.2 | 7.5 | 4.6 | 1.5 | 14.9 | 3.2 | 3.2 | 4.5 | 6.2 | ||
| Number (matched 1:3) | |||||||||||
| Without traditional risk factors | 796 | 286 | 52 | 37 | 132 | 36 | 80 | 90 | 83 | ||
| With traditional risk factors | 2388 | 648 | 150 | 127 | 280 | 234 | 466 | 279 | 204 | ||
| Demographics | |||||||||||
| Age (years), mean ± SD | |||||||||||
| Without traditional risk factors | 45.7 ± 10.1 | 47.6 ± 9.4 | 45.4 ± 9.6 | 48.1 ± 9.5 | 41.2 ± 10.3b | 43.3 ± 11.8 | 45.5 ± 9.6 | 45.3 ± 10.8 | 47.2 ± 9.5 | <0.001 | |
| With traditional risk factors | 46.4 ± 11.9 | 54.3 ± 7.3 | 49.2 ± 8.7b | 48.2 ± 8.3b | 46.5 ± 9.3b | 32.4 ± 14.5 | 38.2 ± 11.4 | 48.1 ± 7.7 | 50.7 ± 6.9 | <0.001 | |
| Male (%) | |||||||||||
| Without traditional risk factors | 588 (73.9)c | 227 (79.4) | 38 (73.1) | 30 (81.1) | 106 (80.3) | 24 (66.7) | 46 (57.5)b | 59 (65.6) | 58 (69.9) | 0.001 | |
| With traditional risk factors | 1410 (59.0) | 484 (74.7) | 97 (64.7) | 94 (74.0) | 177 (63.2) | 96 (41.0) | 203 (43.6) | 136 (48.7) | 123 (60.3) | <0.001 | |
| Height (cm), mean ± SD | |||||||||||
| Without traditional risk factors | 172.4 ± 10.2c | 178.0 ± 9.4 | 172.5 ± 10.1b | 177.5 ± 9.1 | 171.2 ± 7.9b | 168.7 ± 7.7b | 162.9 ± 8.7b | 169.2 ± 7.8b | 166.8 ± 9.0b | <0.001 | |
| With traditional risk factors | 167.9 ± 11.5 | 176.0 ± 10.0 | 167.4 ± 10.5b | 175.7 ± 8.9 | 166.1 ± 9.2b | 160.9 ± 13.0b | 161.7 ± 9.1b | 165.6 ± 8.7b | 165.4 ± 9.5b | <0.001 | |
| Weight (kg), mean ± SD | |||||||||||
| Without traditional risk factors | 79.6 ± 18.7c | 90.4 ± 18.3 | 78.8 ± 12.5b | 84.6 ± 14.8 | 80.1 ± 17.8b | 76.0 ± 19.4b | 64.6 ± 12.9b | 70.7 ± 11.8b | 65.8 ± 12.3b | <0.001 | |
| With traditional risk factors | 75.8 ± 25.5 | 98.2 ± 26.3 | 78.6 ± 18.1b | 91.1 ± 20.3b | 76.9 ± 19.2b | 59.2 ± 20.0b | 57.0 ± 13.2b | 65.2 ± 14.4b | 69.0 ± 15.8b | <0.001 | |
| Body mass index (kg/m2), mean (SD) | |||||||||||
| Without traditional risk factors | 26.7 ± 5.3 | 28.6 ± 6.0 | 26.5 ± 3.9b | 26.7 ± 3.2b | 27.2 ± 5.3 | 26.7 ± 7.2 | 24.3 ± 4.0b | 24.6 ± 3.5b | 23.5 ± 3.3b | <0.001 | |
| With traditional risk factors | 26.6 ± 7.4 | 31.7 ± 8.4 | 28.0 ± 5.7b | 29.5 ± 6.0b | 27.9 ± 6.4b | 22.5 ± 6.1b | 21.7 ± 4.4b | 23.6 ± 4.1b | 25.4 ± 5.2 | <0.001 | |
| eGFR (mL/min*1.73 m2), mean (SD) | |||||||||||
| Without traditional risk factors | 87.9 ± 27.0c | 87.9 ± 25.4 | 66.2 ± 20.0b | 83.1 ± 20.8 | 87.0 ± 22.7 | 86.4±29.2 | 62.1 ± 24.9 | 88.9 ± 25.7 | 100.8 ± 32.8 | <0.001 | |
| With traditional risk factors | 80.0 ± 55.5 | 80.7 ± 54.2 | 66.7 ± 22.2 | 83.7 ± 24.3 | 750 ± 32.3 | 86.8 ± 54.0 | 61.5 ± 21.3b | 79.4 ± 29.6 | 81.0 ± 37.8 | 0.230 | |
| Blood pressure (mmHg), mean ± SD | |||||||||||
| Systolic | |||||||||||
| Without traditional risk factors | 125 ± 20 | 130 ± 21 | 120 ± 18b | 122 ± 15 | 123 ± 20b | 115 ± 16b | 119 ± 17b | 122 ± 21b | 125 ± 18 | <0.001 | |
| With traditional risk factors | 126 ± 23 | 133 ± 23 | 128 ± 25 | 133 ± 22 | 129 ± 26 | 115 ± 21b | 119 ± 19b | 121 ± 22b | 129 ± 21 | <0.001 | |
| Diastolic | |||||||||||
| Without traditional risk factors | 79 ± 14 | 84 ± 15 | 77 ± 13b | 77 ± 8b | 77 ± 14b | 73 ± 12b | 77 ± 11b | 77 ± 13b | 76 ± 12b | <0.001 | |
| With traditional risk factors | 80 ± 15 | 84 ± 17 | 81 ± 16 | 84 ± 12 | 81 ± 17 | 75 ± 16b | 77 ± 11b | 78 ± 15b | 78 ± 16b | <0.001 | |
| Prior diagnosis of AF (%) | |||||||||||
| Without traditional risk factors | 375 (47.1)c | 159 (55.6) | 25 (48.1) | 28 (75.7) | 35 (26.5)b | 6 (16.7)b | 25 (31.3)b | 57 (63.3) | 40 (48.2) | <0.001 | |
| With traditional risk factors | 1428 (59.8) | 435 (67.1) | 101 (67.3) | 90 (70.9) | 152 (54.3)b | 103 (44.0)b | 195 (41.8b | 220 (78.9)b | 132 (64.7) | <0.001 | |
| AF type (%) | |||||||||||
| Paroxysmal | |||||||||||
| Without traditional risk factors | 440 (55.3)c | 175 (61.2) | 18 (34.6)b | 13 (35.1)b | 70 (53.0) | 18 (50.0) | 43 (53.8) | 44 (48.9) | 59 (71.1) | <0.001 | |
| With traditional risk factors | 887 (37.2) | 331 (51.2) | 26 (17.3)b | 48 (37.8) | 68 (24.3)b | 26 (11.1)b | 202 (43.3) | 89 (31.9)b | 97 (47.5) | <0.001 | |
| Persistent | |||||||||||
| Without traditional risk factors | 268 (33.7)c | 99 (34.6) | 26 (50.0) | 20 (54.1) | 42 (31.8) | 8 (22.2) | 28 (35.0) | 33 (36.7) | 12 (14.5)b | <0.001 | |
| With traditional risk factors | 636 (26.6) | 205 (31.7) | 59 (39.3) | 44 (34.6) | 48 (17.1)b | 42 (17.9)b | 124 (26.6) | 66 (23.7) | 48 (23.5) | <0.001 | |
| Permanent | |||||||||||
| Without traditional risk factors | 88 (11.1)c | 12 (4.2) | 8 (15.4) | 4 (10.8) | 20 (15.2)b | 10 (27.8)b | 9 (11.3) | 13 (14.4)b | 12 (14.5)b | <0.001d | |
| With traditional risk factors | 864 (36.2) | 111 (17.2) | 65 (43.3)b | 35 (27.6) | 164 (58.6)b | 166 (70.9)b | 140 (30.0)b | 124 (44.4)b | 59 (28.9)b | <0.001 | |
| Reason for initial ED visit (%) | |||||||||||
| AF | |||||||||||
| Without traditional risk factors | 590 (74.1)c | 207 (72.4) | 45 (86.5) | 32 (86.5) | 108 (81.8) | 20 (55.6) | 52 (65.0) | 75 (83.3) | 51 (61.4) | <0.001 | |
| With traditional risk factors | 1178 (49.3) | 390 (60.2) | 86 (57.3) | 89 (70.1) | 120 (42.9)b | 63 (26.9)b | 238 (51.1)b | 109 (30.1)b | 83 (40.7)b | <0.001 | |
| Less-established or borderline risk factors (%) | |||||||||||
| Borderline hypertension (130–140/80–90 mmHg) | |||||||||||
| Without traditional risk factors | 372 (46.8) | 134 (46.9) | 25 (48.1) | 24 (64.9) | 64 (48.5) | 15 (41.7) | 41 (51.9) | 34 (37.8) | 35 (42.2) | 0.194 | |
| With traditional risk factors | 1040 (43.7) | 279 (43.1) | 70 (47.0) | 85 (66.9)b | 94 (33.6) | 77 (33.) | 227 (49.1) | 125 (45.0) | 83 (40.7) | <0.001 | |
| Chronic kidney disease (eGFR <60) | |||||||||||
| Without traditional risk factors | 456 (57.3)c | 162 (56.6) | 43 (82.7)b | 29 (78.4) | 48 (36.4)b | 20 (55.6) | 75 (93.8)b | 48 (53.3) | 31 (37.3)b | <0.001 | |
| With traditional risk factors | 1563 (65.5) | 355 (54.8) | 115 (76.7)b | 102 (80.3)b | 121 (43.2)b | 155 (66.2)b | 449 (96.4) | 192 (68.8)b | 74 (36.3)b | <0.001 | |
| Obesity (body mass index >30) | |||||||||||
| Without traditional risk factors | 153 (19.2)c | 85 (29.7) | 9 (17.3) | 4 (10.8) | 33 (25.0) | 8 (22.2) | 6 (7.5)b | 5 (5.6)b | 3 (3.6)b | <0.001 | |
| With traditional risk factors | 566 (23.7) | 309 (47.4) | 47 (31.3)b | 50 (39.4) | 81 (28.9)b | 19 (8.1)b | 10 (2.1)b | 19 (6.8)b | 31 (15.2)b | <0.001 | |
| Diabetes mellitus | |||||||||||
| Without traditional risk factors | 36 (4.5)c | 8 (2.8) | 0 (–) | 0 (–) | 14 (10.6)b | 2 (5.6) | 6 (7.5) | 3 (3.3) | 3 (3.6) | 0.011d | |
| With traditional risk factors | 378 (15.8) | 132 (20.4) | 24 (16.0) | 21 (16.5) | 75 (26.8) | 13 (5.6)b | 33 (7.1)b | 28 (10.0)b | 52 (25.5) | <0.001 | |
| Excessive alcohol intake (>14/week) | |||||||||||
| Without traditional risk factors | 31 (3.9) | 18 (6.3) | 0 (–) | 3 (8.1) | 0 (–)b | 8 (22.2)b | 0 (–) | 2 (2.2) | 0 (–) | <0.001d | |
| With traditional risk factors | 62 (2.6) | 43 (6.6) | 2 (1.3) | 5 (3.9) | 1 (0.4)b | 2 (0.9)b | 1 (0.2)b | 4 (1.4)b | 4 (2.0) | <0.001d | |
| Smoking | |||||||||||
| Without traditional risk factors | 197 (24.7) | 65 (22.7) | 14 (26.9) | 14 (37.8) | 42 (31.8) | 8 (22.2) | 6 (7.5)b | 20 (22.2) | 28 (33.7) | <0.001 | |
| With traditional risk factors | 519 (21.7) | 184 (28.4) | 29 (19.3) | 41 (32.3) | 77 (27.5) | 19 (8.1)b | 29 (6.2)b | 81 (29.0) | 59 (28.9) | <0.001 | |
| Sleep apnoea | |||||||||||
| Without traditional risk factors | 15 (1.9)c | 8 (2.8) | 1 (1.9) | 2 (5.4) | 2 (1.5) | 0 (–) | 0 (–) | 2 (2.2) | 0 (–) | 0.371d | |
| With traditional risk factors | 146 (6.1) | 93 (14.4) | 7 (4.7)b | 11 (8.7) | 9 (3.2)b | 0 (–)b | 4 (0.9)b | 20 (7.2)b | 2 (1.0)b | <0.001 | |
AF, atrial fibrillation; ED, emergency department; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LVH, left ventricular hypertrophy; SD, standard deviation.
P-value is from the test of null hypothesis that there is no difference among regions, using analysis of variance test for mean age, Kruskal–Wallis test for median age, and the χ2 test or Monte Carlo estimates of the Fisher's exact test for categorical variables.
Significantly different from North America/Western Europe, P < 0.005.
Significantly different P-value of <0.01 between patients with and without traditional risk factors.
Exact P-value was estimated by Monte Carlo simulation with 100 000 samples.
The most common less-established or borderline risk factors were borderline hypertension (130–140/80–90 mmHg; 47%), chronic kidney disease (eGFR < 60 mL/min; 57%), obesity (BMI >30 kg/m2; 19%), and smoking (25%) (Table 1). In total, 779 of 796 (98%) patients had one or more less-established or borderline risk factors. Less-established or borderline risk factors were present in a comparable or lower number in patients with traditional risk factors (Table 1).
Among patients without traditional risk factors, the prevalence of specific less-established or borderline risk factors differed between regions (Table 1). In North America and Western Europe, obesity was common (30%), and in the Middle East, both obesity (25%) and diabetes mellitus (11%) were frequent. In Eastern Europe, borderline hypertension (65%), excessive alcohol intake (8%), and smoking (38%) were often found. In South America (83%) and India (94%), high percentages of chronic kidney disease were observed, and in Africa, 22% of patients used large amounts of alcohol.
Type and treatment of atrial fibrillation
Patients without traditional risk factors more often had paroxysmal AF (55% vs. 37%, P < 0.001) and were more likely to undergo cardioversion in the emergency department, either spontaneously or through electrical or chemical cardioversion (P < 0.001) (Supplementary material online, Table S2.1). Fewer patients without traditional risk factors left the emergency department in AF compared with patients with traditional risk factors (54% vs. 77%, P < 0.001). Patients without traditional risk factors received less medications, including anticoagulation, anti-arrhythmic drugs, beta-blockade, and diuretics (all P < 0.001) (Supplementary material online, Table S2.1). They experienced more AF recurrences (28% vs. 21%, P < 0.001), but AF persistence was less pronounced after 1 year (21% vs. 49%, P < 0.001) (Supplementary material online, Table S3.1).
Outcomes
Complete 1-year follow-up was available for 793 (99.6%) patients without traditional risk factors and 2374 (99.4%) patients with traditional risk factors (Table 2). Patients without traditional risk factors suffered less strokes [5 (0.6%) vs. 48 (2%); RR 0.31 (95% CI 0.12–0.78, P = 0.013)] and had a lower all-cause mortality within 1 year of initial emergency department visit [13 patients (1.6%) vs. 165 (7%); RR 0.24 (95% CI 0.14–0.41, P < 0.001)]. Reasons for death in patients without traditional risk factors included: cancer (n = 5), unknown (n = 4), heart failure (n = 3), and sudden cardiac death (n = 1). Patients with traditional risk factors were more frequently hospitalized for heart failure (13% vs. 0.9%, P < 0.001). Hospitalizations for AF occurred often in both groups (18% in patients without vs. 21% in patients with traditional risk factors, P = 0.09). The highest rate of repeat hospital visits for AF was in North America and Western Europe (27%) (Supplementary material online, Table S4). Adjustments for sex, chronic kidney disease, diabetes mellitus, and anticoagulation use did not affect the observation of increased death, stroke, and heart failure hospitalization risk in patients with traditional risk factors (Table 2).
Table 2.
Outcomes of patients without traditional risk factors compared with age and region-matched patients with traditional risk factors
| Overall | Without traditional risk factors | With traditional risk factors | Unadjusted |
Adjusted |
|||
|---|---|---|---|---|---|---|---|
| Number of complete follow-up visit | 3167 | 793 | 2374 | RR (95% CI) | P-value | RR (95% CI)a | P-value |
| MACCE, n (%) | 235 (7.4) | 18 (2.3) | 217 (9.1) | 0.25 (0.15–0.40) | <0.001 | 0.26 (0.16–0.43) | <0.001 |
| Death | 178 (5.6) | 13 (1.6) | 165 (7.0) | 0.24 (0.14–0.41) | <0.001 | 0.25 (0.14–0.44) | <0.001 |
| Stroke | 53 (1.7) | 5 (0.6) | 48 (2.0) | 0.31 (0.12–0.78) | 0.013 | 0.35 (0.14–0.89) | 0.027 |
| Systemic embolism | 12 (0.4) | 0 (0.0) | 12 (0.5) | 0.00 (–) | 1.000 | 0.00 (–) | 1.000 |
| Major bleeding | 33 (1.0) | 3 (0.4) | 30 (1.3) | 0.30 (0.09–0.98) | 0.046 | 0.41 (0.12–1.39) | 0.154 |
| Hospitalization, n (%) | 814 (25.7) | 146 (18.4) | 668 (28.1) | 0.66 (0.56–0.77) | <0.001 | 0.72 (0.61–0.85) | <0.001 |
| Hospitalization for heart failure | 303 (9.6) | 7 (0.9) | 296 (12.5) | 0.07 (0.03–0.15) | <0.001 | 0.08 (0.04–0.17) | <0.001 |
| Hospitalization for MI | 23 (0.7) | 2 (0.3) | 21 (0.9) | 0.29 (0.07–1.22) | 0.090 | 0.36 (0.08–1.62) | 0.184 |
| Hospitalization for AF | 630 (19.9) | 141 (17.8) | 489 (20.6) | 0.87 (0.73–1.02) | 0.093 | 0.94 (0.79–1.13) | 0.529 |
Matching conducted 1:3 on age and region.
AF, atrial fibrillation; IQR, interquartile range; MACCE, major adverse cardiac and cerebrovascular event; MI, myocardial infarction; RR, relative risk; SD, standard deviation.
Adjusted for sex, chronic kidney disease, diabetes mellitus, and anticoagulation use.
Discussion
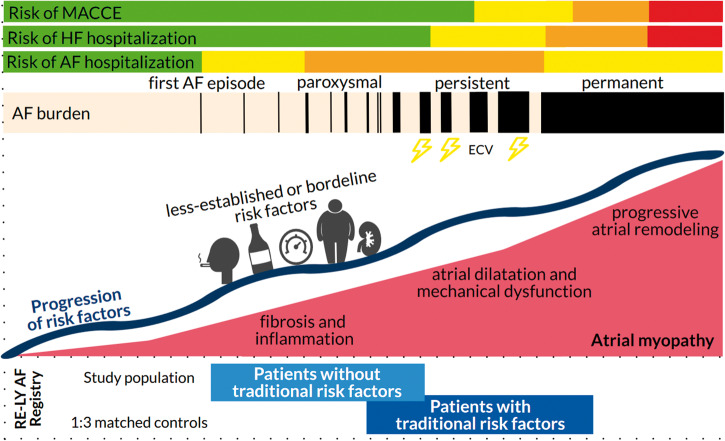
This observational study shows that almost all patients presenting to the emergency department without traditionally defined AF risk factors have less-established or borderline risk factors upon closer examination. These patients without traditional risk factors have predominantly paroxysmal episodes, less AF persistence, and a low 1-year risk of death, stroke, and heart failure hospitalizations (Figure 3). Nevertheless, their risk of AF-related re-hospitalization is high; with nearly one-fifth returning to the emergency department within 1 year. Recognition and management of these non-traditional risk could help improve patient outcomes.13
Figure 3.
In the RE-LY AF registry, patients without traditional risk factors seemed to have less severe AF, with more paroxysmal AF (55% vs. 37%, P < 0.001) and less AF persistence (21% vs. 49%, P < 0.001) compared with matched controls with traditional risk factors. Additionally, their risk of heart failure hospitalizations (0.9% vs. 12.5%) and MACCE during 1-year follow-up (2.3% vs. 9.1%) was low. However, risk of AF-related re-hospitalization was high, almost 18%, similar to patients with traditional risk factors. AF, atrial fibrillation; HF, heart failure; MACCE, major adverse cardiac or cerebrovascular events.
The term ‘lone AF’ was first used in 1954 to describe patients in whom ‘subsequent investigation shows that heart disease is absent’.1 In the last few decades, our understanding of AF pathophysiology and the multitude of systemic aetiologies and risk factors for AF has increased exponentially. We now know that AF without any risk factor is rare.2 Weijs et al.7 have shown that in clinical practice almost half of the patients originally diagnosed with idiopathic AF developed cardiovascular diseases within 5 years.14 Other long-term follow-up studies corroborate these findings and show that almost all patients develop evident cardiovascular risk factors over time.14,15 In the Olmsted study, all patients who had a cerebrovascular event during long-term follow-up had developed at least one overt risk factor for thromboembolism.3,9 The high presence of less-established or borderline risk factors in the RE-LY AF registry (98% had one or more less-established or borderline risk factors) underscores the rarity of ‘lone AF’.2 In the current population, different profiles of less-established or borderline risk factors existed across the world, with obesity being common in North America and Western Europe; borderline hypertension in the Middle East and Eastern Europe; and chronic kidney disease in South America and India.
AF in the absence of traditional risk factors is often considered a benign disease.2 We confirm that our large, global AF population without traditional risk factors has a low short-term risk of morbidity and mortality.7,8,14 This can be explained not only by the lack of cardiovascular conditions in these patients but also by their young age and low rate of AF persistence,16,17 as both morbidity and mortality are increased in patients with AF progression.18 Incident heart failure is common among patients with AF, and many traditional AF risk factors are also independent clinical predictors of heart failure. Additionally, prolongation of AF episodes >24 h is associated with a higher rate of heart failure hospitalizations, and AF type and increased burden have been found to be associated with a higher risk of ischaemic stroke.19
Although patients with AF without traditional risk factors had a lower risk of death and cardiovascular events, they had a substantial risk of repeat hospitalizations for AF. This highlights the importance of initial AF management during the emergency department visit, and the importance of appropriate follow-up for further optimization of AF management to prevent recurrent symptoms due to AF. Additionally, prevention of AF progression and management of new risk factors that may develop during follow-up of patients with AF could help to minimize the risk of adverse outcomes, including heart failure hospitalizations (Figure 3).16,17
Clinical implications
In all patients presenting with AF without an obvious cardiac cause, a thorough initial search for less-established or borderline risk factors, which vary between geographic regions, is recommended.4,5,20 In some cases, no risk factors will be present as AF can occur as a primary electrical disease; however, in many cases, borderline or non-traditional risk factors may be found. These patients seem to have less severe AF and a lower risk of adverse events. However, also these non-traditional risk factors require treatment or careful follow-up since they may contribute to progression of AF and the occurrence of cardiovascular morbidity and mortality.13
Early identification of less-established or borderline risk factors with timely, holistic treatment; targeted, tailored, and adjusted over time according to the individual needs of these patients, may facilitate the maintenance of sinus rhythm and improve cardiovascular outcomes.13 Given the complexity of AF management and the heterogeneity of patients’ risk factor profiles, integrated AF care by a multidisciplinary team in specialized AF clinics is recommended.4,5,20
Strengths and limitations
Selection of sites within regions was not random and might have introduced recruitment bias in comparing the regions, making this a convenience sample. Furthermore, our population without traditional risk factors is determined not only by definition but also by the organization of the health care systems, given differences in the extent of the search for underlying factors, and the robustness of diagnostic tools used in the different world regions. It is conceivable that risk factors or other secondary precipitants have been missed. Detailed echocardiographic and electrocardiogram data were not collected in this study. Follow-up was only 1 year, which limits the comparison of outcomes with low incidence; including stroke and death. Strengths include the relatively large, matched group of patients and the broad global representation of countries, many of which have never been included in previous registries or clinical trials of patients without traditional risk factors of AF.
Conclusion
Almost all patients without traditionally defined AF risk factors have less-established or borderline risk factors. These patients have a lower burden of AF and a more favourable 1-year prognosis but their risk of AF-related re-hospitalization remains high. Greater emphasis should be placed on the recognition and management of these AF risk factors, as this could improve patient outcomes.
Supplementary Material
Acknowledgements
J.S.H. is the Population Health Research Institute Chair of Cardiology Research and the recipient of a Heart and Stroke Foundation of Ontario Mid-Career Award (MC7450). D.C. holds a McMaster University Department of Medicine Mid-Career Research Award. His work was supported by the Hamilton Health Sciences RFA Strategic Initiative Program. I.C.V.G.7’s work was funded by the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, CVON 2014-9: Reappraisal of Atrial Fibrillation: interaction between hyperCoagulability, Electrical remodelling, and Vascular destabilization in the progression of AF (RACE V). S.Y. holds the Marion Burke Chair of the Heart and Stroke Foundation of Canada. W.F.M. holds fellowship awards from the Canadian Stroke Prevention Intervention Network (C-SPIN) and the Canadian Institutes of Health Research (CIHR).
Funding
Funding for the RE-LY AF Registry was provided by a grant from Bohringer-Ingelheim.
Conflict of interest: T.W.B. reports research support for the Centers for Disease Control/Tennessee Department of Health and Portola; paid consulting work with Red Bull. R.P. reports research grants from Abbott Medical, Medtronic and Pfizer. J.S.H. reports research grants from Boehringer Ingelheim, Bristol-Meyers-Squibb, Pfizer, Medtronic, St. Jude Medical, and Boston Scientific. All other authors have no conflict of interest in relation to the present article.
References
- 1. Evans W, Swann P.. Lone auricular fibrillation. Br Heart J 1954;16:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wyse DG, Van Gelder IC, Ellinor PT, Go AS, Kalman JM, Narayan SM. et al. Lone atrial fibrillation: does it exist? J Am Coll Cardiol 2014;63:1715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL. et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation 2007;115:3050–6. [DOI] [PubMed] [Google Scholar]
- 4. Lau DH, Nattel S, Kalman JM, Sanders P.. Modifiable risk factors and atrial fibrillation. Circulation 2017;136:583–96. [DOI] [PubMed] [Google Scholar]
- 5. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 6. Brand FN, Abbott RD, Kannel WB, Wolf PA.. Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham Study. JAMA 1985;254:3449–53. [PubMed] [Google Scholar]
- 7. Weijs B, Pisters R, Nieuwlaat R, Breithardt G, Le Heuzey JY, Vardas PE. et al. Idiopathic atrial fibrillation revisited in a large longitudinal clinical cohort. Europace 2012;14:184–90. [DOI] [PubMed] [Google Scholar]
- 8. Jouven X, Desnos M, Guerot C, Ducimetiere P.. Idiopathic atrial fibrillation as a risk factor for mortality. The Paris Prospective Study I. Eur Heart J 1999;20:896–9. [DOI] [PubMed] [Google Scholar]
- 9. Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR Jr, Ilstrup DM. et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med 1987;317:669–74. [DOI] [PubMed] [Google Scholar]
- 10. Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P. et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation 2014;129:1568–76. [DOI] [PubMed] [Google Scholar]
- 11. Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J. et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet 2016;388:1161–9. [DOI] [PubMed] [Google Scholar]
- 12. Rahman F, Kwan GF, Benjamin EJ.. Global epidemiology of atrial fibrillation. Nat Rev Cardiol 2016;13:501.. [DOI] [PubMed] [Google Scholar]
- 13. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brugemann J. et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J 2018;39:2987–96. [DOI] [PubMed] [Google Scholar]
- 14. Weijs B, de Vos CB, Tieleman RG, Peeters FE, Limantoro I, Kroon AA. et al. The occurrence of cardiovascular disease during 5-year follow-up in patients with idiopathic atrial fibrillation. Europace 2013;15:18–23. [DOI] [PubMed] [Google Scholar]
- 15. Katritsis DG, Toumpoulis IK, Giazitzoglou E, Korovesis S, Karabinos I, Paxinos G. et al. Latent arterial hypertension in apparently lone atrial fibrillation. J Interv Card Electrophysiol 2005;13:203–207. [DOI] [PubMed] [Google Scholar]
- 16. Rienstra M, Van Gelder IC, Hagens VE, Veeger NJ, Van Veldhuisen DJ, Crijns HJ.. Mending the rhythm does not improve prognosis in patients with persistent atrial fibrillation: a subanalysis of the RACE study. Eur Heart J 2006;27:357–64. [DOI] [PubMed] [Google Scholar]
- 17. Chamberlain AM, Alonso A, Gersh BJ, Manemann SM, Killian JM, Weston SA. et al. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: a population-based study. Am Heart J 2017;185:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De With RR, Marcos EG, Van Gelder IC, Rienstra M.. Atrial fibrillation progression and outcome in patients with young-onset atrial fibrillation. Europace 2018;20:1750–7. [DOI] [PubMed] [Google Scholar]
- 19. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P. et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J 2016;37:1591–1602. [DOI] [PubMed] [Google Scholar]
- 20. Mahajan R, Pathak RK, Thiyagarajah A, Lau DH, Marchlinski FE, Dixit S. et al. Risk factor management and atrial fibrillation clinics: saving the best for last? Heart Lung Circ 2017;26:990–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.