Abstract
Aims
Persistent atrial fibrillation (AF) has been explained by multiple mechanisms which, while they conflict, all agree that more disorganized AF is more difficult to treat than organized AF. We hypothesized that persistent AF consists of interacting organized areas which may enlarge, shrink or coalesce, and that patients whose AF areas enlarge by ablation are more likely to respond to therapy.
Methods and results
We mapped vectorial propagation in persistent AF using wavefront fields (WFF), constructed from raw unipolar electrograms at 64-pole basket catheters, during ablation until termination (Group 1, N = 20 patients) or cardioversion (Group 2, N = 20 patients). Wavefront field mapping of patients (age 61.1 ± 13.2 years, left atrium 47.1 ± 6.9 mm) at baseline showed 4.6 ± 1.0 organized areas, each separated by disorganization. Ablation of sites that led to termination controlled larger organized area than competing sites (44.1 ± 11.1% vs. 22.4 ± 7.0%, P < 0.001). In Group 1, ablation progressively enlarged unablated areas (rising from 32.2 ± 15.7% to 44.1 ± 11.1% of mapped atrium, P < 0.0001). In Group 2, organized areas did not enlarge but contracted during ablation (23.6 ± 6.3% to 15.2 ± 5.6%, P < 0.0001).
Conclusion
Mapping wavefront vectors in persistent AF revealed competing organized areas. Ablation that progressively enlarged remaining areas was acutely successful, and sites where ablation terminated AF were surrounded by large organized areas. Patients in whom large organized areas did not emerge during ablation did not exhibit AF termination. Further studies should define how fibrillatory activity is organized within such areas and whether this approach can guide ablation.
Keywords: Atrial fibrillation, Mechanisms, Drivers, Rotational, Focal, Ablation, Multiwavelet re-entry
What’s new?
Atrial fibrillation (AF) reveals organized spatial areas that show 1:1 electrogram activity, separated by disorganized activation.
Catheter ablation can eliminate organized areas and cause remaining organized areas to enlarge, suggesting that persistent AF represents a connected network.
Patients in whom large organized areas emerge with catheter ablation had a higher likelihood of acute AF termination.
Wavefront field mapping is a novel non-phase-based approach to quantify propagation vectors in AF and highlights clinically relevant patterns in persistent AF.
Introduction
Mechanistic understanding of atrial fibrillation (AF) is unclear. PVI is a cornerstone of AF ablation,1 but outcomes remain suboptimal with variability across centres. Several mechanisms are suggested that describe persistent AF as consisting of organized regions of high dominant frequency,2 drivers, or passive activation3,4 or, alternatively, disorganized sites5 of colliding waves represented by complex electrograms. However, it is unclear how to reconcile these mechanisms between different AF patients or studies, to guide therapy.
One notable conceptual agreement between models is that AF exhibits a spectrum from relatively organized to disorganized activity. This organizational AF spectrum was noted by Konings et al.6 and, more recently, organized AF on the electrocardiogram (ECG) has been associated with successful cardioversion and ablation7 as measured in the coronary sinus (CS).8 However, it is currently unclear how to map this spectrum of AF organization clinically to guide or assess the impact of therapy.
We hypothesized that persistent AF comprises areas of organized activity interspersed with zones of disorganization.5,6 We further reasoned that successful ablation may remove disorganized zones so that remaining organized areas cover progressively more of the atrial surface. Ultimately, this may result in the atria activating in a 1:1 fashion and no longer fibrillating, i.e. atrial tachycardia or sinus rhythm. Conversely, we reasoned that ablation that does not progressively organize the atrium may not acutely terminate AF.
We tested our hypothesis by developing and applying a novel wavefront field (WFF) approach to globally map organized areas, which we quantified and tracked over time during cases of successful and unsuccessful ablation.
Methods
Patient inclusion
We recruited consecutive patients with persistent AF at catheter ablation, in whom mapping with basket catheters were used throughout the procedure and in whom ablation acutely terminated AF during defined ablation (n = 20, Group 1). In this same time frame, we identified consecutive patients without AF termination during defined ablation (n = 20, Group 2). Patients were included only if they had atrial basket recordings until the time of termination of AF by ablation or the time of cardioversion. All patients were classified as persistent AF1 and refractory to one or more antiarrhythmic medications.
Electrophysiology study
Patients were brought to the EP lab in the post-absorptive state. All antiarrhythmic medications were discontinued >5 half-lives (>30 days for amiodarone). Catheters were placed in the right atrium (RA), CS, and left atrium (LA) via transseptal puncture. Basket catheters (64 poles, FIRMap, Abbott) were placed in the right then left atria sequentially, or simultaneously (N = 9). Recent studies show that this maps ∼85% of the atrial surface.9 For patients who presented in sinus rhythm, AF was induced with rapid pacing from the CS. If AT was induced, this was mapped and ablated first, followed by AF induction. Ablation was guided prospectively by a clinical mapping system (RhythmViewTM, Abbott). Regions of ablation were recorded relative to electrodes (e.g. CD23 reference means site bracketed by electrodes C2, C3, D2, and D3) and in electroanatomic maps. Analysis in this study then focused on WFF streamlines blinded to sites of delivered ablation and outcome. PVI was performed by guidelines, with wide-area circumferential ablation of the left and right pulmonary veins, with verification of entrance and exit block.
Data export
Unipolar electrograms from the 64-pole basket and 12-lead ECG were recorded (Bard LabSystem, Pro, Boston Scientific, MA, USA; Prucka, GE CardioLab, Boston, MA, USA) with filtering at 0.05–500 Hz, sampled at 1 kHz, with electroanatomic mapping system amplifier disconnected to reduce electromagnetic interference. Data exports were analysed retrospectively.
In patients with acute AF termination (Group 1, n = 20), data were exported for the minute spanning termination and for periods backwards in time to the first recorded epoch (‘baseline AF’). In patients who did not have acute termination of AF, data just prior to cardioversion or ibutilide administration (Group 2, DCCV = 20, ibutilide = 5) and at periods backwards in time to the first recorded epoch (‘baseline AF’) were exported.
Wavefront field mapping
We used a novel approach to map AF propagation globally.10 Wavefront field mapping applies gradient matching to calculate dynamic vector fields that describe AF wavefront propagation over time. These vector fields represent the direction and velocity of conduction across the atrium at each point in time. Raw electrograms from panoramic recordings are used as the input, and vectors are calculated based on activation timings from any method, such as openly available methods online (http://narayanlab.stanford.edu) or clinical mapping systems. The process is displayed as an animated sequence of images containing activation fronts (grayscale background features) and streamlines (green contours).
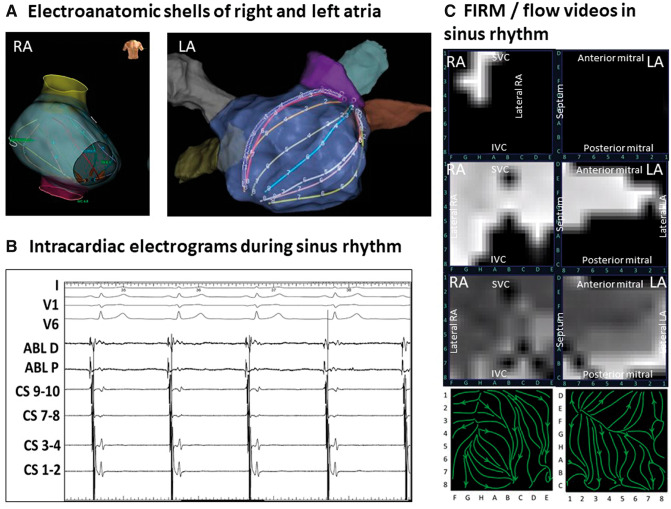
To illustrate WFF mapping, we first evaluated WFFs in sinus rhythm (Figure 1, Supplementary material online, Movie S1) in a 65-year-old Caucasian man. Figure1A and B shows biatrial basket locations, which record unipolar (Figure 1C) electrograms from the majority of the atrial endocardium (>80% in a recent study9). Figure 1D shows WFF mapping of sinus rhythm, shown as white pixels on a black background that decay through shades of grey during repolarization for successive frames (timepoints). Propagation originates from the sinus node during diastole in remaining atria (10 ms). Next, electrical propagation through the posterior RA, through Bachmann’s bundle into the LA is visualized (50 ms). Finally, laminar continuation of electrical propagation is seen at the inferior RA, spreading to the inferior and lateral LA (90 ms). Figure 1E shows a 200 ms summary of sinus wavefront propagation as streamlines (green) with arrows indicating propagation direction.
Figure 1.
WFF mapping of sinus rhythm. Biatrial basket locations in the right (A) and left atria (B) demonstrating appropriate contact and coverage of the atrium. (B) Electrograms in sinus rhythm. (C) Successive snapshots of wavefront propagation over the atria in sinus rhythm, commencing in the sinus region (10 ms), proceeding to the posterior RA and across Bachmann’s bundle (50 ms), then to the inferolateral LA. (D) WFF summary of the sinus wavefront displayed as stream lines (green) with arrows showing propagation direction. LA, left atrium; RA, right atrium; WFF, wavefront field.
Identification of organized areas in atrial fibrillation
Figures 2–4 show WFF mapping of AF. Organized areas were defined when wavefront propagation activated the area in a repeatable, 1:1 fashion, confirmed by electrogram sequence. Streamlines reveal organized areas in which activation could be rotational, focal (centrifugal), or laminar. Streamlines could also diverge from the organized pattern due to competing patch areas, lines of block, or fibrillatory conduction.
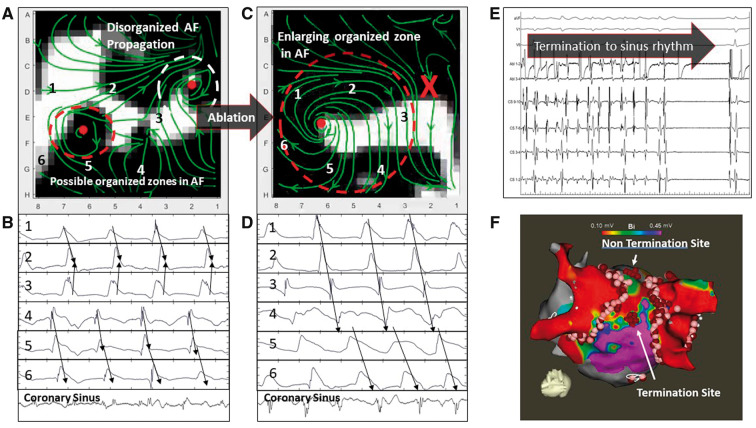
Figure 2.
WFF of persistent AF in a 55-year-old man with termination to sinus rhythm. (A) WFF and corresponding electrograms. WFF shows two organized areas (coloured ellipses) separated by disorganization. Unipolar electrograms at precise points marked on WFF streamlines confirm 1:1 activation near the red ellipse, within disorganized activity seen on bipolar CS electrograms. The white ellipse area was ablated, but AF did not terminate. (B) WFF after ablation of white organized ellipse (marked X), shows enlargement of the residual organized area (red ellipse). Unipolar electrograms confirm 1:1 activation within the red ellipse. (C) Ablation at the centre of this primary area terminated AF to sinus rhythm. (D) Electroanatomic map. AF, atrial fibrillation; CS, coronary sinus; WFF, wavefront field.
Organized areas were quantified as proportions of the mapped field. Three trained observers examined a random sampling of 30 animated image sequences (translating into 47 s videos) of AF and visually estimated the size of the largest organized area (from 0 to 100%, in 5% increments) as a proportion of the mapped field. Intraclass correlation among three observers was 94.2%. The proportion of time, or temporal presence, of each organized area, was calculated by a trained observer. The time present of the identified organized area was divided by the total mapped time in a video of the minute of mapped AF, giving a proportion of the mapped time (0–100%).
Statistical analysis
The primary analysis was based on WFF for the largest organized area in the baseline minute and the minute just prior to termination or cardioversion. Continuous data are represented as mean ± standard deviation, median, or interquartile range as appropriate. Kolmogorov–Smirnov tests did not reveal deviations from normality, so comparisons between two groups were made with Student’s t-tests for independent samples or Welch’s t-test if heterogeneity of various was present. Nominal values were expressed n (%) and compared with χ2 tests or Fisher’s exact tests as appropriate. Inter-rater agreement was quantified by intraclass correlation. A probability of <0.05 was considered statistically significant.
Results
Demographics and clinical flow
Table 1 summarizes demographics. There were no significant differences between patients in Groups 1 and 2. Group 2 patients trended towards higher comorbidities than Group 1 patients (CHADS2-VASc score 3.7 ± 1.3 vs. 3.0 ± 1.2, P = 0.07). Patients presented to the laboratory in AF in 29/40 (72.5%) of cases, and AF was induced in the remaining 11 (27.5%).
Table 1.
Patient demographics
| n = 40 | Termination (n = 20) | Non-termination (n = 20) | P-value |
|---|---|---|---|
| Age (years) | 60.1 ± 9.2 | 62.2 ± 16.5 | 0.63 |
| Gender (male) | 18 (90%) | 20 (100%) | 0.16 |
| Persistent AF | 20 (100%) | 20 (100%) | 1.00 |
| Left atrial diameter (mm) | 46.6 ± 6.0 | 47.5 ± 7.8 | 0.71 |
| Left ventricular ejection fraction | 53.3 ± 12.6 | 46.7 ± 16.9 | 0.17 |
| Hypertension | 15 (75%) | 14 (70%) | 0.73 |
| Coronary artery disease | 7 (35%) | 8 (40%) | 0.75 |
| Diabetes mellitus Type 2 | 4 (20%) | 8 (40%) | 0.17 |
| Transient ischaemia attack or stroke | 3 (15%) | 3 (15%) | 1.00 |
| CHADS2-VASc | 2.95 ± 1.2 | 3.7 ± 1.3 | 0.07 |
| Previous AF ablation | 6 (30%) | 8 (40%) | 0.51 |
AF, atrial fibrillation.
Of patients in Group 1, 14 terminated with guided ablation prior to PVI and 6 by guided ablation after PVI. Of patients in Group 2, five had delivery of ibutilide (after final mapping) to facilitate cardioversion. Atrial fibrillation was analyzed from the earliest mapped segment to AF termination (Group 1) or cardioversion (Group 2) of 124.3 ± 80.0 and 141.1 ± 75.2 min, respectively (P = 0.49).
Wavefront fields in atrial fibrillation: ablation causing termination to sinus rhythm
We applied WFF mapping during ablation of persistent AF. Figure 2 shows WFF maps in a 55-year-old Caucasian man with persistent AF in whom ablation ultimately terminated AF to sinus rhythm (Supplementary material online; Movie S2). Figure 2A shows baseline AF prior to ablation, with two organized areas indicated by red and white ellipses separated by disorganized areas. Unipolar electrograms confirm 1:1 electrogram activation within each ellipse that were asynchronous with each other, despite disorganized CS electrograms (cycle length 176 ms).
Ablation targeted the centre of the white organized site indicated by the white ellipse, where mapping showed rotational activity. Atrial fibrillation did not terminate. Figure 2B indicates WFF of AF after this ablation (marked X). Notably, the organized area indicated by the red ellipse has enlarged. Unipolar electrograms at the same electrode locations now show 1:1 organized electrogram activation in the red ellipse (marked numbers 1–6), and CS electrograms still show AF but cycle length prolonged to 191 ms. Ablation now targeted the centre of the residual large organized area (red ellipse), which terminated AF to sinus rhythm (Figure 2C). Figure 2D shows the electroanatomic map, ablation lesions, and organized sites.
Overall, AF terminated to sinus rhythm in nine cases, in each of which eliminating organized areas caused enlargement of residual areas. Elimination of such primary organized areas terminated AF to sinus rhythm in each case.
Wavefront field during atrial fibrillation ablation with termination to atrial tachycardia
We studied WFF maps in the right atrium(RA) (Supplementary material online, Movie S3) and left atrium(LA) during ablation which terminated persistent AF to atrial tachycardia, in a 74-year-old Caucasian male with persistent AF after prior AF catheter ablation (Supplementary material online, Movie S4).
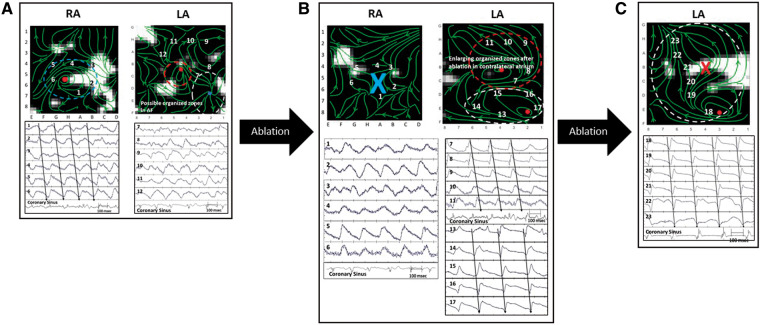
Right atrial WFF mapping of AF at baseline (Figure 3A, left panel) shows an organized area (blue ellipse), confirmed by sequential 1:1 electrograms at sites 1–6, within disorganized AF shown in bipolar CS electrograms (cycle length 179 ms). Simultaneous left atrial WFF mapping (Figure 3A, right panel) revealed two small areas (white ellipse, labels 7–12, and red ellipse) with partial organization on unipolar electrograms (CS cycle length 179 ms).
Figure 3.
Persistent AF in a 74-year-old man with termination to atrial tachycardia. (A) WFF and corresponding unipolar electrograms in right and LA, prior to ablation. WFF in RA shows an organized blue ellipse, where unipolar electrograms show sequential activation. WFF in LA shows two less organized areas (red and white ellipses). Ablation in the centre of the right atrial (blue) ellipse did not terminate AF. (B) WFF remapping of RA shows loss of organized blue ellipse and disorganized electrograms. WFF remapping of LA shows enlargement of organized areas, with 1:1 electrograms in each ellipse (red and white) at different cycle lengths. Ablation within the red ellipse terminated AF to atrial tachycardia. (C) WFF remapping of LA shows full control by the white area with 1:1 activation of electrograms and cycle length 218 ms. This atrial tachycardia was successfully ablated. AF, atrial fibrillation; LA, left atrium; RA, right atrium; WFF, wavefront field.
Ablation at the centre of the right atrial organized area where clinical mapping showed a region of interest (blue ellipse in Figure 3A, left panel) did not terminate AF. However, repeat WFF map in the RA (Figure 3B, left panel) shows loss of the organized blue area (‘X’). Wavefront field in the LA (Figure 3B, right panel) now shows enlargement of both LA areas, each with sequential 1:1 electrograms and slightly different cycle lengths (181 ms in red ellipse labels 7–11 vs. 166 ms in white ellipse labels 13–17).
Ablation guided by clinical mapping targeted the red ellipse, which terminated AF to atrial tachycardia with cycle length 218 ms. Wavefront field remapping in LA (Figure 3C) shows the ablated red ellipse (‘X’) and expansion of the white ellipse with 1:1 electrograms in the entire atrium (marked number 18–23) with cycle length 218 ms. This atrial tachycardia was mapped and successfully ablated.
Overall, 6 of 11 cases where ablation terminated AF to atrial tachycardia (n = 8) or atrial flutter (n = 3) showed two competing primary areas controlling most of the atrium, with ablation of one area leaving the remaining area as an organized atrial tachycardia. Three of the 11 cases terminated to cavotricuspid atrial flutter, whose ablation resulted in sinus rhythm.
Wavefront field maps in cases where atrial fibrillation did not terminate by ablation
We mapped WFF in a 62-year-old Caucasian man with persistent AF with previous failed AF ablation in whom ablation within organized areas failed to terminate AF (Figure 4, Supplementary material online, Movie S5). Figure 4A shows left atrial WFF prior to ablation, with small organized areas (red and yellow ellipses) confirmed by partially organized electrograms (bipolar CS electrogram CL 180 ms).
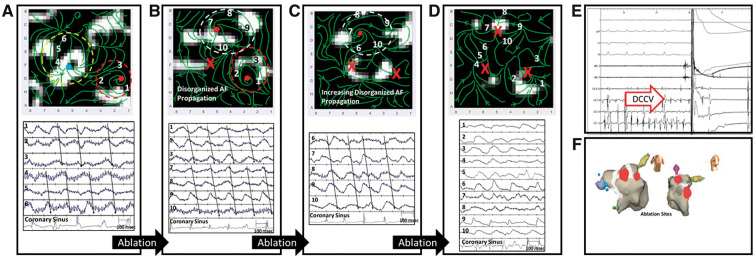
Figure 4.
Persistent AF in a 62-year-old man requiring cardioversion despite extensive ablation. (A) WFF of left atrium shows two organized areas (red and yellow ellipses) with partial organization of unipolar electrograms. Ablation within these sites did not terminate AF. (B) WFF remapping of left atrium (ablation marked X) reveals a new organized area (white ellipse) with minimal change to the previous red labelled area, with unipolar electrograms showing partial organization. Ablation of the red ellipse did not terminate AF. (C) WFF remapping of left atrium (ablated area marked X) shows continuation of white organized area, and unipolar electrograms showing some organization. Ablation within the white area did not terminate AF. (D) WFF mapping after ablation of all organized areas; unipolar electrograms show no organized areas are present (E) AF cardioversion (red arrow labeled DCCV) to sinus rhythm. (F) Electroanatomic map showing ablation lesions within organized zones (red) and PVI (white). AF, atrial fibrillation; DCCV, direct current cardioversion; PVI, pulmonary vein isolation; WFF, wavefront field.
Clinical ablation at the centre of the yellow organized area did not terminate AF. Figure 4B shows WFF after ablation (site marked X). A new organized area (white ellipse) is seen, with minimal change in the previous red area, with electrograms showing partial organization and bipolar CS electrograms (CL 172 ms).
Ablation targeting the original red ellipse also did not terminate AF. Figure 4C shows WFF after second ablation (both sites marked X) showing continuation with no expansion of the prior white organized area and AF CL 173 ms. A significant amount of the atrium was still disorganized.
Ablation of this white organized area also did not terminate AF. Figure 4D shows disorganized WFF with three ablated areas (marked X) and no residual organized areas. The atrium is now qualitatively more disorganized than at baseline, seen also in electrograms with AF CL 144 ms.
Figure 4E shows AF cardioversion to sinus rhythm, and Figure 4F shows the electroanatomic map of sites of organized regions (red circles) and pulmonary vein isolation (white circles).
Of 20 cases in Group 2 without AF termination by ablation, 18 cases showed no expansion of organized areas. The remaining two cases showed progressive enlargement of an organized area that was not ablated because it was not identified prospectively by clinical mapping.
Organized areas between patients in whom atrial fibrillation did/not terminate by ablation
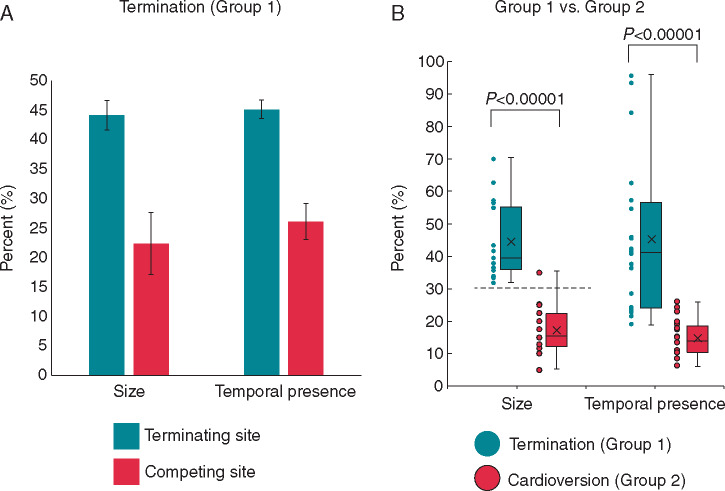
Figure 5 compares organized AF areas between groups. Figure 5A shows, for Group 1 patients, that organized areas surrounding AF termination sites covered larger atria areas (44.1 ± 11.1%) than the average of cotemporaneous competing sites (22.4 ± 7.0%; P < 0.00001). The temporal presence for organized areas was also greater for terminating than competing sites (45.1 ± 23.4% vs. 26.1 ± 13.7% of mapped time; P < 0.005).
Figure 5.
Comparison of organized areas leading to termination. (A) Bar graph showing organized area size (as % of mapped area) and temporal duration(as % of mapped duration) of sites that led to termination (terminating site, green) compared with the average of cotemporaneous competing areas (competing site, red) prior to termination. (B) Histogram of all organized area sizes and temporal duration that led to termination (green) compared with sites prior to cardioversion (red). Error bars represent standard error of means.
For Group 2 patients, in segments prior to cardioversion, there was no difference in the observed size of the largest organized area compared with the average organized area (16.6 ± 6.9% vs. 15.2 ± 5.6% of mapped area; P = 0.376).
Comparing the largest observed organized area just prior to termination and cardioversion in Groups 1 vs. 2, respectively, there was a significant difference in the size of mapped areas (44.1 ± 11.1% vs. 16.6 ± 6.9%, P < 0.0001, Figure 5B). Temporal presence of organized areas was larger in Group 1 than 2 (45.1 ± 23.5% vs. 14.8 ± 5.7% P = 0.0001) but with a less clear separating cut point.
We established a threshold for size of organized area which predicts AF termination by ablation. The final site of AF termination in Group 1 controlled an organized area >30% of mapped atrium in all cases (100%). All but one case in Group 2, the largest organized area covered <30% of mapped area (Figure 5B); the exception showed progressive enlargement of this area yet without termination for unclear reasons.
There were no differences between patients who presented in AF or sinus rhythm in mean AF cycle length (179.7 ± 19.1 ms vs. 182.3 ± 13.9 ms; P = 0.4), in WFF area in Group 1 (45.9 ± 12.3 vs. 43.1 ± 10.5 %, P = 0.30) or in Group 2 (14.1 ± 9.1 vs. 16.1 ± 7.8 %, P = 0.40), respectively.
Temporal progression of organized areas between patient groups
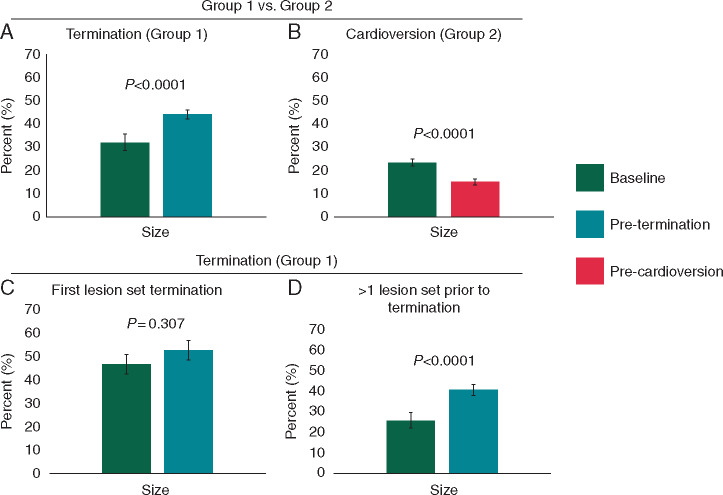
In Group 1, organized area sizes were larger just prior to termination than at baseline (44.1 ± 11.1% vs. 32.2 ± 15.7%, P < 0.0001; Figure 6A). In Group 2 (Figure 6B), there was actually a decrease in organized area size (23.6 ± 6.3 vs. 16.6 ± 6.9%; P < 0.0001) from baseline AF until just before cardioversion.
Figure 6.
Temporal progression of organized areas during ablation in both groups. (A) Bar graph showing organized area size of the terminating site in Group 1 at two different time points: at baseline AF (dark green) and prior to termination (green). (B) Comparison of organized area size at baseline AF (dark green) and prior to cardioversion (red) in Group 2. (C) In patients that had acute termination of AF with first lesion set, comparison of organized area of terminating site at both baseline AF (dark green) and prior to termination (green). (D) In patients receiving more than one ablation prior to termination of AF, comparison of organized area size of termination site at both baseline AF and prior to termination. Error bars represent standard error of means. AF, atrial fibrillation.
Temporal duration of organized areas discriminated groups less well. The temporal presence of organized areas trended to be higher just prior to termination than at baseline in Group 1 (45.1 ± 23.5% vs. 34.3 ± 21.2%, P = 0.07) compared to Group 2 (15.6 ± 7.1% vs. 14.2 ± 6.2%, P = 0.203). The change in temporal presence by ablation was greater for Group 1 than 2 (P < 0.005, Supplementary material online, Figure).
For Group 1 patients, those in whom ablation within the first area terminated AF showed no fluctuation in organized area size (46.9 ± 12.6 vs. 52.7 ± 11.6 % of mapped atrium, P = 0.307, Figure 6C), or temporal presence (53.2 ± 28.1% vs. 58.2 ± 27.9%, P = 0.729, Supplementary material online, Figure) up to this first ablation lesion set. In Group 1 patients who had ablation of multiple areas prior to AF termination, there was a progressive increase in organized areas from baseline to the final site prior to termination (25.9 ± 12.6 vs. 40.5 ± 9.0 % of mapped atrium, P = 0.0001; Figure 6D). Increase in temporal duration (26.2 ± 10.7% vs. 39.5 ± 19.9%, P < 0.05) was also observed in patients who required multiple ablations (Supplementary material online, Figure).
Discussion
This study suggests a novel mechanistic framework in which AF represents the dynamic interaction of organized atrial areas. In patients with acute procedural termination, organized AF areas progressively enlarged as competing areas were eliminated by ablation. Ablation sites surrounded by sufficiently large organized areas resulted in AF termination in all patients. Conversely, patients with smaller organized areas did not experience AF termination. This model may reconcile several proposed mechanisms, by mapping high-level organized and disorganized areas rather than by attempting to identify specific indices, such as complex fractionated electrogram (CFAE), scar, rotational, or focal drivers. Future studies should test whether ablation within larger organized atrial areas is generally more successful than ablation within regions that control less of global atrial activity.
Prior studies of atrial fibrillation organization
To our knowledge, this is the first study of organized areas within the entire mapped atria in AF, within which we show that atrial activation propagates in a coordinated 1:1 fashion. These data are consistent with, and may reconcile, many other indices of AF organization.
Progression of global atrial organization with ablation from AF to atrial flutter has been previously proposed at single sites by recurrence of electrogram shape.11 High spectral organizational index has been seen in sites leading to AF termination; progressive organization occurs after PVI.12 It has been shown that atrial tissue showing repetitive-regular activities are more likely to be sites where ablation acutely terminates AF, with better long-term outcomes.13 Conversely, an increased number of such sites may reduce the chance of termination with ablation.14
A substantial body of work shows focal and rotational organized drivers in AF, including optical mapping of human AF15 and various clinical studies with promising yet variable results.16 One major question is if some driver sites are ‘more important’ than others, such that ablating others may hinder outcomes. In the recently presented REAFFIRM trial,17 on-treatmentgroups receiving driver plus PVI ablation showed a trend for improved outcomes compared to PVI (77.7% vs. 65.5% success), but this trend was extinguished if extensive additional ablation (linear or complex electrograms) was also performed. Although rotational and focal sources in this study were often concordant between WFF and the clinical mapping system, WFF prioritizes sites by quantifying the impact of organized areas on surrounding tissue which distinguishes it from prior AF mapping approaches. This requires further study.
Prior studies of atrial fibrillation disorganization
These data may help to explain why ablation at some sites of disorganization, such as CFAEs, may be effective. Complex fractionated electrogram regions may on occasion represent pivot points for continuous re-entry of fibrillatory waves or pivot points represented by electrogram dispersion. Ablation of such sites may allow re-entry around ablated areas, leading to a larger organized area whose elimination may ultimately terminate AF.18 Organized areas fluctuate in time, and CFAEs may reflect intermittent disruption of organized areas by competing zones or fibrillatory conduction.16 Some suggest that disorganization is perpetuated by endo-epi dissociation or chaotic signals5 which may represent activity that intersperses organized areas. It is currently unclear why organized AF sizes did not increase by ablation in Group 2. It is possible that ablation was suboptimal despite adequate contact and electrogram reduction, which could have anchored instead of eliminating competing sites, or that such patients have different mechanisms with multiple small domain regions.
Mechanistic implications and future directions
These data suggest that persistent AF, a globally disorganized rhythm, exhibits a patchwork of organized areas (‘islands’) which interact to cause disorganization. This avoids the need to address currently controversial debates on identifying complete or partial rotations,19 whether re-entry in human AF is functional or anatomic, whether a focal beat indicates breakthrough from re-entry on the opposite wall,20 or whether activation is passively organized by scar. Future studies should examine why some organized areas did expand on ablation of surrounding sites (Group 1), while others did not (Group 2).
The WFF method used here is attractive for this investigation since it does not use phase mapping or traditional activation mapping16 and can rapidly identify regions of 1:1 organized electrograms. Mechanistic studies should define causes for organized patches which may differ between patients. Clinical studies should define the relationship between ablation of organized patches and long-term outcome. Real-time mapping during ablation may ultimately guide ablation.
Limitations
First, this is a relatively small cohort of patients, although they were well mapped over a long duration. Prospective, larger studies will be needed to accurately predict termination based on atrial organized areas. Organized areas were estimated by blinded, visual analysis. We are currently developing automated methods to quantify organized areas. Patients in this study were part of multiple protocols, and so long-term outcomes are not available. Global mapping is needed to define organized areas and basket catheters are limited by variable contact, electrode spacing, and movement. Nevertheless, they currently provide the highest available resolution for wide-area contact mapping. Non-invasive electrocardiographic imaging provides an alternative approach, although its accuracy has been questioned. True assessment of areas of control is limited by 2-dimensional displays of the basket. We are currently working to reconstruct organized areas in 3 dimensions in patient-specific anatomies.
Conclusion
We use novel global mapping of AF propagation to show that AF can be represented as a dynamic interaction of organized areas of control, interspersed by disorganized activity. In successful cases, ablation enabled residual areas to enlarge, and sites surrounded by a critical atrial area were invariably sites where ablation terminated AF. Patients in whom atrial areas did not enlarge did not exhibit termination. Future studies should test if using these results to guide ablation can improve outcomes.
Supplementary Material
Funding
A.J.R. acknowledges funding from the National Institutes of Health (F32HL144101). T.B. acknowledges funding from the National Institutes of Health (K23 HL145017) and a Josephson-Wellens Heart Rhythm Society Fellowship grant. W.-J.R. acknowledges funding from the National Institutes of Health (R01HL122384). S.M.N. reports research grants from National Institutes of Health (K24HL103800 and R01HL83359).
Conflict of interest: J.M.M. reports consulting fees Biosense Webster and Abbott Electrophysiology (both modest); honoraria from Biosense Webster, Medtronic Inc., and Boston Scientific (all modest); and fellowship support from Biosense Webster, Medtronic Inc., and Boston Scientific (all significant). J.B. reports modest honoraria from Medtronic, Bayer, and Biotronik. P.J.W. reports honoraria/consultant from Janssen, St. Jude Medical, Amgen, and Medtronic; (all modest) fellowship support from Biosense Webster (moderate), Boston Scientific (moderate), Medtronic, and St. Jude Medical (all modest); clinical studies from Medtronic, Siemens, Cardiofocus, and ARCA (all modest); and Stock options from Vytronus (modest). W.-J.R. reports Intellectual Property Rights from University of California Regents. S.M.N. reports consulting compensation from UpToDate, Abbott Laboratories, American College of Cardiology Foundation, Beyond Limits.ai and TDK (all modest); speaking/consulting fees from Medtronic, Inc. (modest) and St. Jude Medical (modest); and intellectual property rights from University of California Regents and Stanford University. M.P.T. reports grants from Janssen Inc., Boehringer Ingelheim, Cardiva Medical, Bristol Myers Squibb, Astra Zeneca, and American Heart Association; consulting fees from Medtronic, Precision Health Economics, iBeat, Abbott, iRhythm, Myokarida, and Biotronik (all modest); and equity from AliveCor (moderate). The remaining authors have no conflict of interest to declare.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L. et al. ; Document Reviewers. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz-Snyder R, Torrecilla EG. et al. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 2009;6:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM.. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Honarbakhsh S, Schilling RJ, Dhillon G, Ullah W, Keating E, Providencia R. et al. A novel mapping system for panoramic mapping of the left atrium: application to detect and characterize localized sources maintaining atrial fibrillation. JACC Clin Electrophysiol 2018;4:124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Groot N, van der Does L, Yaksh A, Lanters E, Teuwen C, Knops P. et al. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circ Arrhyth Electrophysiol 2016;9: 1–7. [DOI] [PubMed] [Google Scholar]
- 6. Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC, Allessie MA.. High-density mapping of electrically induced atrial fibrillation in humans. Circulation 1994;89:1665–80. [DOI] [PubMed] [Google Scholar]
- 7. Lankveld T, Zeemering S, Scherr D, Kuklik P, Hoffmann BA, Willems S. et al. Atrial fibrillation complexity parameters derived from surface ECGs predict procedural outcome and long-term follow-up of stepwise catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2016;9:e003354. [DOI] [PubMed] [Google Scholar]
- 8. Yoshida K, Chugh A, Good E, Crawford T, Myles J, Veerareddy S. et al. A critical decrease in dominant frequency and clinical outcome after catheter ablation of persistent atrial fibrillation. Heart Rhythm 2010;7:295–302. [DOI] [PubMed] [Google Scholar]
- 9. Honarbakhsh S, Schilling RJ, Providência R, Dhillon G, Sawhney V, Martin CA. et al. Panoramic atrial mapping with basket catheters: a quantitative analysis to optimize practice, patient selection, and catheter choice. J Cardiovasc Electrophysiol 2017;28:1423–32. [DOI] [PubMed] [Google Scholar]
- 10. Vidmar D, Narayan SM, Krummen DE, Rappel W-J.. Determining conduction patterns on a sparse electrode grid: implications for the analysis of clinical arrhythmias. Phys Rev E 2016;94:050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iravanian S, Langberg JJ.. Spatiotemporal organization during ablation of persistent atrial fibrillation. Heart Rhythm 2015;12:1937–44. [DOI] [PubMed] [Google Scholar]
- 12. Takahashi Y, Sanders P, Jais P, Hocini M, Dubois R, Rotter M. et al. Organization of frequency spectra of atrial fibrillation: relevance to radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2006;17:382–8. [DOI] [PubMed] [Google Scholar]
- 13. Pappone C, Ciconte G, Vicedomini G, Mangual JO, Li W, Conti M. et al. Clinical outcome of electrophysiologically guided ablation for nonparoxysmal atrial fibrillation using a novel real-time 3-dimensional mapping technique: results from a prospective randomized trial. Circ Arrhythm Electrophysiol 2018;11:e005904. [DOI] [PubMed] [Google Scholar]
- 14. Haissaguerre M, Hocini M, Denis A, Shah Ashok J, Komatsu Y, Yamashita S. et al. Driver domains in persistent atrial fibrillation. Circulation 2014;130:530–8. [DOI] [PubMed] [Google Scholar]
- 15. Hansen BJ, Zhao J, Li N, Zolotarev A, Zakharkin S, Wang Y. et al. Human atrial fibrillation drivers resolved with integrated functional and structural imaging to benefit clinical mapping. JACC Clin Electrophysiol 2018;4:1501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baykaner T, Rogers AJ, Meckler GL, Zaman J, Navara R, Rodrigo M. et al. Clinical implications of ablation of drivers for atrial fibrillation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2018;11:e006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brachmann JHJ, Wilber DJ, Sarver AE, Rapkin J, Shpun S, Szili-Torok T.. Prospective randomized comparison of rotor ablation vs. conventional ablation for treatment of persistent atrial fibrillation—the REAFFIRM Trial. Heart Rhythm 2019;16:963–5. [Google Scholar]
- 18. Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T. et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044–53. [DOI] [PubMed] [Google Scholar]
- 19. Lee S, Sahadevan J, Khrestian CM, Cakulev I, Markowitz A, Waldo AL.. Simultaneous biatrial high-density (510–512 electrodes) epicardial mapping of persistent and long-standing persistent atrial fibrillation in patients: new insights into the mechanism of its maintenance. Circulation 2015;132:2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hansen BJ, Briggs C, Moore BT, Csepe TA, Li N, Zhao J. et al. Human atrial fibrillation drivers seen simultaneously by focal impulse and rotor mapping and high-resolution optical mapping. Am Heart Assoc 2015;132:A18402. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.