Abstract
Mutations in the gene encoding bone morphogenetic protein receptor type II (BMPR2) have been associated with heritable pulmonary arterial hypertension (HPAH), whereas mutations in the gene encoding eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) are associated with heritable pulmonary veno-occlusive disease/pulmonary capillary hemangiomatosis (HPVOD/PCH). We describe two unrelated patients found to carry the same hitherto unreported pathogenic BMPR2 mutation; one of whom presented with typical pulmonary arterial hypertension, whereas the second patient presented with aggressive disease and characteristic clinical features of PVOD/PCH. These two clinically divergent cases representative of the same novel pathogenic mutation exemplify the variable phenotype of HPAH and the variable involvement of venules and capillaries in the pathology of the pulmonary vascular bed in pulmonary arterial hypertension.
Keywords: heritable pulmonary arterial hypertension, pulmonary veno-occlusive disease, pulmonary capillary hemangiomatosis, BMPR2 mutation
Introduction
Heritable pulmonary arterial hypertension (HPAH) is associated with mutations in multiple genes,1 most commonly the gene that encodes bone morphogenetic protein receptor 2 (BMPR2). Bi-allelic mutations in eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) associate with heritable pulmonary veno-occlusive disease/pulmonary capillary hemangiomatosis (PVOD/PCH).2,3 Although HPAH predominantly involves small pulmonary arterioles and heritable PVOD/PCH predominately involves capillaries and small venules, significant pulmonary arterial remodeling accompanies bi-allelic EIF2AK4 mutations, and remodeling of pulmonary septal veins may accompany BMPR2 mutations4 as recognized by the 2018 World Symposium on Pulmonary Hypertension (WSPH)5 and illustrated by the two patients with the same novel BMPR2 mutation presented herein.
Case descriptions
Case 1
A previously healthy 36-year-old Caucasian male presented with exertional dyspnea and lightheadedness. He was adopted, and the health history of his biologic parents was unknown. History, including drug and solvent exposure and all clinical, serologic laboratory and imaging evaluations were negative for congenital heart disease, connective tissue disease, portal hypertension, human immunodeficiency virus (HIV) infection or other conditions or exposures associated with pulmonary hypertension. Physical examination revealed only signs of severe pulmonary hypertension. Pulmonary function tests were normal including the diffusion capacity for carbon monoxide (DLCO). A computerized tomogram (CT) pulmonary angiogram showed no pulmonary embolism or lung parenchymal abnormalities. A transthoracic echocardiogram suggested severe pulmonary hypertension, and right heart catheterization (RHC) confirmed PAH without vasoreactivity: mean pulmonary artery pressure (mPAP) 42 mmHg; pulmonary capillary wedge pressure (PCWP) 6 mmHg; cardiac output (CO) 3.46 L/min; cardiac index (CI) 2.86 L/min/m2; pulmonary vascular resistance (PVR) 10.0 Wood units. Idiopathic pulmonary arterial hypertension (IPAH) was diagnosed, and treatment begun with tadalafil, and subsequently with inhaled treprostinil, with symptom resolution for the subsequent eight years. Subsequent genetic testing showed a novel pathogenic BMPR2 variant, with a mutation in exon 6(c.712C>T; p.Gln238*).
Case 2
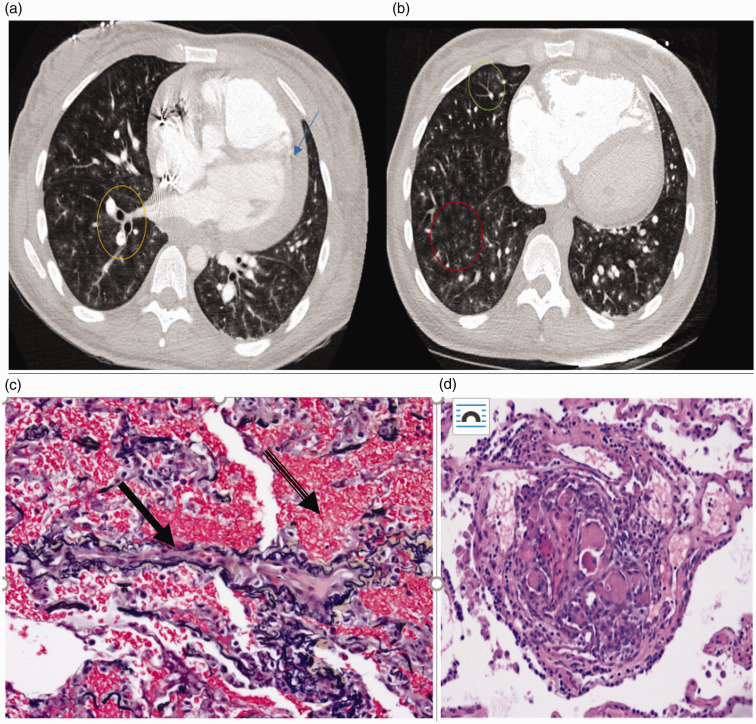
A 26-year-old black female presented with cough and dyspnea on exertion that progressed rapidly to dyspnea at rest. She was a non-smoker with no history of exposure to organic solvents or drugs associated with PAH. She had no symptoms, signs or serology suggestive of connective tissue disease, hepatitis or HIV, or family history of cardiovascular disease or early death. Pulmonary function tests were normal except for a severely reduced DLCO (34% of predicted). Ventilation-perfusion lung scan and initial high-resolution chest CT scan were normal. Transthoracic echocardiogram demonstrated severe pulmonary hypertension confirmed on RHC: mPAP50 mm Hg, PCWP 14 mm Hg, CO 3.09 L/min, CI 1.62 L/min/m2, PVR 11.4 Wood units. Initial dual oral therapy failed to mitigate symptoms, and intravenous epoprostenol was begun with the development of radiological evidence of pulmonary edema and worsening cough, dyspnea and hypoxemia. With every prostacyclin dose increment, cough and hypoxemia worsened and repeat CT scans of the chest demonstrated septal lines, ground glass opacities with centrilobular nodules and mediastinal lymphadenopathy (Fig. 1(a) and (b)). PVOD was suspected and the patient underwent expedited referral, evaluation and subsequent double lung transplantation. Pathologic examination of the explanted lungs showed marked medial arteriolar hypertrophy, concentric vascular intimal fibrosis focally associated with loss of vascular lumen, perivascular sclerosis, dilatation and tortuosity of the vessels and fibrous intimal thickening of septal veins (Fig. 1(c) and (d)). The pathology was reviewed by an external expert in PVOD, and the findings were not thought to be sufficiently extensive or severe to represent PVOD.
Fig. 1.
(a and b) Case 2: Axial images from computed tomography angiogram demonstrating diffuse bilateral centrilobular ground glass opacities (red circle), tree in bud opacities (green circle), central peribronchovascular interstitial thickening (yellow circle), and pericardial effusion (blue arrow). Case 2. (c) Elastic fiber (VVG)-stained section of lung showing a vein (solid arrow) with marked fibrous intimal thickening and almost complete obliteration of the lumen and extensive alveolar hemorrhage (banded arrow). (d): H&E-stained section of the lung showing a plexiform lesion present adjacent to two pulmonary artery branches composed of several slit-like lumens with prominent cellularity and fibrin thrombi.
Two laboratories did not identify EIF2AK4 mutations by sequencing of the coding region to look for pathogenic variants in EIF2AK4. Exonic deletions were not assessed, as to date there are no reports of exonic deletions in EIF2AK4 in PVOD/PCH patients. The same novel pathogenic BMPR2 variant in exon 6 (c.712C>T; p.Gln238*) was identified in case 1 and case 2. The mutation reported herein, the C to T change at nucleotide 712 of the coding sequence results in change of the glutamine residue at codon 238 to a stop codon, and falls into the PVS1 PS4 PM2 Pathogenic (Ia) per American College of Medical Genetics and Genomics (ACMG) guidelines.6
Discussion
We present two patients with the same novel pathogenic BMPR2 mutation but disparate clinical presentations and outcomes. Consistent with HPAH, both patients were younger than most IPAH patients and neither demonstrated a positive vasodilator response. Case 1 demonstrated none of the clinical features of PVOD/PCH, while the second patient's clinical presentation strongly suggested PVOD/PCH.
BMPR2 mutations are the most commonly identified mutations in HPAH. Pathogenic bi-allelic mutations in EIF2AK4 are identified in only 28.7% of clinically ascertained7 PVOD cases. The 2018 WSPH clarifies that pulmonary septal vein involvement can occur in BMPR2 mutation carriers; however, PVOD without arterial plexiform lesions has been reported only once in association with a BMPR2 mutation (c.43-44delC resulting in p.Pro15fs+32 in exon 1).8 The nomenclature for mutation reporting has changed in the 17 years that has intervened since its report, and it is more properly termed c.44 delC resulting in p.Pro15fs*32. That report antedated the identification of EIF2AK4 mutations as a cause of HPVOD/PCH. The deletion reported therein resulted in a frameshift mutation which was predicted to cause premature termination of the protein with the truncated protein thought to cause PVOD by haploinsufficiency or possibly by dominant negative effects. The mutation reported herein falls into the PVS1 PS4 PM2 Pathogenic (Ia) class per ACMG guidelines.
While the heterogeneity in our patients' presentations and disease trajectory with the same genetic mutation could reflect gender,9,10 racial differences, or the presence of genetic factors modifying the impact of BMPR2 mutations,11 these two cases illustrate the emerging concept that PAH and PVOD/PCH are part of a spectrum of pulmonary vascular disease.5 They exemplify the variable clinical expression of PAH caused by a novel pathogenic BMPR2 mutation, in the absence of EIF2AK4 mutations, and further underscore the importance of genetic testing in patients diagnosed with PAH or PVOD/PCH.
Acknowledgements
The authors would like to thank Dr. Carlyne D. Cool for her review and report on the pathology.
Contributorship
Oriaku and LeSieur were residents at the time of their data collection and review of the papers. Nichols and Barrios were involved with the review, critique and approval of the paper and contributed to the genetic and pathological reviews respectively. All authors were equally involved in the preparation, writing and final approval of the article.
Conflicts of interest
The author(s) declare that there is no conflict of interest.
Ethical approval
Covered under GME and QM.
Funding
William C. Nichols PAH Biobank is funded by a grant from the NIH. NHLBI grant, 5R24HL105333.
Guarantor
AF.
ORCID iD
Adaani Frost https://orcid.org/0000-0002-5566-2103
References
- 1.Morrell NW, Aldred MA, Chung WK, et al. Genetics and genomics of pulmonary arterial hypertension, World Symposium on Pulmonary Hypertension Series. Eur Respir J 2019; 53: 1801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Best DH, Sumner KL, Austin ED, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest 2014; 145: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease. Nat Genet 2014; 46: 65–69. [DOI] [PubMed] [Google Scholar]
- 4.Ghigna M-R, Guignabert C, Montani D, et al. BMPR2 mutation status influences bronchial vascular changes in pulmonary arterial hypertension. Eur Respir J 2016; 48: 1553–1555. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1): 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montani D, Girerd B, Jaïs X, et al. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med 2017; 5: 125–134. [DOI] [PubMed] [Google Scholar]
- 8.Runo JR, Vnencak-Jones CL, Prince M, et al. Pulmonary veno-occlusive disease caused by an inherited mutation in bone morphogenetic protein receptor II. Am J Respir Crit Care Med 2003; 167: 889–894. [DOI] [PubMed] [Google Scholar]
- 9.Medrek S, Sahay S, Zhao C, et al. Impact of race on survival in pulmonary arterial hypertension: results from the REVEAL registry. J Heart Lung Transplant 2020; 39(4): 321–330. . [DOI] [PubMed] [Google Scholar]
- 10.Ventetuolo CE, Baird GL, Barr RG, et al. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med 2016; 193: 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu N, Pauciulo MW, Welch CL, et al. Novel risk genes and mechanisms implicated by exome sequencing of 2572 individuals with pulmonary arterial hypertension. Genomc Med 2019; 11: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]