Abstract
Benign prostatic hyperplasia (BPH) is one of the most common diseases of the genitourinary system. The prevalence of BPH increases in men with advancing age. While transurethral resection of the prostate gland entails complications such as retrograde ejaculation, urinary incontinence, hematuria, urethral strictures, bladder neck sclerosis, and other adverse events, it is necessary to apply minimally invasive surgical methods such as superselective embolization of the prostatic arteries (PAE), particularly Proximal Embolization First Then Distal Embolization (PErFecTED). The data from 1,015 BPH patients who underwent endovascular surgery demonstrate the benefits of PErFecTED treatment during 24 months after surgery. Both Quality of Life score and International Prostate Symptom Score were around three times better in the PErFecTED group and remained stable during the entire observation period. However, the technique needs to be improved due to the high risk of postembolization syndrome.
Keywords: BPH, minimally invasive surgical methods, PAE, embolization, superselective embolization of prostatic arteries
Study Relevance
Benign prostatic hyperplasia (BPH) is one of the most common diseases of the genitourinary system. It affects about 50% of 60-year-olds and almost 90% by the age of 85 years.
Medicine offers a patient several effective treatment methods, the most important of which remains conservative therapy. When drug treatment is ineffective, however, surgical treatment is indicated.
The best method of surgical treatment of BPH is an endoscopic surgery. Transurethral resection of the prostate gland (TURP) is a “gold standard” for surgical treatment of BPH. However, despite many years of surgical experience, TURP might entail complications such as retrograde ejaculation, urinary incontinence, hematuria, urethral strictures, and bladder neck sclerosis. As a consequence, it cannot be performed in a patient with high surgical and anesthetic risks. Modern methods of laser transurethral surgery as well as other surgical methods of managing BPH, such as retropubic and laparoscopic adenomectomy, have similar limitations. The implementation of surgical treatment is often impossible due to severe somatic status. Minimally invasive surgical approach for treating BPH is especially important, because it reduces anesthetic intervention, duration of surgery, and likelihood of postoperative complications.
Among other many minimally invasive surgical methods for treating BPH, superselective embolization of the prostate arteries (PAE) is noteworthy. The main advantage of this method is that it can be performed under local anesthesia. It also might be used in the treatment of elderly patients with severe concomitant pathology.
The Use of PAE in Clinical Practice
In 2000, DeMeritt et al. (2000) performed the embolization of the right lower cystic artery in a 76-year-old man with incurable macrohematuria caused by BPH. Transcatheter arterial embolization of the right lower cystic artery was performed using polyvinyl alcohol (PVA) until the prostate was completely devascularized. Based on the results of a 12-month follow-up, it was noted that the International Prostate Symptom Score (IPSS) improved from 24 to 13 points and prostate volume decreased by 40% from 305 to 190 ml.
In Russia, the first results of the clinical use of PAE for BPH treatment were presented by Yakovets et al. (2010) in 2010. From 2004 to 2010, 38 patients with BPH and severe concomitant pathology, which did not allow traditional surgical treatment, underwent PAE. Embolization was successfully performed for all 38 patients; the quality of urination improved in all patients, prostate volume decreased, and 4 patients had cystostomy drainage removed.
Carnevale et al. (2010) performed PAE in two patients using 300–500 µm microspheres. Six months later in both cases, a decrease of infravesical obstruction, prostate volume, and volume of residual urine was noted. Later in 2011, the same authors presented the medium-term results of treatment of the same patients: The volume of the prostate did not increase and the quality of urination after 30 months of observation was similar to the value at the 6-month point (Carnevale et al., 2011, 2013).
In 2011, Pisco et al. (2011) published the results of a study evaluating the effectiveness of PAE in patients with lower urinary tract symptoms (LUTS) because of BPH. The procedure was technically successful in 14 of 15 patients; IPSS score decreased significantly, Quality of Life (QoL) score improved, peak urination rate increased, and prostate volume decreased. Subsequently, a significant decrease in obstructive and irritative symptoms was confirmed in 104 patients (Pisco et al., 2013).
In 2011, Kurbatov, Sitkin, et al. (2011) at the Congress of the American Urological Association (AUA) were the first from Russia to present a report on joint work with A.I. Neymark (Kurbatov, Sitkin, et al., 2011) dedicated to the intercenter investigation of PAE treatment of large volume BPH. Later in 2014, 2015, and 2018, Kurbatov et al. published data on PAE treatment of 106 patients with a prostate volume of more than 80 cm3 (Kurbatov et al., 2014; Russo et al., 2015; Sitkin et al., 2018).
In 2014, Gao et al. (2014) published the results of a randomized controlled study in 114 patients with BPH. Patients were divided into two groups: the TURP (n = 57) and the PAE (n = 57) groups. In patients of both groups, 24 months after the operation, almost identical values of maximum urination flow rate (Qmax) and postvoid residual as well as similar indices on the IPSS and QoL scales were noted. (Gao et al., 2014).
In 2015, Neymark et al. (2015) reported the results of PAE from 2004 to 2014 in 59 patients with BPH; the average age of patients was 68.2 ± 6.2 years and the prostate volume varied from 35 to 296 cm3. After embolization the severity of symptoms according to the IPSS scale dramatically decreased, Qmax increased, prostate volume decreased by 53% (p < .01), and the volume of nodular formation decreased by 47% (p < .01; Neymark et al., 2017).
In a study by Carnevale et al. (2014) three groups of patients underwent TURP, classical PAE, and prostate embolization using an advanced technology— Proximal Embolization First Then Embolize Distal (PErFecTed). It demonstrated a significant advantage of PErFecTed over TURP in the assessment of LUTS according to the IPSS scale after 12 months of follow-up time: 3.6 ± 2.9 in the PErFecTed embolization group versus 6.1 ± 8.6 in patients who underwent TURP. Patients after PAE and PErFecTed embolization did not have any complications that occurred after TURP and included urinary incontinence (26.7%), prostatic capsule rupture (6.7%), retrograde ejaculation (100%), and rehospitalization because of hematuria (Carnevale et al., 2016).
From 2013 to 2019, the collaborators of the Moscow Research and Education Center of Lomonosov Moscow State University (MSU) and the Department of Urology and Andrology of Lomonosov Moscow State University in conjunction with Research Institute of Clinical Surgery of Pirogov Russian National Research Medical University (RNRMU) performed superselective embolization of the prostatic arteries in 143 patients with BPH. Bilateral superselective embolization of prostatic arteries was successfully performed in 123 patients, in 14 cases unilateral PAE was performed, and 6 patients underwent repeated PAE in a period of up to 16 months (Karpov et al., 2019).
As of December 2019, the cumulative experience of the research groups of Lomonosov MSU Moscow Research and Education Center, Department of Urology and Andrology of Lomonosov MSU, Research Institute of Clinical Surgery of Pirogov RNMRU, Department of Urology and Andrology with a course in specialized surgery of Altaic State Medical University (Barnaul), and Department of Andrology and Urology of National Medical Research Center of Endocrinology comprises more than 1,000 surgical interventions.
Materials and Methods
A multicenter study included 1,015 patients treated from 2004 to 2019. The average age of the patients was 68.1 ± 9.2 years and the average prostate volume was 96 ± 24.7 cm3. In 114 cases, urination was carried out through cystostomy drainage. The follow-up period was 24 months with control examinations at 3, 6, 12, and 24 months after PAE. The algorithm of preoperative examination and further observation included determining the volume of the prostate by performing a transurethral ultrasound or MRI, determining the volume of residual urine using ultrasound, a blood test for prostate-specific antigen (PSA), uroflowmetry, and determining the LUTS using the IPSS and QoL questionnaires. Hydrogel microspheres with a diameter of 100–300 μm and 300–500 μm as well as PVA microparticles with a diameter of 100–500 μm were used as embolization material. The type of discharge of the prostate arteries from the internal iliac artery was analyzed intraoperatively.
Indications for PAE included prostate volume greater than 60 cm3, no effect of conservative therapy after 6 months, index values on the IPSS scale >18 points and on the QoL scale >3 points, Qmax <13 ml/s, anamnesis of acute or chronic urinary retention, the presence of cystostomy, age over 65 years, patient’s desire to avoid open or transurethral intervention, and desire of the patient to maintain antegrade ejaculation. The main indication for PAE was the incapacity to perform surgical treatment because of high anesthetic risk, severe somatic status, and the inability to perform open or endoscopic treatment. Contraindications for PAE were drug intolerance of an x-ray contrast drug, severe atherosclerosis, and vascular malformations in the area of aortic bifurcation and external or internal iliac arteries. For statistical processing of the results, IBM SPSS Statistics 23 software was used.
Results
Bilateral embolization of the prostatic arteries was successfully performed in 931 (91.7%) cases; in 49 (4.8%) patients, puncture of the contralateral femoral artery was required due to technical difficulties. Unilateral PAE was performed in 69 (6.8%) cases as a result of anatomical features of the patients. At the early stages of the study, patients underwent selective embolization of the prostatic arteries without superselective catheterization of the capsular and stromal branches of the prostatic artery (PA). This technique was used in 283 (27.9%) cases. During the 24-month control, the observed patients showed positive dynamics in comparison with the initial results in terms of IPSS (22 ± 2.4 vs. 10 ± 2.9), maximum urination rate (7.6 ± 1.2 vs. 15.7 ± 4.9), residual urine volume (132 ± 19.4 vs. 72 ± 15.1), prostate volume (96 ± 24.7 vs. 62 ± 11.6), and PSA (5.56 ± 2.7 vs. 2.8 ± 0.5; Table 1).
Table 1.
Clinical Results of the Effectiveness of PAE in Patients Over a 24-Month Follow-Up Period.
| Indicator | Follow-up period | |||||
|---|---|---|---|---|---|---|
| Before PAE (n = 1015) | 3 months (n = 846) | 6 months (n = 783) | 12 months (n = 572) | 24 months (n = 282) | p value | |
| PV (ml) | 96 ± 24.7 | 63 ± 21.3 | 61 ± 17.4 | 58 ± 15.2 | 62 ± 11.6 | <.001 |
| PVR (ml) | 132 ± 19.4 | 11 9 ± 15.2 | 76 ± 13.4 | 66 ± 15.7 | 72 ± 15.1 | <.001 |
| IPSS | 22 ± 2.4 | 14 ± 4.3 | 12 ± 5.4 | 12 ± 4.3 | 10 ± 2.9 | <.001 |
| QoL | 4.3 ± 0.7 | 2.5 ± 1.1 | 2.3 ± 1.3 | 2.3 ± 1.2 | 2.2 ± 0.9 | <.001 |
| Qmax (ml/s) | 7.6 ± 1.2 | 15.4 ± 3.4 | 16.4 ± 3.1 | 16.2 ± 4.2 | 15.7 ± 4.9 | <.001 |
| PSA (ng/ml) | 5.56 ± 2.7 | 3.6 ± 1.3 | 2.1 ± 1.1 | 2.5 ± 0.7 | 2.8 ± 0.5 | <.001 |
Note. IPSS = International Prostate Symptom Score; PAE = prostatic artery embolization; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = Maximum urination flow rate; QoL = Quality of Life.
The PErFecTED technique was preferred for use in all observations after 2015; however, its use was not always possible due to structural features of the PA. PErFecTED embolization was successfully performed in 276 patients (27.2%). As part of the study, a comparative analysis of PErFecTED and classical PAE was carried out. When selecting patients from the control groups, patients who underwent selective embolization of the PA without superselective catheterization of the capsular and stromal branches, patients with cystostomy, as well as patients after unilateral PAE were excluded. A comparative analysis of the clinical efficacy of classical PAE and PErFecTED revealed greater efficacy of PErFecTED in the clinical groups of 393 (38.7%) and 188 (18.5%) people, respectively (Table 2).
Table 2.
Clinical Efficacy Indicators of Classical Superselective PAE and PErFecTED Over a 24-Month Follow-Up Period.
| Baseline | IPSS | QoL | Qmax (ml\s) | PV (ml) | PVR (ml) | PSA (ng\ml) | |
|---|---|---|---|---|---|---|---|
| PAE | Baseline (n = 393) | 23.0 ± 2.9 | 4.2 ± 0.9 | 8.1 ± 1.7 | 102 ± 19.8 | 153 ± 26.3 | 4.87 ± 2.1 |
| 3 months (n = 319) | 14.0 ± 4.9 | 2.7 ± 1.4 | 15.1 ± 4.1 | 69 ± 25.1 | 122 ± 17.6 | 3.6 ± 2.8 | |
| 6 months (n = 286) | 13.4 ± 4.8 | 2.5 ± 1.4 | 16.4 ± 3.9 | 66 ± 19.9 | 81 ± 15.2 | 3.5 ± 2.5 | |
| 12 months (n = 194) | 12.3 ± 5.0 | 2.4 ± 1.2 | 16.3 ± 3.5 | 62.4 ± 14.4 | 65.4 ± 21.4 | 2.6 ± 1.5 | |
| 24 months (n = 108) | 12.8 ± 8.0 | 2.7 ±1.2 | 16.1 ± 6.5 | 66.9 ± 19.0 | 77.3 ± 21.0 | 3.1 ± 1.1 | |
| PErFecTED | Baseline (n = 188) | 22 ± 1.8 | 4.7 ± 1.1 | 7.5 ± 1.1 | 99 ± 22.3 | 119 ± 18.5 | 5.21 ± 2.2 |
| 3 months (n = 156) | 11 ± 2.3 | 2.1 ± 1.3 | 17.2 ±1.1 | 57± 22.3 | 79 ± 22.1 | 2.4 ± 2.6 | |
| 6 months (n = 114) | 7 ± 4.2 | 1.9 ± 0.9 | 18.5 ± 2.2 | 53 ± 17.9 | 55 ± 16.5 | 2.1 ± 2.2 | |
| 12 months (n = 85) | 3.7 ± 2.3 | 1.4 ± 1.1 | 18.8 ± 4.9 | 51.2 ± 14.6 | 44.2 ± 21.1 | 1.8 ± 2.3 | |
| 24 months (n = 51) | 4.1 ± 3.2 | 1.3 ± 1.4 | 19.1 ± 3.9 | 54.3 ± 15.4 | 45.2 ± 19.1 | 1.8 ± 2.1 | |
Note. IPSS = International Prostate Symptom Score; PAE = prostatic artery embolization; PErFecTED = Proximal Embolization First Then Embolize Distal; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = Maximum urination flow rate; QoL = Quality of Life.
As part of the study, the issue of choosing the optimal embolization drug to maximize the effectiveness of procedure of the prostate and reduce the incidence of complications was additionally studied. To perform PAE, hydrogel microspheres with a diameter of 100–300 μm and 300–500 μm as well as PVA microparticles with a diameter of 100–500 μm were used. For analysis, we selected patients who underwent classical superselective embolization of the prostatic arteries. When selecting patients from the control groups, patients who underwent selective embolization of the PA without superselective catheterization of the capsular and stromal branches, patients with cystostomy, patients after unilateral PAE, and patients who underwent PErFecTED-PAE were excluded. The formed control groups included 124 (12.2%) patients for whom hydrogel microspheres with a diameter of 100–300 μm/300–500 μm were used and 269 (26.5%) patients for whom PVA microparticles with a diameter of 100–500 μm were used as embolization substrate. The obtained results indicate that the clinical efficacy of both types of embolization substrate is comparable, with a slight advantage of the microspherical embolizate (Table 3).
Table 3.
Clinical Efficacy Indicators of Classical PAE Using Microspherical Hydrogel Embolisate and PVA Embolization Microparticles During the 24-Month Follow-Up Period.
| Baseline | IPSS | QoL | Qmax (ml\s) | PV (ml) | PVR (ml) | PSA (ng\ml) | |
|---|---|---|---|---|---|---|---|
| Microspheres 100–300 µm/300–500 µm | Baseline (n = 124) | 23.1 ± 2.7 | 4.4 ± 1.0 | 8.3 ± 2.2 | 98 ± 20.4 | 149 ± 25.4 | 4.70 ± 2.2 |
| 3 months (n = 109) | 13.4 ± 4.5 | 2.3 ± 1.6 | 15.7 ± 4.8 | 67 ± 24.5 | 119 ± 19.4 | 3.5 ± 2.9 | |
| 6 months (n = 101) | 11.6 ± 4.9 | 2.2 ± 1.5 | 16.8 ± 3.5 | 64 ± 23.5 | 76 ± 16.6 | 3.3 ± 2.7 | |
| 12 months (n = 55) | 11.9 ± 5.2 | 2.3 ± 1.6 | 16.9 ± 3.9 | 59.7 ± 16.3 | 63.2 ± 24.1 | 2.4 ± 1.7 | |
| 24 months (n = 26) | 12.3 ± 7.3 | 2.5 ± 1.4 | 16.5 ± 7.2 | 63.4 ± 21.4 | 73.6 ± 24.2 | 2.9 ± 1.4 | |
| PVA from 100 to 500 µm | Baseline (n = 269) | 22.9 ± 3.2 | 4.1 ± 1.2 | 7.8 ± 2.1 | 103 ± 17.5 | 154 ± 27.1 | 4.9 ± 1.8 |
| 3 months (n = 210) | 14.4 ± 4.4 | 2.8 ± 1.5 | 14.8 ± 4.6 | 70 ± 22.7 | 123 ± 14.7 | 3.6 ± 3.2 | |
| 6 months (n = 185) | 13.7 ± 4.3 | 2.6 ± 1.9 | 16.1 ± 4.2 | 68 ± 17.4 | 85 ± 14.8 | 3.6 ± 2.1 | |
| 12 months (n = 139) | 12.5 ± 4.3 | 2.5 ± 1.5 | 16.0 ± 4.1 | 64.3 ± 17.2 | 66.9 ± 19.3 | 2.7 ± 0.8 | |
| 24 months (n = 82) | 12.9 ± 8.4 | 2.8 ± 1.8 | 15.8 ± 6.2 | 68.1 ± 21.3 | 79.4 ± 22.4 | 3.3 ± 1.6 | |
Note. IPSS = International Prostate Symptom Score; PAE = prostatic artery embolization; PErFecTED = Proximal Embolization First Then Embolize Distal; PSA = prostate-specific antigen; PV = prostate volume; PVA = polyvinyl alcohol; PVR = postvoid residual; Qmax = Maximum urination flow rate; QoL = Quality of Life.
When analyzing the structure of early postoperative complications, it was noted that in the group of patients who underwent embolization with PVA microparticles, complications associated with unintentional embolization of PA anastomoses were observed more often (Table 4). This effect is probably associated with the frequent use of small-diameter PVA microparticles (100 µm and 200 µm) as an embolization substrate, which is confirmed by results of other studies (Gonçalves et al., 2016).
Table 4.
The Structure of Early Postoperative Complications of PAE in the Control Groups of Patients Depending on the Type of Embolization Substrate Used.

|
Note. PAE = prostatic artery embolization; PVA = polyvinyl alcohol.
None of the patients participating in the study had complications of superselective embolization of the prostatic arteries exceeding grade II according to the Clavien–Dindo classification. All complications identified during the observation period were completely resolved as a result of conservative therapy and did not require active surgical tactics. The most common complications of PAE in the early postoperative period were postembolization syndrome (hyperthermia, dysuria, perineal pain) observed in 546 (53.8%) patients and acute urinary retention (AUR) that was observed in 221 (21.8%) patients. Urinary tract infection was observed in 42 (4.1%) patients. In the early postoperative period, 21 (2%) patients had an inguinal hematoma that resolved after repeated compression of arterial puncture site. A limited number of complications associated with unintentional embolization of prostatic anastomoses were also noted (Table 5).
Table 5.
Early Postoperative Complications of PAE.
| Complication type | Number (n) |
|---|---|
| Postembolization syndrome | 556 (54.7%) |
| Hyperthermia | 266 (26.2%) |
| ●Perineal pain | 133 (13.1%) |
| ●Dysuria | 248 (24.4%) |
| Acute urinary retention | 221 (21.8%) |
| Urinary tract infection | 42 (4.1%) |
| Complications associated with unintentional embolization of prostatic artery anastomoses | 124 (12.2%) |
| ●Pain in the rectum, an admixture of blood in the stool | 89 (8.8%) |
| ●Trophic damage to the skin of the glans penis | 34 (3.4%) |
| ●Ischemic damage to the bladder mucosa | 1 (0.09%) |
| Inguinal hematoma | 21 (2.2%) |
Note. PAE = prostatic artery embolization.
In 38 (3.7%) cases in patients with LUTS due to BPH, after bilateral and unilateral PAE a satisfactory clinical result was not obtained; that is most likely because of the large number of collaterals of the PA with other pelvic arteries. Among patients who had cystostomy drainage, adequate restoration of self-urination was recorded in 99 patients (87% of all patients with cystostomy); in 15 patients (13% of all patients with cystostomy), a satisfactory clinical effect was not achieved and that is most likely due to prolonged drainage of the bladder before PAE. The clinical efficacy of superselective embolization of the prostatic arteries in the treatment of BPH was 94.8%.
X-Ray Anatomy of the PAs
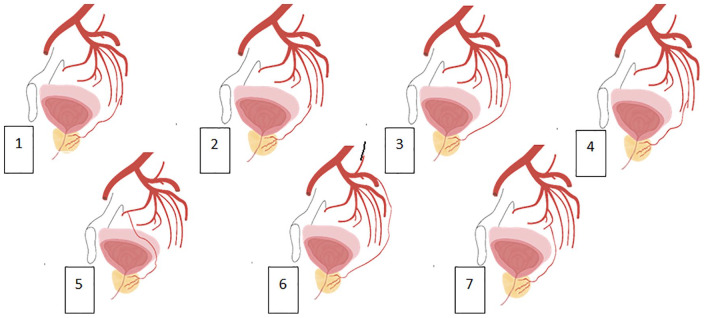
When analyzing angiograms for x-ray navigation in the pelvis, the algorithm for identifying prostatic arteries, PROVISO (Moya et al., 2016) was used. During the observation, seven types of prostate arteries branching from the internal iliac artery were identified: Type I—PA originates from the anterior portion of the internal iliac artery (20.7%), Type II—PA branches from the a. (arteria) obturatoria (5.2%), Type III—PA branches from the gluteal artery (27.5%), Type IV—PA branches from the a. pudenda interna (29.3%), and Type V—PA branches from the middle rectal artery (15.5%). Other PA branching options were combined into Type VI–VII (Figure 1). Received experience correlates with world observations (Bilhim, 2012).
Figure 1.
Options for the anatomy of the prostatic arteries: (1) a.prostatica from a.pudenda interna, (2) a.prostatica from a.vesicalis inferior, (3) a.prostatica from a.glutea inferior, (4) a.prostatica from a.rectalis media, (5) a.prostatica from a.obturatoria, (6) a.prostatica from a.glutea superior, and (7) a.prostatica from a.vesicalis superior.
In most cases, the left and right prostatic arteries are the primary source of prostate blood supply. The prostatic arteries bend around the gland from two sides, forming a characteristic rhomboid plexus, which is represented by numerous terminal branches going to the center of the prostate gland. In the area of the urethra, there is a slight decrease in the contrast density of the arteries (Figure 2).
Figure 2.
Left (1) and right (2) prostatic arteries.
The prostatic arteries branch from the anterior portion of the internal iliac artery and the PA itself can branch from the main arterial trunk. In most cases, the prostate gland has blood supply from the so-called vesico-prostatic trunk (truncus vesico-prostatic)—a vessel formed by the fusion of the lower cystic artery and the PA. In some cases, the prostate gland has additional blood supply from the middle rectal artery (a.rectalis media).
On selective angiograms, three groups of vessels can be clearly seen: The first group directly supplies the gland tissue itself—the stromal branches; the second group of arteries are mainly located on the surface of the gland—capsular branches; and the third group of vessels— periurethral vessels are located along the length of the prostate part of the urethra. Some publications (Carnevale et al., 2011, 2013) use the conditional separation of the arteries that supply the prostate gland into the cranial vesico-prostatic trunk (analogue of stromal branches) and caudal prostatic trunk (analogue of capsular branches).
It should be noted that selective embolization of the cranial branch with PAE provides the main effect of the intervention, although embolization of the caudal branch is quite important for achieving a positive clinical result. However, it must be emphasized that, as a rule, the caudal branch of the PA has direct anastomoses with both the rectal and penile arteries. This largely determines tactics of endovascular embolization in favor of the concepts “effectiveness of the intervention—the risk of operation complications.”
The number and caliber of arteries involved in the blood supply of the prostate are highly variable. Depending on the preoperative or intraoperative examination, a single artery that supplies the prostate can be found in 57–96.4% of cases. In 3.6% −7.4% −27.5% −43% two unilateral prostatic arteries that supply the gland are detected, and in 2.5% of cases even three prostatic arteries are identified.
Features of Anastomoses of PAs
Due to the anatomical location in the region of the pelvic organs, the prostatic arteries can anastomose with (a) the cystic arteries, (b) the rectal arteries, (c) the penile arteries, and (d) the terminal branches of the internal genital and a.obturatoria (Figure 3).
Figure 3.
Anastomoses of the prostatic artery with various branches of the internal iliac arteries (scheme): with a.dorsalis penis, a.pudenda interna, a.rectalis media, and a.vesicalis inferior.
The most common anastomosis is a direct communication of the PA with the cystic arteries. As mentioned earlier, in half of the cases, PA branches as part of the one trunk with the inferior cystic artery (truncus vesico-prostatici), which determines the need for the precise technique of PAE (Figure 4). The nontargeted “incidental” embolization of the lower cystic artery is usually not accompanied by any subsequent clinical symptoms because patency of the contralateral artery is maintained; however, in the world literature there are isolated observations of ischemia of the bladder wall requiring surgical treatment (Pisco, 2016). In this study, one episode (0.09%) of transient ischemia of the bladder wall was observed, but it resolved after conservative treatment.
Figure 4.
Anastomosis of the PA with the a. vesicalis inferior (1). Superselective catheterization of PA (2). PA = prostatic artery.
Anastomoses of PA with arteries that supply the rectum, which include superior rectal artery (branch of the inferior mesenteric artery) and middle and lower rectal arteries (branches of the internal genital artery), are detected only after intensive administration of the contrast directly into the internal iliac artery. Moreover, often these anastomoses appear only after administration of a contrast solution with embolization drug particles (Figure 5).
Figure 5.

Identification of anastomosis of the prostatic and rectal arteries after the first stage of PAE. PAE = prostatic artery embolization.
The ingress of embolus particles into these anastomoses can cause ischemic damage to the intestine, paresis and even necrosis of the sphincter of the rectum (Pisco et al., 2016); however, the risk of such a complication is rather small. If such anastomoses are detected, their preliminary dissociation can be performed by microspirales injection, which, however, dramatically increases the duration and cost of endovascular intervention. In this observation, 89 patients (8.8%) in the early postoperative period had symptoms associated with inappropriate embolization of prostate anastomoses with rectal arteries—pain during defecation and the appearance of blood streaks in the stool. In all patients, these symptoms resolved after conservative therapy.
The most noticeable clinical manifestations of nontargeted embolization of PA anastomoses occur when they are directly connected with the penile arteries.
In this case, emboli penetrating into the peripheral branching of the dorsal artery of the penis (a.dorsalis penis) can lead to a transient disturbance of soft tissue trophism (Figure 6) and erectile dysfunction (Abt et al., 2018). Clinically similar manifestations occur in the case of anastomosis of PA with terminal branches of the internal genital and a.obturatoria.
Figure 6.
Inadvertent embolization of the anastomosis of the prostatic artery (1) with the arteries of the penis (a.dorsalis penis) (2). Violation of trophism of the glans penis (3).
In this study, trophic damage to the skin of the glans penis was noted in 34 cases (3.4%) and in all cases it completely resolved after conservative therapy. The development of erectile dysfunction in the study was not observed.
Conclusion
The data of our study, as well as international experience, indicate that the superselective embolization of the prostatic arteries is an effective and safe method for minimally invasive treatment of BPH. PErFecTED embolization is a more effective method than classical PAE. The matter of choosing an optimal drug for embolization is still relevant. The data from 1,015 BPH patients who underwent endovascular surgery demonstrate the benefits of PErFecTED treatment during 24 months after surgery. Both QoL and IPSS scores were around three times better in the PErFecTED group and remained stable during the entire observation period. However, the technique needs to be improved due to the high risk of postembolization syndrome.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: This study was approved by the National medical research center of endocrinology Ethics Committee (approval no. 114/04) and Clinical Hospital “Russian Railways-Medicine” of the city of Barnaul Ethics Committee (Approval no. 4-03/09).
According to Russian law, from 2012 prostatic artery embolization is allowed for use in the territory of the Russian Federation (order of the Ministry of Health No. 1629n of 29.12.2012)
All observations in Moscow Research and Education Center of the Lomonosov Moscow State University were after 2016. All observations in the Moscow City Clinical Hospital No. 31 were after 2013.
Informed Consent Obtained for Research: All participants provided written informed consent prior to enrolment in the study.
ORCID iD: Boris Shaparov  https://orcid.org/0000-0002-0232-1567
https://orcid.org/0000-0002-0232-1567
References
- Abt D., Hechelhammer L., Mullhaupt G., Müllhaupt G., Markart S., Güsewell S., Kessler T. M., Schmid H. P., Engeler D. S., Mordasini L. (2018, June 19). Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: Randomised, open label, noninferiority trial. British Medical Association, 361, k2338. doi:10.1136/bmj.k2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilhim T., Pisco J. M., Rio Tinto H., Fernandes L., Pinheiro L. C., Furtado A., Casal D., Duarte M., Pereira J., Oliveira A. G., O’Neill J. E. (2012). Prostatic arterial supply: Anatomic and imaging findings relevant for selective arterial embolization. Society of Cardiovascular and Interventional Radiology, 23(11), 1403–1415. [DOI] [PubMed] [Google Scholar]
- Carnevale F. C., Antunes A. A., da Motta Leal Filho J. M., de Oliveira Cerri L. M., Baroni R. H., Marcelino A. S., Freire G. C., Moreira A. M., Srougi M., Cerri C. G. (2010). Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: Preliminary results in two patients. Cardiovascular and Interventional Radiology, 33(2), 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale F. C., da Motta-Leal-Filho J. M., Antunes A. A., Baroni R. H., Marcelino A. S., Cerri L. M., Yoshinaga E. M., Cerri G. G., Srougi M. (2013). Quality of life and clinical symptom improvement support prostatic artery embolization for patients with acute urinary retention caused by benign prostatic hyperplasia. Journal of Vascular and Interventional Radiology : JVIR. 24(4), 535–542. [DOI] [PubMed] [Google Scholar]
- Carnevale F. C., da Motta-Leal-Filho J. M., Antunes A. A., de Oliveira Cerri L. M., Baroni R. H., Marcelino A. S., Freire G. C., Moreira A. M., Srougi M., Cerri G. G. (2011). Midterm follow-up after prostate embolization in two patients with benign prostatic hyperplasia. Cardiovascular and Interventional Radiology, 34(6), 1330–1333. [DOI] [PubMed] [Google Scholar]
- Carnevale F. C., Iscaife A., Yoshinaga E. M., Moreira A. M., Antunes A. A., Srougi M. (2016). Transurethral resection of the prostate (TURP) versus original and PErFecTED prostate artery embolization (PAE) due to benign prostatic hyperplasia (bph): Preliminary results of a single center, prospective, urodynamic-controlled analysis. Cardiovascular and Interventional Radiology, 39(1), 44–52. [DOI] [PubMed] [Google Scholar]
- Carnevale F. C., Moreira A. M., Antunes A. A., Baroni R. H., Marcelino A. S., Cerri L. M., Yoshinaga E. M., Cerri G. G., Srougi M. (2014). The ‘‘PErFecTED technique’’: Proximal embolization first, then embolize distal for benign prostatic hyperplasia. Journal of Vascular and Interventional Radiology : JVIR., 37(6), 1602–1605. [DOI] [PubMed] [Google Scholar]
- DeMeritt J. S., Elmasri F. F., Esposito M. P., Rosenberg G. S. (2000). Relief of benign prostatic hyperplasia-related bladder outlet obstruction after transarterial polyvinyl alcohol prostate embolization. Journal of Vascular and Interventional Radiology : JVIR., 11(6), 767–770. doi:10.1016s1051-0443(07)61638-8 [DOI] [PubMed] [Google Scholar]
- Gao Y. A., Huang Y., Zhang R., Yang Y. D., Zhang Q., Hou M., Wang Y. (2014) Benign prostatic hyperplasia: Prostatic arterial embolization versus transurethral resection of the prostate—A prospective, randomized, and controlled clinical trial. Radiology, 270(3), 920–928. [DOI] [PubMed] [Google Scholar]
- Gonçalves O. M., Carnevale F. C., Moreira A. M., Antunes A. A., Rodrigues V. C., Srougi M. (2016). Comparative study using 100-300 versus 300-500 mm microspheres for symptomatic patients due to enlarged-BPH prostates. Cardiovascular and Interventional Radiology, 39(10), 1372–1378. [DOI] [PubMed] [Google Scholar]
- Karpov V. K., Kapranov S. A., Shaparov B. M., Osmolovskiy B. E., Kamalov D. M., Kamalov A. A. (2019). [Super-selective prostatic artery embolization for bph treatment.] Urologiia, (3), 134–141. [PubMed] [Google Scholar]
- Kurbatov D., Russo G. I., Lepetukhin A., Dubsky S., Sitkin I., Morgia G., Rozhivanov R., Cimino S., Sansalone S. (2014). Prostatic artery embolization for prostate volume greater than 80 cm3: Results from a single-center prospective study. Urology, 84(2), 400–404. doi:10.1016/j.urology.2014.04.028 [DOI] [PubMed] [Google Scholar]
- Kurbatov D., Sitkin I., Lepetukhin A. (2011). Endovascular superselective embolization of prostatic arteries as the new method of BPH less invasive treatment [Poster presentation]. Abstracts of AUA Annual Meeting 14–19 May, Washington, DC USA p. 105. [Google Scholar]
- Moya C., Cuesta J., Friera A., Gil-Vernet Sedó J. M., Valderrama-Canales F. J. (2017). Cadaveric and radiologic study of the anatomical variations of the prostatic arteries: A review of the literature and a new classification proposal with application to prostatectomy. Clinical Anatomy, 30(1), 71–80. doi:10.1002/ca.22746 [DOI] [PubMed] [Google Scholar]
- Neymark A. I., Neymark B. A., Tachalov M. A., Arzamascev D. D., Torbik D. V. (2015). Superselective prostatic artery embolization as a preparatory step before turp in the treatment of benign prostatic hyperplasia in patients with large prostates. Urologiia, (2), 60–62, 64. [Russian] [PubMed] [Google Scholar]
- Neymark A. I., Tachalov M. A., Neymark B. A., Torbik D. V., Arzamastsev D. D. (2017). [X-ray-guided endovascular surgery in patients with benign prostatic hyperplasia and prostate cancer]. Urologiia, (1), 54–60. [DOI] [PubMed] [Google Scholar]
- Pisco J., Campos Pinheiro L., Bilhim T., Duarte M., Mendes J. R., Oliveira A. G. (2013). Prostatic arterial embolization for benign prostatic hyperplasia: Short- and intermediate-term results. Radiology, 266(2), 668–677. [DOI] [PubMed] [Google Scholar]
- Pisco J. M., Bilhim T., Pinheiro L. C., Fernandes L., Pereira J., Costa N. V., Duarte M., Oliveira A. G. (2016). Medium- and long-term outcome of prostate artery embolization for patients with benign prostatic hyperplasia: results in 630 patients. Journal of Vascular and Interventional Radiology: JVIR, 27(8), 1115–1122. [DOI] [PubMed] [Google Scholar]
- Pisco J., Pinheiro L. C., Bilhim T., Duarte M., Mendes J. R., Oliveira A. G. (2011). Prostatic arterial embolization to treat benign prostatic hyperplasia. Journal of Vascular and Interventional Radiology: JVIR., 22(1), 11–19. [DOI] [PubMed] [Google Scholar]
- Russo G. I., Kurbatov D., Sansalone S., Lepetukhin A., Dubsky S., Sitkin I., Salamone C., Fiorino L., Rozhivanov R., Cimino S., Morgia G. (2015). Prostatic arterial embolization vs open prostatectomy: A 1-year matched-pair analysis of functional outcomes and morbidities. Urology, 86(2), 343–348. [DOI] [PubMed] [Google Scholar]
- Sitkin I. I., Lepetukhin A. E., Dubsky S. A., Kurbatov D. G., Kozlov K. L., Oleksyuk I. B., Kudryavtsev O. I. (2018). [Embolization of prostate arteries - An alternative technology for treatment of prostatic adenoma in patients with diabetes mellitus.] Advances in Gerontology = Uspekhi gerontologii / Rossiĭskai͡a akademii͡a nauk, Gerontologicheskoe obshchestvo, 31(6), 983–987. [PubMed] [Google Scholar]
- Yakovets E. A., Neymark A. I., Karpenko A. A., Yakovets Y. V. (2010). Embolization of the prostate arteries in the treatment of patients with pancreatic adenoma with high surgical risk. Andrology and Genital Surgery, (1), 38–43. [Google Scholar]