Abstract
Recently, polysaccharides from Bletilla striata, a member of the orchidaceous family, aroused the wide interest of people, especially their isolation, chemical properties, and bioactivities. It is reported that these polysaccharides are the most important biologically active components of B. striata, exhibiting various biological activities, such as immunomodulatory, antioxidant, antifibrotic, and hemostatic effects. This review appraised the available literatures which described different aspects of B. striata polysaccharides, including the extraction, separation, purification, structural characterization, and biological activities. We expect to lay the foundation for further investigation of the application of B. striata polysaccharides in the field of functional foods and biomedicine.
1. Introduction
Bletilla striata (Thunb.) Reichb. f. (Orchidaceae), named “Bai Ji” in Chinese, belongs to the orchidaceous family and is distributed widely in eastern Asian countries, including China, North Korea, Japan, and Burma [1, 2]. B. striata has been widely used in traditional Chinese herbal medicine (TCM) for thousands of years and is one of the “seven white” in ancient China, mainly distributed in the Qinling Mountains and Yangtze River region [3, 4]. TCM holds that B. striata is bitter, astringent, neutral, and warm and has bioactivities in alimentary canal mucosal damage, ulcers, bleeding, bruises, and burns [4, 5]. Also, the functions of hemostasis, detumescence, and improving one's health of B. striata were recorded by Chinese pharmacopeia (2010) [2, 6].
Increasing numbers of modern chemical and pharmacological studies revealed that B. striata has many beneficial effects, including healing, hemostasis, antioxidation, anti-inflammation, antifibrotic, and immunomodulatory activity in vitro [4, 7, 8]. These multiple pharmacological effects of B. striata are attributed to its various functional ingredients, such as polysaccharides, saponins, flavonoids, terpenoids, trace elements, and other chemicals [1, 4], among which the polysaccharides (BSPs) have gradually received attention and are now considered to be the main active substance of B. striata for its beneficial effects [9–11]. BSPs isolated using different extraction and purification methods have been confirmed to be structurally diverse biomacromolecules with various functions [2]. Their anti-inflammatory [12], antitumor [13], immunomodulatory [14], and antifibrotic [15] activities have been well recognized. Moreover, BSPs have been used as a therapeutic agent for the treatment of bleeding [16, 17]. So for, BSPs with multiple functional applications have been widely used in food and medical industries [2].
Throughout the available literatures, there has been no systematic review of B. striata polysaccharides. Neither has there been a systematic review on the extraction and purification techniques nor on the structural characteristics and biological activities. In this review, we intend to systematically summarize the research findings about BSPs in the past decades to provide a comprehensive insight into the extraction and purification techniques as well as the structure and pharmacological effects of BSPs.
The main purpose of this review is to provide informative knowledge which is expected to promote better utilization of these polysaccharides and to preliminarily illustrate the relationships between the structure and bioactivities of BSPs, as well as pave the way for further exploitation of these compounds in the field of biomedicine.
2. Extraction and Purification Methods
In the past decades, diverse methods have been described for the extraction, isolation, and purification of polysaccharides from B. striata [9, 18–20]. Extraction methods of BSPs from pretreated dry powders are summarized in Table 1. Generally, water extraction at a certain temperature is the classic and also most convenient method for laboratory extraction and even industrial extraction [21–23]. Extraction time and temperature have significant influences on the yield of conventional water extraction, and solid-liquid ratio is usually in the range of 1 : 5 to 1 : 50. Different extraction conditions, such as the source of B. striata, extraction temperature, time, solvent, and the raw material to solvent ratio, have significant effects on the extraction rate of BSPs [2, 3, 23].
Table 1.
A summary of the extraction of polysaccharides from Bletilla striata.
| No. | Times (min) | Solid-liquid ratio | Temperature (°C) | Other conditions | Yield (%) | References |
|---|---|---|---|---|---|---|
| Routine extraction | ||||||
| 1 | 150 | 1 : 30 | 80 | 2 times | [18] | |
| 2 | 180 | 1 : 10 | 60 | 18.50 | [24] | |
| 3 | 240 | 1 : 5 | 80 | 1 time | [66] | |
| 4 | 180 | 1 : 15 | 90 | Cold soaking extraction 6 h, first 2 times | 28.43 | [67] |
| 5 | 90 | 1 : 30 | 80 | 26.45 | [43] | |
| 6 | 180 | 1 : 10 | 90 | 3 times | 10.00 | [68] |
| 7 | 90 | 1 : 10 | 90 | 2 times | 7.64 | [69] |
| 8 | 180 | 1 : 6 | 90 | Alkali solution, 3 times | [70] | |
| 9 | 240 | 95 | 0.2 M NaOH, 3 times | 62.50 | [36] | |
| Ultrasound-assisted extraction | ||||||
| 1 | 50 | 1 : 50 | 60 | 400 W, 2 times | 26.023 | [25] |
| 2 | 5 | 1 : 20 | 50 W | 3.23 | [54] | |
| 3 | 20 | 1 : 25 | 100 W | 12.343 | [71] | |
| 4 | 80 | 1 : 30 | 30 | 126 W | 19.94 | [72] |
| Microwave-assisted extraction | ||||||
| 1 | 5 | 1 : 63 | 504 W, pH 7.0 | 32.48 | [26] | |
| 2 | 5 | 1 : 20 | 200 W | 6.14 | [54] | |
| 3 | 5 | 1 : 20 | Ultrasonic 50 W Microwave 200 W |
6.98 | [54] | |
| Infrared-assisted extraction | ||||||
| 1 | 150 | 1 : 53 | 75 | 1 time | 43.95 | [20] |
Wang found the following optimum conditions for extracting BSPs using distilled water: extraction temperature—60°C; extraction time—3 h; and water : raw material ratio—10 : 1. With this method, only one round of extraction produced a final yield of 18.50% [24]. However, hot water extraction still has the disadvantages of being time consuming and having high temperature, low efficiency, and possible polysaccharide degradation.
To improve the yield of extracted BSPs, other techniques have also been applied, such as ultrasonic- [3, 25], microwave- [3, 6], and infrared-assisted [20] extraction, by promoting the breakdown of the B. striata cell walls [23]. For example, He et al. reported that ultrasound-assisted extraction based on an orthogonal array design could improve the extraction efficiency of polysaccharides. They also reported the following optimal extraction conditions with two rounds of extraction: ultrasonic power—400 W; extraction time—0 min; extraction temperature—60°C; and water to raw material ratio (w : w)—50 : 1. Under these conditions, the yield of crude polysaccharides from B. striata harvested in Shanxi province increased to 26.02% [25]. Microwave-assisted extraction has also been explored to maximize the yield of BSPs. The most favorable conditions were found to be the following: microwave power—504 W; extraction time—5 min; and water (pH 7.0) to raw material ratio (w : w)—63 : 1 [26]. In addition, with the help of infrared-assisted extraction for BSPs and the Box-Behnken design, a considerable improvement of the extraction yield has been realized. An extraction yield of 43.95 ± 0.26% was achieved using an extraction temperature of 75°C, a water to solid ratio of 53 mL/g, and an extraction time of 2.5 h [20]. In summary, we could find that each of these assisted extraction methods could contribute to shorten the processing time, reduce solvent consumption, and further decrease the economic cost while improving the extraction efficiency of BSPs [3, 6, 27].
In order to illuminate the composition, molecular mass, and basic chemical structure of BSPs, further purification of crude polysaccharides is needed. The main purification method is column chromatography with various adsorbents and eluents. In this process, fractions containing polysaccharide products are collected, dialyzed, concentrated, and lyophilized to receive pure polysaccharides [28, 29]. As reported before, ion exchange chromatography (DEAE-cellulose and DEAE-Sepharose Fast Flow) could separate neutral polysaccharides from acidic ones using a salt gradient for elution, whereas gel filtration (Sephadex G column and Bio-Gel P column) could separate polysaccharides according to the differences of their molecular weights (Figure 1) [9, 19, 30]. For instance, Wang et al. fractionated four different parts with a DEAE-cellulose column (3.0 × 55 cm), preequilibrated with distilled water and eluted with 0, 0.05, 0.2, and 1.0 M of NaCl at a flow rate of 1.5 mL/min. The collected fraction was further purified on a Sephadex G-200 column (1 cm × 100 cm) and eluted with distilled water (10 mL/tube). Sugar-positive parts were gathered, concentrated, and lyophilized to obtain two homogeneous polysaccharides, BSP-1 and BSP-2, with an average molecular weight of 83.54 kDa and 12.60 kDa, respectively [9].
Figure 1.
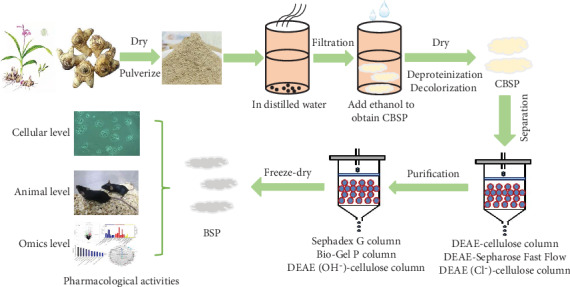
Schematic representation of the extraction, purification, and bioactivity of polysaccharides from Bletilla striata.
The procedures used to separate and purify polysaccharides from B. striata are summarized as follows [2]. The tubers of B. striata were overdried, finely pulverized, and sieved through a 30-mesh sieve, and then the powders were homogenized and dispersed in hot double-distilled water, then filtered to remove impurities. The crude extracts were precipitated by the addition of three vol. of 95% (v/v) ethanol and left to stand overnight. The resultant precipitate was collected by centrifugation. The last two steps were repeated to resuspend the precipitate in double-distilled water. Deproteinization was done by adding 1/3 vol. Sevage reagent (chloroform/n-butanol, 4 : 1) to the solution and stirring overnight, until the absorption of the solution at 260 nm and 280 nm was zero, then the aqueous phase was collected by centrifugation at 3500 rpm for 10 min. After decolorization, dialysis, evaporation, precipitation with ethanol, filtration, and lyophilization, the crude B. striata polysaccharides are obtained [2, 3, 5]. The crude extracts are redissolved and applied to different chromatographic columns described above, eluted with appropriate running buffers, collected, dialyzed, concentrated, and lyophilized, to produce the pure B. striata polysaccharide (BSP) [2]. Then, the polysaccharide contents are determined using the phenol-sulfuric acid method [31], and proteins in the polysaccharides are quantified with the Bradford method [32].
3. Physiochemical and Structural Features
The structural characteristics of plant polysaccharides, including molecular weight; monosaccharide composition and sequence; and types, positions, and configurations of glycosidic linkages as well, are the key to determine their unique physiochemical activity [21, 33, 34]. There are polysaccharides with various monosaccharide constituents and chemical structures isolated from B. striata. Meanwhile, the monosaccharide constituents and basic chemical structures of BSPs have been determined by several research groups through infrared spectroscopy (IR), nuclear magnetic resonance (NMR), gas chromatography-mass spectroscopy (GC-MS), gas chromatography (GC), high-performance liquid chromatography (HPLC), acid hydrolysis, methylation analysis, periodate oxidation, and Smith's degradation [7, 9, 18, 35]. The primary structural characteristics of BSPs and also their biological activities are summarized in Table 2, together with their related bibliographies.
Table 2.
The polysaccharides isolated from Bletilla striata.
| No. | Compound name | Molecular weight | Monosaccharide composition | Structures | Biological activities | Reference |
|---|---|---|---|---|---|---|
| 1 | BSP-1 | 8.354 × 104 Da | Man and Glc in the ratio of 4.0 : 1.0 | Backbone composed of β-1,4-linked Manp | Immunomodulation | [9] |
| BSP-2 | 1.26 × 104 Da | Man and Glc in the ratio of 3.0 : 1.0 | Backbone composed of β-1,4-linked Glcp | [9] | ||
| 2 | BSP | Mw : 3.73 × 105 g/mol Mn : 6.75 × 104 g/mol |
Man and Glc in the ratio of 2.946 : 1 | Backbone composed of 1,4-linked Glcp. Branches composed of 1,3-linked Manp and 1,3-linked Glcp | Anti-inflammatory | [7] |
| 3 | BSP | 1.98 × 105 Da | β-Glucopyranose and α-mannopyranose | Antitumor | [18] | |
| 4 | BFPS | Mw : 9.545 × 104 g/mol Mn : 7.297 × 104 g/mol |
Man and Glc in the ratio of 7.4 : 2.6 | [8] | ||
| BVPS | Mw : 1.472 × 105 g/mol Mn : 1.218 × 105 g/mol |
Man and Glc in the ratio of 7.37 : 2.63 | [8] | |||
| 5 | BSPF2 | 2.35 × 105 Da | Man, Glc, and Gal in the ratio of 9.4 : 2.6 : 1.0 | Backbone composed of 1,4-linked Manp and 1,4-linked Glcp in a molar ratio of 2 : 1 | Immunomodulation | [14] |
| 6 | BSP | Man and Glc in the ratio of 1.616 : 1.962 | Backbone composed of 1,3,4-linked Manp and 1-linked Manp. Branches composed of 1-linked Glcp | [37] | ||
| 7 | BSPb | 2.60 × 105 Da | Glc and Man in the ratio of 3 : 1 | Backbone composed of 1,2-linked Manp and 1,4-linked Glcp | Antifibrotic effect Anti-inflammatory |
[12, 15] |
| 8 | BSP | 1.82 × 105 Da | Man and Glc in the ratio of 3 : 1 | 1,4-Linked aldohexopyranosyl residues | [24] | |
| 9 | BOP | 4.93 × 105 Da | Man and Glc in the ratio of 7.88 : 2.12 | Backbone composed of 1,4-linked Manp and 1,4-linked Glcp in a molar ratio of 4 : 1 | Immunomodulation Antitumor |
[43] |
| 10 | BSP | ~1.35 × 105 Da | Man and Glc in the ratio of 2.4 : 1 | Backbone composed of 1,4-linked Manp | Anti-inflammatory | [37, 73] |
| 11 | BSPI-A | >4.0 × 105 Da | Man and Glc in the ratio of 8.09 : 1 | Backbone composed of 1,4-linked Manp and 1,4-linked Glcp | [74] | |
| 12 | BT | Man and Glc in the ratio of 2.88 : 1 | Backbone composed of 1,4-linked Manp and 1,4-linked Glcp | [75] | ||
| 13 | BSP | ~1.0 × 105 Da | Man and Glc in the ratio of 1.6 : 1 | Cell proliferation | [76] | |
| 14 | BSP | ~2.0 × 105 Da | Man and Glc in the ratio of 3.76 : 1 | Backbone composed of 1,4-linked Manp and 1,4-linked Glcp | [77] | |
| 15 | BSP | Man and Glc | Backbone composed of 1,4-linked Manp and 1,4-linked Glcp | Cell proliferation | [17] | |
| 16 | pFSP | 9.1 × 104 Da | Man, Glc, and Gal in the ratio of 3.45 : 1.00 : 2.03 | Backbone composed of (1→4)-linked-α-D-Glcp, (1→4)-linked-β-D-Manp, and (1→3,6)-linked-β-D-Manp. Branches composed of (1→6)-linked-β-D-Galp and terminated with (1→)-linked-β-D-Manp | Antioxidant | [78] |
| 17 | BSP | 1.46 × 105 Da | Man and Glc in the ratio of 2.4 : 1.0 | α-Man and β-Glc residues | Anti-inflammatory | [79] |
3.1. Average Molecular Weights
HPLC and high-performance gel permeation chromatography (HPGPC) are mainly used to determine the average molecular weights of BSPs [8, 12]. Wang demonstrated that molecular weights of BSPs from different isolating fractions were 355.8 kDa (BSPS-I), 174.3 kDa (BSPS-II), 52.8 kDa (BSPS-III), 174.0 kDa (BSPS-IV), and 59.9 kDa (BSPS-V) [36]. It should be noticed that different experimental conditions may have impacts on the molecular weights of polysaccharides. Reports about the Mw of various B. striata polysaccharides ranged from 104 to 105 Da.
3.2. Monosaccharide Compositions
In general, with a process of acid-mediated cleavage of glycosidic linkages, derivatization, detection, and quantification with GC and HPLC, people could get the information of monosaccharide compositions [9, 14, 36]. And owing to different raw materials and purification processes, different monosaccharide compositions of BSPs are derived.
It is reported that most of BSPs are composed of mannose and glucose with a similar molar ratio [2, 14, 37]. Wang et al. separated two polysaccharides, BSP-1 and BSP-2, from B. striata and analyzed their monosaccharide compositions with GC [9]. The results are shown in Table 2. Diverse BSPs might have similar monosaccharide compositions in various molar ratios.
3.3. Chemical Structures
Besides monosaccharide components and molecular weights, information about structure or conformation of BSPs has been demonstrated. So far, all available evidence (Table 2) indicated that BSPs were a sort of glucomannan [2, 5]. An early publication in 1993 first reported that BSPs contained glucose and mannose in a molar ratio of 1 : 3, with the backbone consisting of 1,4-linked aldohexopyranosyl residues [37]. In addition, the structural features of a water-soluble polysaccharide (BSP-2) were studied by methylation analysis, GC-MS analysis, Fourier transform-infrared spectroscopy, 1H, 13C, HSQC, COSY, and HMBC NMR spectroscopy [9]. It was shown that the primarily BSP-2 structure was deduced to be →4)-β-D-Glcp (1→4)-β-D-Manp (1→4)-β-D-Manp (1→4)-β-D-Manp(1→. A schematic structure is shown in Figure 2(c). The primary structures of the B. striata polysaccharide (designated BSPF2), with a molecular weight of 2.35 × 105 Da, containing mannose, glucose, and galactose in a molar ratio of 9.4 : 2.6 : 1.0, were determined with a combination of chemical and instrumental analyses, including methylation analysis, GC, methylation, and FT-IR. The backbone of BSPF2 consisted of (1→4)-linked mannosyl residues and (1→4)-linked glucosyl residues in a molar ratio of 2 : 1 [14].
Figure 2.
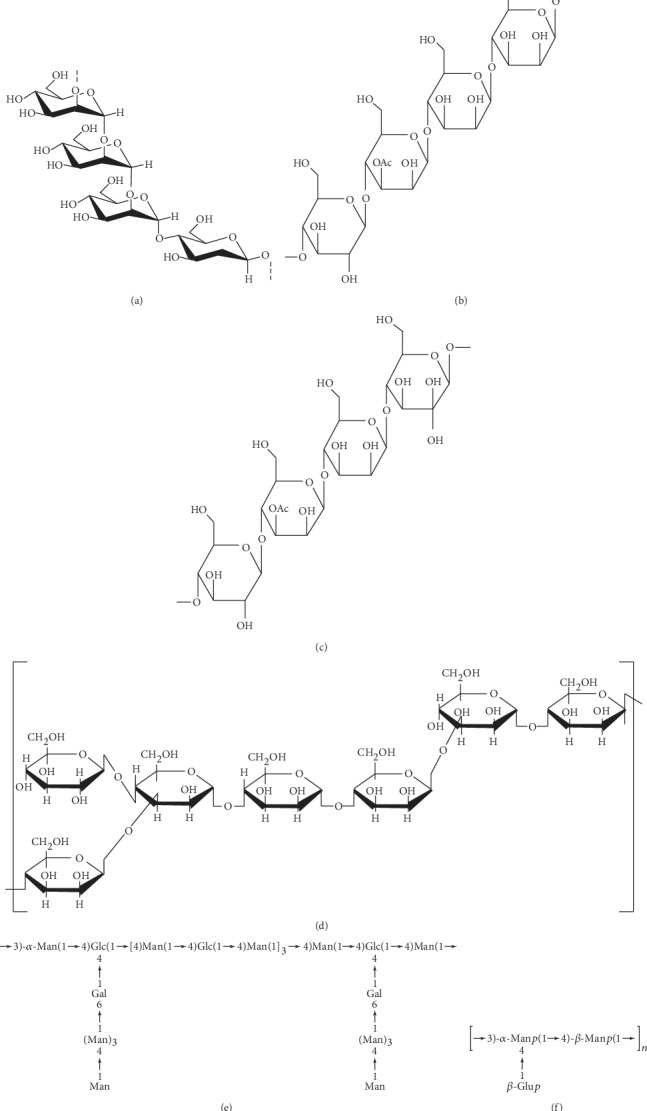
Schematic structures of Bletilla striata polysaccharides. (a) Bletilla striata polysaccharide b (BSPb) [15]. (b) Polysaccharide from the tuber of Bletilla striata (BSP-1) [9]. (c) Polysaccharide from the tuber of Bletilla striata (BSP-2) [9]. (d) Polysaccharide of Bletilla striata (BSP) [19]. (e) polysaccharide from the tuber of Bletilla striata (BSPF2) [14]. (f) Bletilla striata polysaccharide [37].
As illustrated in Figure 2, the hypothesized structure of BSP fractions consists of two types, the linear chain and the branched chain, respectively, and the branched polysaccharides composed of mannose and glucose with different ratios are common [2].
3.4. Conformational Features
The activities of polysaccharides have a close relationship with their molecular weights, chemical structures, and chain conformations as well [21, 38]. However, few reports are available on the solution properties or chain conformations of BSPs. As for the advanced structure of BSPs, Qu et al. discovered that a shift in the maximum absorption wavelength (503-512 nm) of BSPs that appeared in distilled water was longer than that examined in the Congo red solution without BSPs (487-498 nm), indicating that BSPs exhibited a triple helical conformation [20]. The surfaces of BSP fractions exhibited a loose filamentous network, although there were large differences depending on concentration. Besides, some angular irregular voids were observed in the network structure. These voids possibly represent spaces that remained after ice crystals sublimated during the freeze-drying process [8].
The B. striata polysaccharide (BSP) could be used for preparing a fiber with mechanical properties through the environment-friendly method of phase inversion with ethanol as the nonsolvent. The prepared BSP fibers were woven into a fabric with excellent flexibility and had excellent flexibility with potential application in the curing of skin wounds [39]. This BSP hydrogel represented a preferable swelling ability and an appropriate water vapor transmission rate, and has been proven to control the inflammatory responses and accelerate the wound closure and thus has potential application in wound healing [40].
It is difficult to determine the definite relationship of the chain conformations, solution properties of BSPs, and their biological activities [41]. Further confirmation of the chain conformations of BSPs in an aqueous solution should require other investigations with advanced techniques, such as viscosity analyses, static and dynamic light scattering, circular dichroism, transmission electron microscopy, scanning electron microscopy, and atomic force microscopy in future research [21, 42].
4. Biological Activities
Based on the theory of traditional Chinese medicine, B. striata is widely used to treat alimentary canal mucosal damage, ulcers, bleeding, bruises, and burns [2, 36, 43]. In recent years, owing to their various biological properties and pharmacological functions, plant polysaccharides attract extensive attention in the field of biology and medicine. Similarly, more and more studies indicated that polysaccharides were a major class of bioactive compounds in B. striata, both in its beneficial effects on human health and its pharmacological value [2, 44, 45]. The multiple bioactivities and health benefits of BSPs are summarized and compared in detail below.
4.1. Immunomodulatory Activity
Working as immunomodulators or biological response modifiers, natural polysaccharides play a role in immunomodulation, which is considered to be an important biological function [46, 47]. The immunomodulatory activities of plant polysaccharides could be correlated with their structures, including molecular weights, chemical compositions, and glycosidic linkages [21, 48, 49]. Peng et al. reported that the B. striata polysaccharide fraction (BSPF2) could concentration-dependently induce spleen cell proliferation, and it was characterized by a strong stimulation of proliferation of mouse spleen cells [14].
The immunomodulatory activities of B. striata polysaccharides (BSP-1 and BSP-2) were previously investigated in rats treated with cyclophosphamide by Wang et al. [9]. Their results indicated that the thymus index inhibition rates of BSP-1 and BSP-2 were -23.5% and 3.3%, and the spleen index inhibition rates of mice with immune dysfunction were also increased, with inhibition rates of -36.0% and -24.4%, respectively. Furthermore, BSP-1 could significantly improve the immunological function of mice that had been immunosuppressed by cyclophosphamide and increase the thymus index in in vivo experiments [9, 24].
4.2. Antioxidant Activity
To study the mechanisms of traditional Chinese medicines as potential nutraceutical and therapeutic agents, investigating antioxidant activities is the focus of many researches, with various assay methods and activity indices [50, 51]. It has been reported that a wide range of biofunctional components of plants, fungi, and animals have strong antioxidant activities, among which polysaccharide is quite a representative one [52, 53].
Some research groups have demonstrated the antioxidative activities of B. striata polysaccharides in vitro. For example, Qu et al. recently demonstrated that BSPs have a definite antioxidative activity, estimated in 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, hydroxyl radical, and superoxide anion systems. BSPs display a dose-dependent DPPH radical scavenging effect of 42.20%, a hydroxyl radical scavenging effect of 35.97%, and a superoxide radical scavenging effect of 43.70% at the tested concentration of 5 mg/mL [20]. Besides, Cai et al. found that BSPs achieved by ultrasonic-microwave synergistic extraction had significantly higher hydroxyl radical and DPPH radical scavenging effects [54]. However, the in vivo antioxidant activities of BSPs are reported to be less marked.
4.3. Anti-Inflammatory Activity
It is reported that the anti-inflammatory effects of polysaccharides could be influenced by many factors, including their molecular size, form, degree of branching, and solubility in water [21, 53]. As we have mentioned before, many previous studies have suggested that polysaccharides exert strong anti-inflammatory activity through different mechanisms [53, 55]. The study reported by Yue et al. demonstrated that BSPb exhibited significant anti-inflammatory and antioxidative activities in Ang II-induced HMCs via the NOX4 and TLR2/MyD88 pathways, efficiently mediating the expression of key factors, such as NOX4 and TLR2, to attenuate the generation of ROS and inflammatory cytokines [12]. Lai et al. previously reported that BSPs could significantly downregulate inflammatory markers, viz., L-1β, TNF-α, COX-2, and iNOS, and upregulate proinflammatory cytokines, like TIMP-1 and TGF-β1, and the anti-inflammatory properties of BSPs might be involved with the regulation of oxidative stress [18].
4.4. Antifibrotic Activity
Only few studies have demonstrated the direct antifibrotic effects of B. striata polysaccharides. More detailed studies are required to clarify the compositional features and antifibrotic activities of BSPs. Wang et al. reported that BSPb, isolated from the roots of B. striata, showed a dose-dependent effect on the proliferation of human mesangial cells (HMCs) and played an important role in protecting against the renal fibrosis effect, which is probably mediated by downregulating TGF-β RI, TGF-β RII, and α-SMA in vitro [15].
4.5. Hemostatic Activity
Pharmacological studies of plant polysaccharides have shed some light on a novel aspect of drug delivery in treating bleeding, bruises, and burns [2, 56]. Polysaccharides could promote tissue repair through different growth factors and produce healing effects by inducing endothelial cell proliferation and vascular endothelial growth factor expression or proinflammatory cytokine expression in macrophages [57, 58].
The research of Wang et al. previously reported that B. striata polysaccharides could upregulate vascular endothelial growth factor expression and enhance vascular endothelial cell (EC) proliferation in vitro in 2006 [37], and two years later they elucidated that the wound healing mechanism might be that the BSPs could induce coordinate changes in the mRNA levels of inducible nitric oxide synthase (iNOS), tumor necrosis factor alpha (TNF-α), and interleukin 1 beta (IL-1β) and then enhance the expression of these cytokines, but that the BSPs have no effect on interferon gamma (IFN-γ) level [59]. BSPs enhanced the proliferation and migration of L929 cells and significantly accelerated the wound healing process in vivo in full-thickness skin defect wounded models. The BSP hydrogel could accelerate wound healing and promote reepithelialization and collagen deposition by means of TGF-β/Smad signal pathway activation [60].
4.6. Gastrointestinal-Protective Effect
B. striata polysaccharides showed bioactivities in protecting the gastrointestinal mucosa. Such polysaccharides could promote tissue repair through different growth factors and produce anti-inflammatory effects by suppressing the neutrophil/cytokine cascade in intestinal epithelial organization. Luo et al. previously reported that BSPs alleviated intestinal epithelial barrier disruption in rats with thioacetamide- (TAA-) induced liver cirrhosis. They found that BSPs markedly reduced endotoxin levels, inhibited the inflammatory cytokines IL-6 and TNF-α, elevated expression of zonula occludens- (ZO-) 1 and occludin at tight junctions, and thereafter, improved the intercellular tight junctions [61, 62]. BSPs had the capacity to protect IEC-18 cells from LPS-induced injury, and the mechanisms could be associated with decreasing the inflammatory cytokine levels of IL-6 and TNF-α and elevating the expression of ZO-1 and occludin, which might serve as a new protective agent for LPS-induced intestinal epithelial barrier disruption [63]. The gastroprotective mechanisms of BSP could be related to the mitigation of oxidative stress, neutrophil infiltration, and inflammatory cytokine accumulation. The inhibition of MAPK/NF-κB signaling pathway activation was mediated by BSP, and pretreatment with BSP promoted the production of acid mucus and upregulated endogenous PGE 2 production, which protected the gastric mucosa from damage induced by ethanol [64].
4.7. Other Biological Activities
B. striata polysaccharides were shown to have significant in vivo and in vitro antitumor activities [2]. Li and Bai previously investigated the in vivo and in vitro effects of paclitaxel nanoparticle- (PTX-) loaded BSPs on human gastric cancer cells. The results suggested that BSP-loaded paclitaxel nanoparticles could realize enhanced drug delivery and exert an antiproliferative effect on the human gastric gland cancer cell line (MKN45) effectively and safely both in vivo and in vitro [10].
The lifespan of C. elegans was extended, and its locomotion ability and stress resistance were increased and mRNA levels of age-1 and hcf-1 were reduced after BSP treatment. BSP could produce an antiaging effect on C. elegans through the insulin/IGF signaling pathway [65].
5. Conclusion and Future Perspectives
As a classical traditional Chinese medicine, B. striata has been extensively investigated in the past decades. Among the various active ingredients of this plant, polysaccharides attract broad attention by virtue of their excellent biological properties and pharmacological functions. Early research has been done and published on the extraction, purification, and preliminary structural identification as well as on the biological functions of B. striata polysaccharides. In this paper, we reviewed recent advances in research on the structure and bioactivities of polysaccharides from B. striata.
Over the past decades, phytochemical studies have isolated a variety of B. striata polysaccharides. The structural diversity and heterogeneity make research challenging in terms of structural perspectives.
Several reports have systematically explored the chemical structures of BSPs. The main methods used for characterization in these studies were still HPGPC, partial acid, oxidation with periodic acid, Smith's degradation, HPLC, GC, IR, 1H-13C-NMR, and GC-MS. It is reported that the glycan backbone of BSPs is mainly represented by 1,4-linked mannosyl residues and 1,4-linked glucosyl residues, with few side chains attached to O‐2, O‐3, or O‐6, accompanied by different branching and terminal sites.
Besides, several studies have focused on the pharmacological functions of B. striata polysaccharides, e.g., antioxidant, antifibrotic, and hemostatic activities, while few studies focused on the relationship between chemical structure and biological activity. Further investigations are required to fully unfold these mysteries. Similarly, more preclinical and clinical trials are required to confirm the reliability and effectiveness of B. striata polysaccharides in human health, as in vitro observations cannot accurately reflect in vivo effects.
On the other hand, the development of new “omic” technologies, such as microbiomics, transcriptomics, metabolomics, and proteomics, and current techniques including genomics and bioinformatics will definitely contribute to uncover the mechanisms of various biological activities of B. striata polysaccharides.
As more attention is given and new research methods have arisen, the significant medicinal value of Bletilla striata polysaccharides will be featured, and their wide exploitation and utilization in the field of biomedicine and functional food can be expected in the near future.
Acknowledgments
This research was financially supported by National Key R&D Program of China (2018YFD0901003), the National Natural Science Foundation of China (21365011), the Youth Teachers' Ability Promotion Project of Guangxi (2018KY0561), the High Level Innovation Teams of Guangxi Colleges & Universities/Outstanding Scholars Program (Guijiaoren (2018)35), Hezhou Innovation-Driven Development Project (He Ke Chuang PT1907006).
Contributor Information
Hui Nie, Email: 763008174@qq.com.
Yanqi Liu, Email: liuyanqi@zzuli.edu.cn.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.He X., Wang X., Fang J., et al. Bletilla striata: medicinal uses, phytochemistry and pharmacological activities. Journal of Ethnopharmacology. 2017;195:20–38. doi: 10.1016/j.jep.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z., Cheng L., He Y., Wei X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: a review. International Journal of Biological Macromolecules. 2018;120:2076–2085. doi: 10.1016/j.ijbiomac.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y., Wang Y. Research progress of polysaccharides from Bletilla striata. Traditional Medical Science and Technology. 2015;22:479–482. [Google Scholar]
- 4.Zhang M., Shao Q., Xu E., Wang Z., Wang Z., Yin L. Bletilla striata: a review of seedling propagation and cultivation modes. Physiology and Molecular Biology of Plants. 2019;25(3):601–609. doi: 10.1007/s12298-019-00644-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J., Lv L., Li Y., et al. Preparation and evaluation of Bletilla striata polysaccharide/graphene oxide composite hemostatic sponge. International Journal of Biological Macromolecules. 2019;130:827–835. doi: 10.1016/j.ijbiomac.2019.02.137. [DOI] [PubMed] [Google Scholar]
- 6.Cai J., Liang Y., Wu Q., Hu J., Liang H., Wei K. Optimization of extraction of polysaccharide from Bletilla striata and its biological activity. Food industry. 2018;39:45–49. [Google Scholar]
- 7.Liao Z., Zeng R., Hu L., Maffucci K. G., Qu Y. Polysaccharides from tubers of Bletilla striata: physicochemical characterization, formulation of buccoadhesive wafers and preliminary study on treating oral ulcer. International Journal of Biological Macromolecules. 2019;122:1035–1045. doi: 10.1016/j.ijbiomac.2018.09.050. [DOI] [PubMed] [Google Scholar]
- 8.Kong L., Yu L., Feng T., Yin X., Liu T., Dong L. Physicochemical characterization of the polysaccharide from Bletilla striata: effect of drying method. Carbohydrate Polymers. 2015;125:1–8. doi: 10.1016/j.carbpol.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Han S., Li R., et al. Structural characterization and immunological activity of polysaccharides from the tuber of Bletilla striata. International Journal of Biological Macromolecules. 2019;122:628–635. doi: 10.1016/j.ijbiomac.2018.10.201. [DOI] [PubMed] [Google Scholar]
- 10.Xuchen L., Guang B. In vivo and in vitro effects of Bletilla striata polysaccharide-loaded paclitaxel nanoparticles on human gastric cancer cells. Tropical Journal of Pharmaceutical Research. 2019;18(1):13–17. doi: 10.4314/tjpr.v18i1.2. [DOI] [Google Scholar]
- 11.Wang L., Wu Y., Li J., Qiao H., Di L. Rheological and mucoadhesive properties of polysaccharide from Bletilla striata with potential use in pharmaceutics as bio-adhesive excipient. International Journal of Biological Macromolecules. 2018;120:529–536. doi: 10.1016/j.ijbiomac.2018.08.127. [DOI] [PubMed] [Google Scholar]
- 12.Yue L., Wang W., Wang Y., et al. Bletilla striata polysaccharide inhibits angiotensin II-induced ROS and inflammation via NOX4 and TLR2 pathways. International Journal of Biological Macromolecules. 2016;89:376–388. doi: 10.1016/j.ijbiomac.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Zhan X., Jia L., Niu Y., et al. Targeted depletion of tumour-associated macrophages by an alendronate-glucomannan conjugate for cancer immunotherapy. Biomaterials. 2014;35(38):10046–10057. doi: 10.1016/j.biomaterials.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Peng Q., Li M., Xue F., Liu H. Structure and immunobiological activity of a new polysaccharide from Bletilla striata. Carbohydrate Polymers. 2014;107:119–123. doi: 10.1016/j.carbpol.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Liu D., Chen S., Wang Y., Jiang H., Yin H. A new glucomannan from Bletilla striata: structural and anti-fibrosis effects. Fitoterapia. 2014;92:72–78. doi: 10.1016/j.fitote.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Hu L., Liao Z., Hu Q., Maffucci K. G., Qu Y. Novel Bletilla striata polysaccharide microneedles: fabrication, characterization, and in vitro transcutaneous drug delivery. International Journal of Biological Macromolecules. 2018;117:928–936. doi: 10.1016/j.ijbiomac.2018.05.097. [DOI] [PubMed] [Google Scholar]
- 17.Wang C., Luo W., Li P., et al. Preparation and evaluation of chitosan/alginate porous microspheres/Bletilla striata polysaccharide composite hemostatic sponges. Carbohydrate Polymers. 2017;174:432–442. doi: 10.1016/j.carbpol.2017.06.112. [DOI] [PubMed] [Google Scholar]
- 18.Lai Y. L., Lin Y. Y., Sadhasivam S., et al. Efficacy of Bletilla striata polysaccharide on hydrogen peroxide-induced apoptosis of osteoarthritic chondrocytes. Journal of Polymer Research. 2018;25(2):49–59. doi: 10.1007/s10965-018-1448-z. [DOI] [Google Scholar]
- 19.Cui X., Zhang X., Yang Y., Wang C., Zhang C., Peng G. Preparation and evaluation of novel hydrogel based on polysaccharide isolated from Bletilla striata. Pharmaceutical Development and Technology. 2016;22:1001–1011. doi: 10.1080/10837450.2016.1221422. [DOI] [PubMed] [Google Scholar]
- 20.Qu Y., Li C., Zhang C., Zeng R., Fu C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydrate Polymers. 2016;148:345–353. doi: 10.1016/j.carbpol.2016.04.081. [DOI] [PubMed] [Google Scholar]
- 21.Ji X., Peng Q., Yuan Y., Shen J., Xie X., Wang M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): a review. Food Chemistry. 2017;227:349–357. doi: 10.1016/j.foodchem.2017.01.074. [DOI] [PubMed] [Google Scholar]
- 22.Ji X., Han L., Liu F., Yin S., Peng Q., Wang M. A mini-review of isolation, chemical properties and bioactivities of polysaccharides from buckwheat (Fagopyrum Mill) International Journal of Biological Macromolecules. 2019;127:204–209. doi: 10.1016/j.ijbiomac.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 23.Shang J. Progress on extraction of polysaccharide from Bletilla striata and its application in food. Modern Food. 2018;1:124–127. [Google Scholar]
- 24.Wang Y. Isolation, characterization and immunological activity of polysaccharides from the tuber of Bletilla striata (M.S. thesis) Beijing, China: Chinese Academy of Medical Sciences and Peking Union Medical College; 2019. [Google Scholar]
- 25.He X., Gu F., Huang S., Han B., Chen N. Ultrasonic-assisted extraction and in vitro antioxidant activities evaluation of polysaccharides from Bletilla striata. Western Anhui University. 2017;33:1–5. [Google Scholar]
- 26.Han W., Peng R., Zhao S. Extraction optimization of total polysaccharide from Bletilla striata by response surface methodology with Plackett-Burman design. Journal of Xuzhou Institute of Technology. 2018;33:19–26. [Google Scholar]
- 27.Tai R., Yin X. Extraction and analysis of polysaccharide from Bletilla Striata. China Pharmaceutical. 2014;23:35–37. [Google Scholar]
- 28.Zhang J., Wen C., Duan Y., Zhang H., Ma H. Advance in Cordyceps militaris (Linn) link polysaccharides: isolation, structure, and bioactivities: a review. International Journal of Biological Macromolecules. 2019;132:906–914. doi: 10.1016/j.ijbiomac.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Ji X., Hou C., Guo X. Physicochemical properties, structures, bioactivities and future prospective for polysaccharides from Plantago L. (Plantaginaceae): a review. International Journal of Biological Macromolecules. 2019;135:637–646. doi: 10.1016/j.ijbiomac.2019.05.211. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J., Guo X., Guo T., et al. Novel pH-responsive and self-assembled nanoparticles based on Bletilla striata polysaccharide: preparation and characterization. RSC Advances. 2018;8(70):40308–40320. doi: 10.1039/C8RA07202G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DuBois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 32.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Masci A., Carradori S., Casadei M. A., et al. Lycium barbarum polysaccharides: extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chemistry. 2018;254:377–389. doi: 10.1016/j.foodchem.2018.01.176. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Xu F., Zheng M., Xi X., Cui X., Han C. Maca polysaccharides: a review of compositions, isolation, therapeutics and prospects. International Journal of Biological Macromolecules. 2018;111:894–902. doi: 10.1016/j.ijbiomac.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 35.Guan Q., Zhang G., Sun D., et al. In vitro and in vivo evaluation of docetaxel-loaded stearic acid-modified Bletilla striata polysaccharide copolymer micelles. PloS One. 2017;12(3):p. e0173172. doi: 10.1371/journal.pone.0173172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W. Physicochemical properties and pharmacological activities of polysaccharides alkali extracted from Bletilla striata (M.S. thesis) Xian, China: Shaanxi Normal University; 2015. [Google Scholar]
- 37.Wang C., Sun J., Luo Y., et al. A polysaccharide isolated from the medicinal herb Bletilla striata induces endothelial cells proliferation and vascular endothelial growth factor expression in vitro. Biotechnology Letters. 2006;28(8):539–543. doi: 10.1007/s10529-006-0011-x. [DOI] [PubMed] [Google Scholar]
- 38.Nie S. P., Xie M. Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocolloids. 2011;25(2):144–149. doi: 10.1016/j.foodhyd.2010.04.010. [DOI] [Google Scholar]
- 39.Zhuang Y., Wang L., Liu C., et al. A novel fiber from Bletilla striata tuber: physical properties and application. Cellulose. 2019;26(9):5201–5210. doi: 10.1007/s10570-019-02472-3. [DOI] [Google Scholar]
- 40.Luo Y., Diao H., Xia S., Dong L., Chen J., Zhang J. A physiologically active polysaccharide hydrogel promotes wound healing. Journal of Biomedical Materials Research Part A. 2010;94A(1):193–204. doi: 10.1002/jbm.a.32711. [DOI] [PubMed] [Google Scholar]
- 41.Yan J. K., Wang W. Q., Wu J. Y. Recent advances in Cordyceps sinensis polysaccharides: mycelial fermentation, isolation, structure, and bioactivities: a review. Journal of Functional Foods. 2014;6:33–47. doi: 10.1016/j.jff.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L., Zhang L. M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydrate Polymers. 2009;76(3):349–361. doi: 10.1016/j.carbpol.2008.12.015. [DOI] [Google Scholar]
- 43.Wang S. Structural analysis and antitumor activity of polysaccharides from Bletilla Ochracea Schltr (M.S. thesis) Xian, China: Shaanxi Normal University; 2017. [Google Scholar]
- 44.Lv H., Zhang T., Li Q. Advances in pharmacological action of polysaccharides from Bletilla striata. China Pharm. 2015;26:4014–4016. [Google Scholar]
- 45.Qu Y., Zhang C., Liao Z., Hu L., He Y. Exploration on pharmaceutical applications of Bletilla striata polysaccharide in medical biomaterials. Pharmacy and Clinics of Chinese Materia Medica. 2017;8:54–58. [Google Scholar]
- 46.Ramberg J. E., Nelson E. D., Sinnott R. A. Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutrition Journal. 2010;9(1):54–75. doi: 10.1186/1475-2891-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahbar Saadat Y., Yari Khosroushahi A., Pourghassem Gargari B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydrate Polymers. 2019;217:79–89. doi: 10.1016/j.carbpol.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 48.Liu J., Bai R., Liu Y., Zhang X., Kan J., Jin C. Isolation, structural characterization and bioactivities of naturally occurring polysaccharide-polyphenolic conjugates from medicinal plants—a review. International Journal of Biological Macromolecules. 2018;107:2242–2250. doi: 10.1016/j.ijbiomac.2017.10.097. [DOI] [PubMed] [Google Scholar]
- 49.Ji X., Shen Y., Guo X. Isolation, structures, and bioactivities of the polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino: a review. BioMed Research International. 2018;2018:14. doi: 10.1155/2018/6285134.6285134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J., Hu S., Nie S., Yu Q., Xie M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Medicine and Cellular Longevity. 2016;2016:13. doi: 10.1155/2016/5692852.5692852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z. J., Xie J. H., Nie S. P., Xie M. Y. Review on cell models to evaluate the potential antioxidant activity of polysaccharides. Food & Function. 2017;8(3):915–926. doi: 10.1039/C6FO01315E. [DOI] [PubMed] [Google Scholar]
- 52.Alam M., Bristi N., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceutical Journal. 2013;21(2):143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu F., Du B., Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Critical Reviews in Food Science and Nutrition. 2017;58(8):1260–1270. doi: 10.1080/10408398.2016.1251390. [DOI] [PubMed] [Google Scholar]
- 54.Cai J., Xiong J., Huang Y., et al. Study on ultrasonic-microwave synergistic extraction of polysaccharose from Bletilla striata and its antioxidant activity. Food Industry Science & Technology. 2016;37:274–284. [Google Scholar]
- 55.Muszynska B., Grzywacz-Kisielewska A., Kala K., Gdula-Argasinska J. Anti-inflammatory properties of edible mushrooms: a review. Food Chemistry. 2018;243:373–381. doi: 10.1016/j.foodchem.2017.09.149. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Liu J., Li Q., Wang Y., Wang C. Two natural glucomannan polymers, from Konjac and Bletilla, as bioactive materials for pharmaceutical applications. Biotechnology Letters. 2015;37(1):1–8. doi: 10.1007/s10529-014-1647-6. [DOI] [PubMed] [Google Scholar]
- 57.Yang X., Liu W., Li N., et al. Design and development of polysaccharide hemostatic materials and their hemostatic mechanism. Biomaterials Science. 2017;5(12):2357–2368. doi: 10.1039/C7BM00554G. [DOI] [PubMed] [Google Scholar]
- 58.Cui X., Wang S., Cao H., et al. A review. The bioactivities and pharmacological applications of Polygonatum sibiricum polysaccharides. Molecules. 2018;23(5):p. 1170. doi: 10.3390/molecules23051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diao H., Li X., Chen J., et al. Bletilla striata polysaccharide stimulates inducible nitric oxide synthase and proinflammatory cytokine expression in macrophages. Journal of Bioscience and Bioengineering. 2008;105(2):85–89. doi: 10.1263/jbb.105.85. [DOI] [PubMed] [Google Scholar]
- 60.Zhang C., He Y., Chen Z., Shi J., Qu Y., Zhang J. Effect of polysaccharides from Bletilla striata on the healing of dermal wounds in mice. Evidence-Based Complementary and Alternative Medicine. 2019;2019:9. doi: 10.1155/2019/9212314.9212314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo L., Zhou Z., Xue J., et al. Bletilla striata polysaccharide has a protective effect on intestinal epithelial barrier disruption in TAA-induced cirrhotic rats. Experimental and Therapeutic Medicine. 2018;16(3):1715–1722. doi: 10.3892/etm.2018.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H. Protective effect of polysaccharide of Bletilla striata on ethanol-induced gastric mucosal injury (M.S. thesis) Xian, China: Shaanxi Normal University; 2018. [Google Scholar]
- 63.Yang F., Luo L., Liu Y., et al. Bletilla striata polysaccharides ameliorates lipopolysaccharide-induced injury in intestinal epithelial cells. Saudi Journal of Gastroenterology. 2019;25(5):302–308. doi: 10.4103/sjg.sjg_520_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Q., Wei X., Tang Y. To investigate the effect of Rhizoma Bletillae polysaccharide and its combination with 5-Fu on human gastric cancer cell line MKN45. Inner Mongol Journal of Traditional Chinese Medicine. 2014;2:46–48. [Google Scholar]
- 65.Zhang Y. S., Lv T., Li M., et al. Anti-aging effect of polysaccharide from Bletilla striata on nematode Caenorhabditis elegans. Pharmacognosy Magazine. 2015;11(43):449–454. doi: 10.4103/0973-1296.160447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Q., Wang N., Hu R., et al. Wet spinning of Bletilla striata polysaccharide/silk fibroin hybrid fibers. Materials Letters. 2015;161:576–579. doi: 10.1016/j.matlet.2015.09.031. [DOI] [Google Scholar]
- 67.Li Q., Li K., Huang S. S., Zhang H. L., Diao Y. P. Optimization of extraction process and antibacterial activity of Bletilla striata polysaccharides. Asian Journal of Chemistry. 2014;26(12):3574–3580. doi: 10.14233/ajchem.2014.16500. [DOI] [Google Scholar]
- 68.Han L., Fu Y., Shen X., Zhang L. Optimization of the extraction technology of polysaccharide from Rhizoma Bletiliae. China Pharm. 2008;19:1620–1622. [Google Scholar]
- 69.Zhao N., Li Z., Zhang Q., Zhang C. Study on extraction technology of Bletilla striata polysaccharide. Applied Chemical Industry. 2015;44:2212–2215. [Google Scholar]
- 70.Peng R., Zhao S., Wang C., Han W. Optimization of flocculating purification on Bletilla striata polysaecharide by desirability function and response surface methodology combined with Plackett-Burman test. Journal of Nanjing Tech University. 2019;41:57–63. [Google Scholar]
- 71.Xiang Y., Ye Q., Li W., Xu W., Yang H. Preparation of wet-spun polysaccharide fibers from Chinese medicinal Bletilla striata. Materials Letters. 2014;117:208–210. doi: 10.1016/j.matlet.2013.05.098. [DOI] [Google Scholar]
- 72.Liu Y., Han W. Ultrasonic-assisted extraction of polysaecharide from Bleailla striata. Mechanical and Electrical Information. 2017;32:35–41. [Google Scholar]
- 73.Dong L., Xia S., Luo Y., et al. Targeting delivery oligonucleotide into macrophages by cationic polysaccharide from Bletilla striata successfully inhibited the expression of TNF-α. Journal of Controlled Release. 2009;134(3):214–220. doi: 10.1016/j.jconrel.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Wang B., Sha X., Huang L., Wang Z. Isolation, purification and structural characterization of a polysaccharide fraction from stem tuber of Bletilla striata, named BSPI-A. Food Science. 2010;31:120–123. [Google Scholar]
- 75.Liu J., Wang H., Yin Y., Li N., Cai P., Yang S. Controlled acetylation of water-soluble glucomannan from Bletilla striata. Carbohydrate Polymers. 2012;89(1):158–162. doi: 10.1016/j.carbpol.2012.02.065. [DOI] [PubMed] [Google Scholar]
- 76.Wu X.-g., Xin M., Chen H., Yang L.-n., Jiang H.-r. Novel mucoadhesive polysaccharide isolated from Bletilla striata improves the intraocular penetration and efficacy of levofloxacin in the topical treatment of experimental bacterial keratitis. Journal of Pharmacy and Pharmacology. 2010;62(9):1152–1157. doi: 10.1111/j.2042-7158.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhang M., Sun L., Zhao W., et al. Cholesteryl-modification of a glucomannan from Bletilla striata and its hydrogel properties. Molecules. 2014;19(7):9089–9100. doi: 10.3390/molecules19079089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Z., Zhao Y., Zhang M., et al. Structural characterization and antioxidant activity of a new polysaccharide from Bletilla striata fibrous roots. Carbohydrate Polymers. 2020;227:p. 115362. doi: 10.1016/j.carbpol.2019.115362. [DOI] [PubMed] [Google Scholar]
- 79.Zhang C., Gao F., Gan S., et al. Chemical characterization and gastroprotective effect of an isolated polysaccharide fraction from Bletilla striata against ethanol-induced acute gastric ulcer. Food and Chemical Toxicology. 2019;131:p. 110539. doi: 10.1016/j.fct.2019.05.047. [DOI] [PubMed] [Google Scholar]


