Abstract
We report a case series of seven mechanically ventilated patients with acute respiratory distress syndrome (ARDS) caused by coronavirus disease (COVID‐19) who received early treatment with high‐dose, short‐term systemic corticosteroids to prevent cytokine overproduction. Of the seven patients, four were male and median age was 69 years. They were intubated within seven days after admission when their respiratory status rapidly worsened. At that time, we administered 1000 or 500 mg/day for three days of methylprednisolone intravenously, followed by 1 mg/kg and tapered off. The median duration for the total administration of corticosteroids was 13 days. This high‐dose, short‐term corticosteroid therapy enabled extubation of the patients within seven days. Many questions on the clinical management of COVID‐19 remain unanswered, and data on corticosteroid therapy as a choice of treatment are mixed. We present the clinical course of our cases, review the previous evidence, and discuss management.
Keywords: ARDS, corticosteroid therapy, COVID‐19, mechanical ventilation
Many questions on the clinical management of coronavirus disease (COVID‐19) remain unanswered, and data on corticosteroid therapy as a choice of treatment are mixed. We present the clinical course of seven patients, review the previous evidence, and discuss management.
Introduction
A novel coronavirus was identified in 2019 as the cause of a cluster of pneumonia cases in Wuhan, China. It has since rapidly spread, resulting in a pandemic. Although most patients have a favourable prognosis, some patients may have worse outcomes. Acute respiratory distress syndrome (ARDS) is the most common complication and occurs in 60–70% of patients admitted to the intensive care unit (ICU) [1, 2, 3]. Indeed, patients with severe illness may develop dyspnoea and hypoxaemia within one week of disease onset, and this may rapidly progress to ARDS [4]. It can progress to refractory respiratory failure and, in such cases, extracorporeal membrane oxygenation or other forms of whole‐body management may be considered as a rescue therapy [4]. In spite of intensive care and rescue therapy, the mortality from coronavirus disease (COVID‐19) appears to be driven by the presence of severe ARDS and is approximately 50% [2, 3, 5, 6]. There is an urgent need to find a way of preventing the progress of ARDS with COVID‐19 to improve mortality rate.
It has been theorized that ARDS‐like states, caused by cytokine overproduction [7], are implicated in the worsening of the illness. The underlying factors in disease progression remain elusive, and effective treatments have not been established [5]. Among the treatments, corticosteroid therapy has been employed in many cases, but its efficacy remains unclear; therefore, it has not been officially recommended [5, 8]. Moreover, there are some reports in which the effectiveness of corticosteroid therapy for COVID‐19 is discussed, but its dose and duration are not clear in most cases. We summarized the clinical course and treatment timeline of seven critically ill patients with ARDS with COVID‐19 who were mechanically ventilated and treated with high‐dose, short‐term corticosteroid therapy. None of the patients have been reported in another manuscript.
Case Series
At St. Luke's International Hospital in Japan in March 2020, seven were admitted to the ICU with COVID‐19‐related ARDS when their oxygenation had been deteriorating rapidly. The diagnosis of COVID‐19 is made by detection of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) RNA by real‐time polymerase chain reaction. High‐flow oxygen therapy such as high‐flow nasal cannula and non‐invasive positive pressure ventilation were not used because they could cause aerosol exposure and lead to the disposal of medical devices. The order of oxygen delivery device up was nasal cannulas, face masks, and reservoir masks. When PaO2/FiO2 ratio < 150 and a worsening trend continued despite the reservoir mask, we performed tracheal intubation. During ventilator management, appropriate amount of maintenance fluids was administered and diuresis was considered. Fluid balance was managed to minus balance as much as possible to prevent worsening of oxygenation. Respiratory settings were with a limit tidal volume of 6–8 mL/kg (predicted body weight), end‐inspiratory plateau pressure < 30 cmH2O, and driving pressure < 15 cmH2O. Positive end‐expiratory pressure was initially set at 8–10 cmH2O and increased to an upper limit of 14–15 cmH2O when pulmonary compliance was decreased and air content was low. No patient was taken with prone positioning.
Four patients were male. The patients had a median age and body mass index of 69 years (range: 41–77) and 25.1, respectively. The median Brinkman index was 40, and comorbidities included asthma, chronic atrial fibrillation, diabetes, hypertension, and dyslipidaemia. The median time between fever onset and intubation was 11 days, the median PaO2/FiO2 ratio before intubation was 117, the median PaO2/FiO2 ratio after corticosteroid administration was 142, and the median fraction of inspired oxygen (FiO2) at 24 h after intubation was 0.4. At the exact moment when the patients' respiratory failure suddenly progressed, we started intravenous pulse methylprednisolone: 1000 or 500 mg/day of methylprednisolone for three days intravenously, followed by 1 mg/kg once daily, then tapered by 10 or 20 or 30 mg/day of prednisolone orally, finishing at 10 mg of prednisolone. The median duration of corticosteroids administration was 13 days. After the corticosteroid therapy, fever and oxygen demand decreased. The median C‐reactive protein levels before and after the therapy were 12.3 and 1.7 mg/dL, respectively. All patients were successfully extubated without reintubation and discharged from our hospital. The median time of mechanical ventilation was five days (range: 2–7). No therapies other than antibiotics ((piperacillin/tazobactam + azithromycin or levofloxacin) for seven days) and systemic corticosteroids for COVID‐19 were used. Venous thromboembolism prophylaxis was given to all patients by subcutaneous injection of unfractionated heparin. Secondary infections related to corticosteroid use were not observed. Side effects included hyperglycaemia in five patients and delusion in two patients. Table 1 and Figure 1 show the clinical features and timeline of cases. Figure 2 shows unenhanced computed tomography images of each patient on admission day.
Table 1.
Clinical features of cases.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
|---|---|---|---|---|---|---|---|
| Age | 72 | 49 | 77 | 57 | 71 | 41 | 69 |
| Sex | Male | Male | Female | Female | Male | Male | Female |
| BMI | 22.7 | 25.1 | 19.6 | 30.8 | 21.2 | 31.7 | 25.5 |
| Brinkman index | 1600 | 480 | 150 | Never | Never | 40 | Never |
| Initial symptoms | Fever, stomach ache | Fever, diarrhoea | Fever, loss of appetite | Fever, cough | Fever, cough | Fever, cough | Fever, dyspnoea |
| Chest CT findings on admission | Multiple GGO in bilateral lungs | Non‐segmental, patchy GGO in bilateral lungs | GGO of peripheral predominance in bilateral lungs | Non‐segmental, patchy GGO in peripheral predominance of bilateral lungs | GGO and consolidation in the bilateral inferior lungs | Multiple panlobular consolidation with fine reticular opacities, vascular thickening | Widespread GGO in bilateral lungs, mainly subpleural |
| Comorbidities | None | Asthma | Diabetes, hypertension, dyslipidaemia | Diabetes, hypertension, dyslipidaemia | Chronic atrial fibrillation | Diabetes, hypertension, asthma | None |
| Other respiratory pathogen infection | None | None | None | None | None | None | None |
| PaO2/FiO2 ratio before intubation | 114 | 156 | 100 | 117 | 76 | 140 | 133 |
| PaO2/FiO2 ratio after intubation | 182 | 170 | 108 | 127 | 120 | 160 | 142 |
| FiO2 at 24 h after intubation | 0.4 | 0.4 | 0.4 | 0.35 | 0.45 | 0.35 | 0.4 |
| Corticosteroid therapy | mPSL 1000 mg × three days followed by 1 mg/kg/day | mPSL 1000 mg × three days followed by 1 mg/kg/day | mPSL 1000 mg × three days followed by 1 mg/kg/day | mPSL 1000 mg × three days followed by 1 mg/kg/day | mPSL 500 mg × three days followed by 1 mg/kg/day | mPSL 500 mg ×three days followed by 1 mg/kg/day | mPSL 500 mg × three days followed by 1 mg/kg/day |
| Corticosteroid period (days) | 13 | 16 | 15 | 13 | 11 | 13 | 11 |
| Admission to intubation (days) | 6 | 1 | 0 | 1 | 6 | 0 | 0 |
| Initial symptoms to intubation (days) | 11 | 10 | 11 | 12 | 12 | 4 | 13 |
| Intubation period (days) | 2 | 7 | 7 | 3 | 5 | 5 | 4 |
| Clinical outcomes | Improved | Improved | Improved | Improved | Improved | Improved | Improved |
| RT‐PCR positive to negative (days) | 16 | 14 | 13 | 13 | 14 | 23 | 14 |
BMI, body mass index; CT, computed tomography; FiO2, fraction of inspired oxygen; GGO, ground‐glass opacity; mPSL, methylprednisolone; PaO2, partial pressure of arterial oxygen; RT‐PCR, real‐time polymerase chain reaction.
Figure 1.
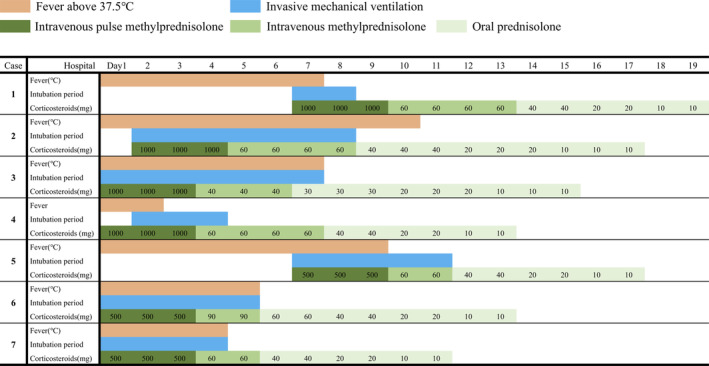
Timeline of disease course according to days from hospital admission. For each case, fever, intubation period, and the amount of corticosteroids were described. Regarding the fever, orange represents a fever of 37.5°C or higher. In terms of corticosteroids, dark green indicates intravenous methylprednisolone and light green indicates oral prednisolone.
Figure 2.
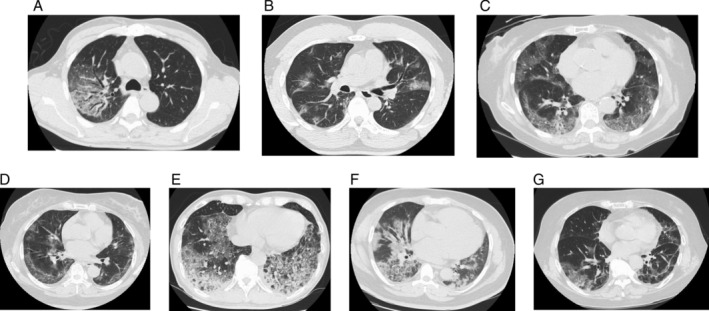
Unenhanced computed tomography images of seven patients on admission day. A–G corresponds to Case 1–7, respectively.
Discussion
Herein, we report a series of seven mechanically ventilated patients with ARDS‐related COVID‐19 who were administered early high‐dose, short‐term corticosteroid therapy.
Early studies have shown that increased amounts of serum proinflammatory cytokines were associated with pulmonary inflammation and extensive lung damage in patients with SARS‐CoV and Middle East respiratory syndrome coronavirus (MERS‐CoV) infections [9, 10]. In COVID‐19, Huang et al. noted the cytokine storm to be associated with disease severity [1]. According to Yang et al. [2], the non‐survivors will likely expire within one to two weeks after ICU admission, and ARDS increases the risk of death. In view of the high levels of cytokines induced during SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2 infections, corticosteroids have been used frequently for the treatment of severe illness, for the possible benefit of reducing inflammation‐induced lung injury. However, current evidence in patients with SARS and MERS suggests that corticosteroids had no effect on mortality, but instead delayed viral clearance [11, 12]. Therefore, glucocorticoids should not be routinely administered to patients with COVID‐19, unless there is a separate evidence‐based indication (e.g. asthma or chronic obstructive lung disease exacerbation, refractory septic shock, and adrenal insufficiency) [13]. However, administration of glucocorticoids in critically ill patients with COVID‐19‐related ARDS is controversial. Wu et al. reported a retrospective cohort analysis of patients with COVID‐19 who had developed ARDS [5]. They revealed methylprednisolone treatment to be associated with decreased risk of death (hazard ratio: 0.38; 95% CI: 0.20–0.72). Furthermore, Villar et al. noted that early administration of dexamethasone could reduce duration of mechanical ventilation and overall mortality in patients with severe ARDS [14].
We hypothesized that high‐dose corticosteroid therapy could prevent tissue damage, thereby mitigating the degree of lung injury. We began high‐dose corticosteroid therapy early in the process of respiratory failure before any progression of viral pneumonia‐related ARDS. As some reports of corticosteroid therapy for SARS and MERS indicated that such a treatment could be damaging and worsen patient prognosis [15, 16], we limited our patients to short‐term regimens. The initiation of methylprednisolone intravenously reduced patients' fever and led to weaning from mechanical ventilation. As a result, a 100% survival rate was achieved, and reintubation rates were 0%, followed by complete withdrawal of ventilator support in all cases within seven days.
The findings from this case series suggest that high‐dose, short‐term corticosteroid therapy early in respiratory failure may provide a good prognosis of patients with COVID‐19‐related ARDS without critical side effects of corticosteroids. This study is a single‐centre report and the number of cases is limited. Further studies are required to clarify the effect of corticosteroid treatment in COVID‐19.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case series and accompanying images.
At the time when this report was accepted for publication, the authors declared that the patients in this report had not been included in any previously published report on COVID‐19 that they had authored.
Acknowledgments
We thank Editage for translation check and constructive criticism. We also thank all participants for their patience and for agreeing to participate in this study.
So, C , Ro, S , Murakami, M , Imai, R , Jinta, T . (2020) High‐dose, short‐term corticosteroids for ARDS caused by COVID‐19: a case series. Respirology Case Reports, 8(6), e00596 10.1002/rcr2.596
Associate Editor: Bei He
References
- 1. Huang C, Wang Y, Li X, et al. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang X, Yu Y, Xu J, et al. 2020. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single centered, retrospective, observational study. Lancet Respir. Med. 8:475–481. 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arentz M, Yim E, Klaff L, et al. 2020. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington state. JAMA 323:1612–1614. 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, et al. 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu C, Chen X, Cai Y, et al. 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. e200994 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grasselli G, Zangrillo A, Zanella A, et al. 2020. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA 323:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta P, McAuley DF, and Brown M. 2020. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Russell CD, Millar JE, and Baillie JK. 2020. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet 395(10223):473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong CK, Lam CWK, Wu AK, et al. 2004. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahallawi WH, Khabour OF, Zhang Q, et al. 2018. MERS‐CoV infection in humans is associated with a pro‐inflammatory Th1 and Th17 cytokine profile. Cytokine 104:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stockman LJ, Bellamy R, and Garner P. 2006. SARS: systematic review of treatment effects. PLoS Med. 3(9):e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arabi YM, Mandourah Y, Al‐Hameed F, et al. 2018. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 197(6):757–767. [DOI] [PubMed] [Google Scholar]
- 13. WHO . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/internal-publications-detail/clinicalmanagement-of-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected (accessed 19 January 2020).
- 14. Villar J, Ferrando C, Martinez D, et al. 2020. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 8:267–276. [DOI] [PubMed] [Google Scholar]
- 15. Peiris JSM, Chu CM, Cheng VCC, et al. 2003. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet 361(9371):1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arabi YM, Balkhy HH, Hayden FG, et al. 2017. Middle East respiratory syndrome. N. Engl. J. Med. 376(6):584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]


