Abstract
Background
Allergic rhinitis (AR) is a global disease that affects a huge proportion of people around the world especially in the Middle East, where multiple allergy-promoting factors can be found. Although AR is not fatal, it severely affects the quality of life. However, it is usually overlooked in developing countries due to resource scarcity.
Methods
An online questionnaire on social media was used which included demographics, smoking, socioeconomic-status (SES), war-related questions, and the score for allergic rhinitis (SFAR), a simple self-reporting tool with the cut-off point at 7. Findings. This study included 968 subjects with 721 (74.5%) females. The mean age was 24.69 years with AR prevalence at 47.9%. AR was associated with male gender [P = 0.001 (OR, 1.677; 95% CI 1.249-2.253)], having a job [P = 0.049 (OR, 1.309; 95% CI 1.001-1.713)], the having a chronic medical condition (P < 0.0001) mainly other allergies [P < 0.0001 (OR, 9.199; 95% CI 3.836-22.063)] and asthma [P = 0.006 (OR, 5.060; 95% CI 1.396-18.342)], using medications (P < 0.0001) and living in particular provinces (P = 0.010). However, no significant correlation was found with type of work and war factors except being distressed by war sounds [P = 0.027 (OR, 1.348; 95% CI 1.034-1.757)]. Finally, no associations were found with age, consanguinity, SES, educational level, and cigarette or/and shisha smoking (P > 0.05). Interpretation. Approximately half of the sample displayed AR symptoms, indicating a potentially high burden of AR in the community. A correlation to being distressed from war noises was found with AR which could reflect a psychological aspect. In addition, in war harmful allergens are released which can be an additional AR risk factor which adds to the environment in the Middle East that is associated with AR. However, we need further studies to discover and minimize this huge prevalence of AR.
1. Introduction
Rhinitis is a respiratory disorder in the upper respiratory airway, characterized by rhinorrhoea, itching, sneezing, and nasal obstruction. Allergic rhinitis (AR) and nonallergic rhinitis (NAR) are the classifications that were previously used according to the clinical manifestations and allergic sensitization to common allergens [1]. AR occurs when nasal mucosa becomes inflamed due to allergens. Its prevalence varies from 5% to 40% of the human population worldwide [2]. AR is known to have chronic effects on more than one function of the body. Although AR is not fatal, it affects the quality of life of patients and disrupts their daily life, both socially and financially [3–5]. Risk factors may vary from smoking and drinking, domestic pet adoption, education environments, and family history to demographic factors [6–10]. Many studies suggest that the Middle East has many allergens and a higher prevalence of allergic diseases that may contribute to the high prevalence of AR [11–13]. AR can have a huge burden on the health system; in 2001 in the United States (US), AR prompted 12 million physician office visits, and the direct medical costs of AR were estimated to be around 4.5 billion US dollars per year and approximately 3.8 million lost work and school days [14]. The high prevalence of AR and its effect on many aspects of life intensify the importance of thoroughly studying AR and its risk factors, particularly in developing countries where it often goes underdiagnosed and is treated with over the counter (OTC) medications. Hence, this study aimed to assess the prevalence of AR among the developing Syrian population which has been poorly documented this far.
2. Materials and Methods
2.1. Study Design
We conducted an online cross-sectional study in Syria from 26/03/2019 to 22/04/2019. Online Arabic surveys were used that included subjects who lived in Syrian provinces. We posted the questionnaires online twice each day at 10 AM and 10 PM in several groups that covered different topics such as educational, cuisine, merchandise, entertainment, cultural, and musical. Any participant who lived in Syria, agreed on participating in the study, answered the basic demographic questions, and AR questionnaire was enrolled in this study. No further criteria was applied as it is an epidemiological study.
2.2. Questionnaires
Socioeconomic status (SES): SES was assessed through three questions: the education of the person or the working family member, monthly family income, and the profession. As a result, SES was divided into 5 different categories: lower, upper-lower, lower-middle, upper-middle, and upper.
Allergic rhinitis: We used an Arabic version of the score for allergic rhinitis (SFAR), a simple self-reporting tool with a cut-off point of 7 [2, 15]. SFAR has a sensitivity of 74% and specificity of 83% [2].
War-related questions: We asked several questions, both directly and indirectly, about the war including changing place of residence due to war, losing someone close, and being distressed by war noises.
Smoking: We only assessed the current smoking status for cigarettes and shisha as getting more details would be beyond this study goal. We only asked two questions in this regards, “do you regularly smoke cigarettes” and “do you regularly smoke shisha”. We did not assess for individuals who quitted smoking and the amount and time of smoking.
Other questions: We asked basic demographic questions including gender, age, educational level, province of residency, and having consanguineous parents. We asked the participants to declare having any medical condition and whether they used to take any medication. Age was divided into groups according to the national classification that was approved by Damascus University
Other definitions: Respiratory disease in this study was indicate to chronic bronchitis and chronic obstructive pulmonary disease (COPD). We defined low educational level as having a high school degree or less.
2.3. Ethical Commute Approval and Consents
Informed consent was taken from the participant before proceeding with the survey for participating in the research and for using and publishing the data. Confidentiality was assured and no questions indicative for the person were asked.
Our study protocol and ethical aspect were reviewed and approved by Damascus University deanship, Damascus, Syria.
2.4. Data Process
Data was processed using IBM SPSS software version 26 for Windows (SPSS Inc, IL, USA). Chi-square and one-way ANOVA tests were performed to determine statistical significance between the groups. Pearson's correlation was also calculated. We calculated odds ratios (ORs) and the 95% confidence intervals for the groups using the Mantel–Haenszel test by using the same software. Values of less than 0.05 for the two-tailed P values were considered statistically significant.
3. Results
3.1. Characteristics of the Sample
Our study included 968 subjects with 247 (25.5%) being male and 721 (74.5%) being female. The characteristics of the subjects are demonstrated in Table 1. The mean age was 24.69 ± 7.603years (CI 95%: 24.23-25.19). The mean SES score was 14.75 ± 5.280 (14.42-15.09 at CI = 95%). The mean SFAR score was 6.34 ± 3.649 (CI 95%: 6.11-6.57) and the percentage of subjects with AR was 47.9% (CI 95%: 44.7%-51.1%). Characteristics of war, the current medical conditions and medications, and SFAR score in the subjects are demonstrated in Table 2. Among university students, the mean SFAR score was 6.30 ± 3.651 (CI 95%: 5.98-6.59), and the valid percentage of subjects with AR was 47.4% (CI 95%: 43.6%-51.3%). There was no statistically significant difference between university students and the other subjects (P > 0.05).
Table 1.
Characteristics of subjects and their demographic data.
| Characteristic | Frequency (n = 968) | Percentage% |
|---|---|---|
| Age | ||
| 0–17 | 21 | 2.2 |
| 18–30 | 825 | 85.2 |
| 31–45 | 96 | 9.9 |
| 46+ | 26 | 2.7 |
| Gender | ||
| Male | 247 | 25.5 |
| Female | 721 | 74.5 |
| Place of living | ||
| Damascus | 525 | 54.2 |
| Rif-Dimashq | 50 | 5.2 |
| Aleppo | 62 | 6.4 |
| Homs and Hama | 127 | 14.3 |
| Al-Jazira region | 5 | 0.6 |
| Southern Syria | 24 | 2.7 |
| Syrian coast | 94 | 10.6 |
| Idlib | 4 | 0.4 |
| Smoking cigarettes | ||
| No | 822 | 84.9 |
| Yes regularly | 146 | 15.1 |
| Smoking shisha | ||
| No | 686 | 71.0 |
| Yes regularly | 280 | 29.0 |
| Educational level | ||
| Primary school | 1 | 0.1 |
| High school | 61 | 6.3 |
| Intermediate or higher Institute certificate | 767 | 79.5 |
| Master or PhD | 136 | 14.1 |
| SES level | ||
| Intermediate or higher Lower | 24 | 2.5 |
| Upper lower | 215 | 22.2 |
| Lower middle | 242 | 25.0 |
| Upper middle | 466 | 48.1 |
| Upper | 21 | 2.2 |
| Employment status | ||
| Unemployed | 615 | 63.9 |
| Employed | 348 | 36.1 |
Table 2.
Other characteristics of war, the current medical conditions and medications, and SFAR score in the subjects.
| Characteristic | Count | Percentage% |
|---|---|---|
| Changing the living area | ||
| No | 474 | 49.6 |
| Yes, but not due to the war | 178 | 18.6 |
| Yes | 304 | 31.8 |
| A relative being endangered by the war | ||
| No | 307 | 32.1 |
| Yes | 650 | 67.9 |
| Losing someone due to the war | ||
| No | 550 | 57.4 |
| Yes | 408 | 42.6 |
| Being afraid of the war sounds | ||
| No | 347 | 36.1 |
| Yes | 614 | 63.9 |
| Medical condition | ||
| No | 546 | 64.7 |
| Digestive | 76 | 9.0 |
| Pulmonary | 7 | 0.8 |
| Cardiac | 16 | 1.9 |
| Endocrine | 71 | 8.4 |
| Urinary | 12 | 1.4 |
| Neurological | 26 | 3.1 |
| Skeletal | 30 | 3.6 |
| Asthma | 14 | 1.7 |
| Allergic reaction | 46 | 5.5 |
| Drugs | ||
| No | 510 | 59.1 |
| Yes, some the supplements | 45 | 5.2 |
| Yes, over the counter drugs | 83 | 9.6 |
| Yes, prescribed drugs | 225 | 26.1 |
| AR | ||
| No | 504 | 52.1 |
| Yes | 464 | 47.9 |
3.2. AR Correlations with Other Factors
Comparing subjects with positive or negative AR is demonstrated in Table 3. We found that being male was correlated with having AR [P = 0.001 (OR, 1.677; 95% CI 1.249-2.253)]. Having a job was also correlated with having AR more frequently [P = 0.049 (OR, 1.309; 95% CI 1.001-1.713)]. However, it was not correlated with any type of work (P > 0.05). Having other medical conditions was also correlated with having AR (P < 0.0001), especially with having other allergic reactions [P < 0.0001 (OR, 9.199; 95% CI 3.836-22.063)] and having asthma [P = 0.006 (OR, 5.060; 95% CI 1.396-18.342)]. This was also the case with being distressed from war noises as it was correlated with having AR more frequently [P = 0.027 (OR, 1.348; 95% CI 1.034-1.757)].
Table 3.
Comparing subjects with positive and negative AR with other factors.
| Characteristic | Positive SFAR score | Percentage (CI 95%) | Negative SFAR score | Percentage (CI 95%) | P value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 95 | 9.8 | 152 | 15.7 | 0.001 |
| Female | 369 | 38.1 | 352 | 36.4 | |
| Consanguinity | |||||
| Negative | 349 | 75.9 | 380 | 76.3 | 0.422 |
| Positive | 111 | 24.1 | 118 | 23.7 | |
| SES | |||||
| Lower | 12 | 2.6 | 12 | 2.4 | 0.880 |
| Upper lower | 100 | 21.6 | 115 | 22.8 | |
| Lower middle | 113 | 24.4 | 129 | 25.6 | |
| Upper middle | 227 | 48.9 | 239 | 47.4 | |
| Upper | 12 | 2.6 | 9 | 1.8 | |
| Educational level | |||||
| Low | 28 | 6.0 | 34 | 6.8 | 0.646 |
| High | 435 | 94.0 | 468 | 93.2 | |
| Cigarette smoking | |||||
| No | 395 | 85.1 | 427 | 84.7 | 0.860 |
| Yes daily | 69 | 14.9 | 77 | 15.3 | |
| Shisha smoking | |||||
| No | 319 | 69.0 | 367 | 72.8 | 0.197 |
| Yes regularly | 143 | 31.0 | 137 | 27.2 | |
| Age groups | |||||
| 0–17 | 8 | 1.7 | 13 | 2.6 | 0.886 |
| 18–30 | 396 | 85.3 | 429 | 85.1 | |
| 31–45 | 47 | 10.1 | 49 | 9.7 | |
| 46+ | 13 | 2.8 | 13 | 2.6 | |
| Employment status | |||||
| Unemployed | 283 | 62.1 | 332 | 68.2 | 0.049 |
| Employed | 173 | 37.9 | 155 | 31.8 | |
| Type of work | |||||
| Labourer | 11 | 6.4 | 16 | 10.4 | 0.289 |
| Clerk or in a restaurant | 14 | 8.2 | 15 | 9.7 | |
| Technician | 58 | 33.9 | 45 | 29.2 | |
| Specialist | 82 | 48.0 | 71 | 46.1 | |
| Employee | 6 | 3.5 | 7 | 4.5 | |
| Medical condition | |||||
| Negative | 230 | 56.7 | 316 | 72.1 | <0.0001 |
| Digestive | 42 | 10.3 | 34 | 7.8 | |
| Respiratory | 3 | 0.7 | 4 | 0.9 | |
| Cardiac | 8 | 2.0 | 8 | 1.8 | |
| Endocrine | 38 | 9.4 | 33 | 7.5 | |
| Urinary | 6 | 1.5 | 6 | 1.4 | |
| Neurological | 14 | 3.4 | 12 | 2.7 | |
| Skeletal | 14 | 3.4 | 16 | 3.7 | |
| Asthma | 11 | 2.7 | 3 | 0.7 | |
| Allergic reaction | 40 | 9.9 | 6 | 1.4 | |
| Drugs taken | |||||
| No | 210 | 51.6 | 300 | 65.8 | <0.0001 |
| Over the counter | 61 | 15.0 | 67 | 14.7 | |
| Prescribed | 136 | 33.4 | 89 | 19.5 | |
| Losing someone close due to war | |||||
| No | 270 | 61.2 | 280 | 58.9 | 0.758 |
| Yes a loved one or loved friend | 4 | 0.9 | 2 | 0.4 | |
| Yes a relative | 167 | 37.9 | 193 | 40.6 | |
| Distress from war noises | |||||
| Negative | 150 | 32.5 | 197 | 39.4 | 0.027 |
| Positive | 311 | 67.5 | 303 | 60.6 | |
| Changing place of living due to war | |||||
| Negative | 322 | 70.2 | 330 | 66.4 | 0.213 |
| Positive | 137 | 29.8 | 167 | 33.6 |
CI: confidence interval.
Furthermore, subjects who had AR took more medications, either prescribed or OTC (P < 0.0001). However, we did not find a statistically significant difference when comparing having AR with age, consanguinity, SES, educational level, cigarette and shisha smoking, and losing someone or changing place of living due to war (P > 0.05). When Pearson's correlation was calculated, no correlation was found between SFAR scores and age, SES scores, and the number of times of changing place of living due to war. No correlation was found with SES classification and SFAR scores when one-way ANOVA was used (P > 0.05).
3.3. AR Symptoms
The mean scores and prevalence of each symptom of the SFAR items in positive AR subjects are demonstrated in Table 4. More than 65% of subjects had symptoms of sneezing, blocked nose, runny nose, and itchy eyes. Pollen season allergy was declared by 25.2% and perennial allergy by 48.1%. 75.2% of AR subjects declared that pollen, house dust, or mite triggered their symptoms.
Table 4.
Mean score for each SFAR item in subjects with AR.
| Characteristic | Mean ± SD | CI (95%) |
|---|---|---|
| Sneezing | 0.64 ± 0.479 | 0.465–0.489 |
| Runny nose | 0.65 ± 0.478 | 0.462–0.489 |
| Blocked nose | 0.66 ± 0.474 | 0.457–0.487 |
| Nasal symptoms plus itchy eyes | 1.41 ± 0.911 | 0.867–0.946 |
| Time of occurrence | 1.21 ± 0.839 | 0.808–0.865 |
| Triggers | 1.85 ± 0.783 | 0.718–0.842 |
| Perceived allergic status | 0.81 ± 0.396 | 0.369–0.423 |
| Result of the allergic test | 0.65 ± 0.481 | 0.427–0.503 |
| Previous medical diagnosis | 0.62 ± 0.487 | 0.474–0.495 |
| Familial history of allergy | 1.53 ± 0.846 | 0.791–0.895 |
3.4. AR Distribution in Provinces
A statistically significant difference was found when comparing having AR with the province of origin (P = 0.005) and the province of currently living (P = 0.010) with Idlib, Daraa, and As-Suwayda having less AR but Tartus and Deir ez-Zur as provinces of origin having more AR. Furthermore, Hama, Idlib, Homs, Daraa, and As-Suwayda as provinces of current living had less frequency of AR (Figure 1).
Figure 1.
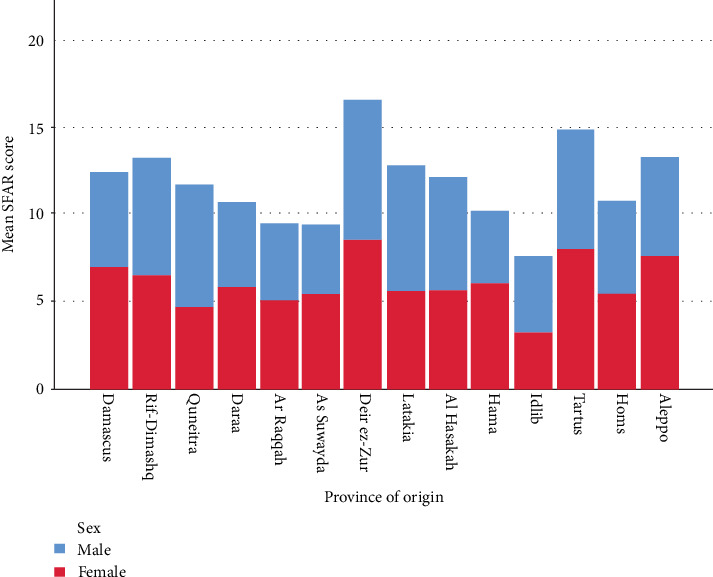
Showing male and female distribution in provinces with their mean SFAR score.
4. Discussion
4.1. AR Categorisation and Burden
AR has been previously categorised as perennial, intermittent, and occupational. However, the new classification is having symptoms either intermittently or persistently [16–18]. We found that AR is associated with being employed, but not to a specific type of work. Although around 75% of subjects declared having allergies from pollen which is in particular seasons in the year, around half of the subjects also declared having allergies throughout the year. AR affects nearly 20 to 40 million people in the United States alone, and these numbers are increasing; an estimated 20% of cases are seasonal, 40% of cases are perennial rhinitis, and 40% of cases are odf both [14]. Risk factors that were highly studied were pollens, drugs, domestic pets, and family history effects on AR [19–21]. The prevalence of AR was found to be approximately 1.4% to 39.7% in 13-14 years old worldwide [22]. In the US, prevalence ranged between 11.9% and 30.2% depending on symptoms and physician diagnosis [23, 24]. In developing countries, AR is especially poorly documented due to a lack of appropriate diagnostic tools [25]. In Europe, AR remains a significant health problem in the community due to the high burden of symptoms and its negative impact on the quality of life, affecting one in five Europeans [26, 27]. It was also found in another study that AR prevalence reached 10% in the Middle East, while in Egypt it was 11% and in Lebanon 9% [28]. However, the prevalence in Syria was much higher, reaching up to 47.9% according to our research which is significantly different from the previous studies (P < 0.0001). The high prevalence of AR in Syria could be due to the war as it exposed the population to various substances. We found a statistically significant correlation between AR and distress from war noises. However, this estimation was based on a screening tool rather than a medical diagnosis due to lack of resources, especially at this time of conflict.
4.2. AR and Its Correlations
In a regional country close to Syria, there was no statistical difference between having AR and gender, smoking, place of living, and other housing and economic conditions [29]. This was also found in another country in the region where gender, smoking, and domestic exposure did not have a significant correlation with AR, but age and area of residency were found to be correlated [30]. In Turkey, AR was found to be the most common allergic disease, and the prevalence rate of clinical symptoms was 11.4% with a higher rate among females and in urban areas [31]. However, it was found in a review that AR was more closely correlated with male gender, tobacco smoke, aspirin, and higher socioeconomic status [32]. Although smoking was found in Syria to be more common in males [33], AR, which was found to be more common in females, was correlated with smoking. Furthermore, AR was found to be correlated with laryngopharyngeal reflux (LPR) disease [34], the prevalence of LPR was also found to be in more than one-third in on study Syria [35] which could explain the high prevalence of AR.
Another study found that the coexistence of asthma and AR was more common in female adolescents [36]. Another study found a slight female predominance in adulthood with AR [37]. In addition, the risk for AR was inversely correlated with urbanisation [38]. However, many other studies found that the higher the SES, the higher AR symptoms were [39–41], but asthma correlation with SES had conflicting data [41, 42]. Smoking was not found to alter nasal symptoms of AR or quality of life [43] although a significant increase of self-reported rhinitis symptoms was found in adult smokers [44]. In our study, AR had a female predominance. However, with the previous conflicting data, our results showed that smoking shisha or cigarettes, SES, and educational level were not correlated with AR although we cannot to compare Syrian SES with the afore mentioned studies as there is a significant gap between SES levels in Syria and the population of the previouse studies.
In contrast, being employed increased the prevalence of AR in our subjects. However, no significant association was found with any particular type of employment. We also found a significant difference in AR prevalence depending on the province of residence. AR was found to increase the risk of asthma and atopic diseases.
4.3. The Significance of the Findings
When AR is not aggressively and early treated, it can lead to an increase in the incidence of asthma [32]. This was also found in our study as subjects with AR reported having asthma and allergies more often. Such as high prevalence can have severe ramifications in the future and can add to suffer from people and make the medical sector suffers even further.
While rates of diagnosis and treatment appear to be high worldwide, there are few reports on patient satisfaction and potential unmet needs in AR sufferers. There is also a need for more data on the diagnosis of respiratory allergies, the use of symptomatic medications, and the potential role of a guideline-recommended treatment option, for example, allergen immunotherapy (AIT) [28, 45, 46]. Distress from war noise was found to be correlated with AR and was found in another study to be correlated with mental distress and that over than 75% of participants were distressed from war noise [47]. We need to dedicate many resources to the health sector in Syria and upgrade the current system, so we can maximise the efficacy and enable better care, mainly as Syria has unique environment and population practices and habits may expose them to harmful substances that may be the cause of the high prevalence of allergies and AR [48].
5. In Conclusion
AR has complicated interactions with different risk factors and increases the risk of asthma if left untreated. It can also severely affect the quality of life. AR has a particularly high prevalence in the Syrian population which suggests underlying factors leading to this incline. This evidence reinforces the importance of studying AR and its risk factors, particularly in Syria, in order to have a comprehensive approach to treatment for such a prevalent health issue which contributes a significant burden on the health sector.
6. Limitations
This study was online based and, therefore, could not exactly determine the studied population. AR diagnosis was based on a screening tool, not a medical diagnosis. However, the effect of these two issues was minimized by the sample size and the high prevalence of AR found as it is a reflective result of the problem
Smoking could only be simply addressed and correlated with AR
No further details could be collected on SES and war-related events as this may raise some concerns and it would not be accepted by Damascus University ethical commutee as it will not be nationally acceptable questions. SES measurement is also different from other countries as the average income and standards are different,
Acknowledgments
We did not receive any support in forms of grants, equipment, or drugs.
Data Availability
Data can be made available upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Bousquet J., Khaltaev N., Cruz A. A., et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Supplement 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 2.Annesi-Maesano I., Didier A., Klossek M., Chanal I., Moreau D., Bousquet J. The score for allergic rhinitis (SFAR): a simple and valid assessment method in population studies. Allergy. 2002;57(2):107–114. doi: 10.1034/j.1398-9995.2002.1o3170.x. [DOI] [PubMed] [Google Scholar]
- 3.Woolcock A. J., Bastiampillai S. A., Marks G. B., Keena V. A. The burden of asthma in Australia. The Medical Journal of Australia. 2001;175(3):141–145. doi: 10.5694/j.1326-5377.2001.tb143062.x. [DOI] [PubMed] [Google Scholar]
- 4.Beasley R. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The Lancet. 1998;351(9111):1225–1232. doi: 10.1016/S0140-6736(97)07302-9. [DOI] [PubMed] [Google Scholar]
- 5.Trikojat K., Buske-Kirschbaum A., Plessow F., Schmitt J., Fischer R. Memory and multitasking performance during acute allergic inflammation in seasonal allergic rhinitis. Clinical & Experimental Allergy. 2017;47(4):479–487. doi: 10.1111/cea.12893. [DOI] [PubMed] [Google Scholar]
- 6.Li C. W., Chen D. H., Zhong J. T., et al. Epidemiological characterization and risk factors of allergic rhinitis in the general population in Guangzhou City in China. PLoS One. 2014;9(12, article e114950) doi: 10.1371/journal.pone.0114950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim F. L., Hashim Z., Than L. T. L., Md Said S., Hisham Hashim J., Norbäck D. Asthma, airway symptoms and rhinitis in office workers in Malaysia: associations with house dust mite (HDM) allergy, cat allergy and levels of house dust mite allergens in office dust. PLoS One. 2015;10(4, article e0124905) doi: 10.1371/journal.pone.0124905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phathammavong O., Ali M., Phengsavanh A., et al. Prevalence and potential risk factors of rhinitis and atopic eczema among schoolchildren in Vientiane capital, Lao PDR: ISAAC questionnaire. BioScience Trends. 2008;2(5):193–199. [PubMed] [Google Scholar]
- 9.Ziyab A. H. Prevalence and risk factors of asthma, rhinitis, and eczema and their multimorbidity among young adults in Kuwait: a cross-sectional study. BioMed Research International. 2017;2017:10. doi: 10.1155/2017/2184193.2184193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donohue K. M., Miller R. L., Perzanowski M. S., et al. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. Journal of Allergy and Clinical Immunology. 2013;131(3):736–742.e6. doi: 10.1016/j.jaci.2012.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goronfolah L. Aeroallergens, atopy and allergic rhinitis in the Middle East. European Annals of Allergy and Clinical Immunology. 2016;48(1):5–21. [PubMed] [Google Scholar]
- 12.Waibel K. H. Allergic rhinitis in the Middle East. Military Medicine. 2005;170(12):1026–1028. doi: 10.7205/MILMED.170.12.1026. [DOI] [PubMed] [Google Scholar]
- 13.John L. J., Ahmed S., Anjum F., et al. Prevalence of allergies among university students: a study from Ajman, United Arab Emirates. ISRN Allergy. 2014;2014:5. doi: 10.1155/2014/502052.502052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skoner D. P. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. The Journal of Allergy and Clinical Immunology. 2001;108(1):S2–S8. doi: 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- 15.Alharethy S., al Wedami M., Syouri F., et al. Validation of the Arabic version of the score for allergic rhinitis tool. Annals of Saudi Medicine. 2017;37(5):357–361. doi: 10.5144/0256-4947.2017.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bousquet J., Khaltaev N., Cruz A. A., et al. Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy. 2008;63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 17.Bousquet J., van Cauwenberge P., Khaltaev N. Allergic rhinitis and its impact on asthma. Journal of Allergy and Clinical Immunology. 2001;108(5):S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 18.Cingi C., Songu M., Ural A., et al. The score for allergic rhinitis study in Turkey. American Journal of Rhinology & Allergy. 2011;25(5):333–337. doi: 10.2500/ajra.2011.25.3665. [DOI] [PubMed] [Google Scholar]
- 19.Kuyucu S., Saraclar Y., Tuncer A., et al. Epidemiologic characteristics of rhinitis in Turkish children: the International Study of Asthma and Allergies in Childhood (ISAAC) phase 2. Pediatric Allergy and Immunology. 2006;17(4):269–277. doi: 10.1111/j.1399-3038.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 20.Sultész M., Katona G., Hirschberg A., Gálffy G. Prevalence and risk factors for allergic rhinitis in primary schoolchildren in Budapest. International Journal of Pediatric Otorhinolaryngology. 2010;74(5):503–509. doi: 10.1016/j.ijporl.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Tamay Z., Akcay A., Ones U., Guler N., Kilic G., Zencir M. Prevalence and risk factors for allergic rhinitis in primary school children. International Journal of Pediatric Otorhinolaryngology. 2007;71(3):463–471. doi: 10.1016/j.ijporl.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Strachan D., Sibbald B., Weiland S., et al. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC) Pediatric Allergy and Immunology. 1997;8(4):161–168. doi: 10.1111/j.1399-3038.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 23.Nathan R., Meltzer E., Seiner J., Storms W. Prevalence of allergic rhinitis in the United States. Journal of Allergy and Clinical Immunology. 1997;99(6):S808–S814. doi: 10.1016/S0091-6749(97)80040-1. [DOI] [PubMed] [Google Scholar]
- 24.Nathan R. A., Meltzer E. O., Derebery J., et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy and Asthma Proceedings. 2008;29(6):600–608. doi: 10.2500/aap.2008.29.3179. [DOI] [PubMed] [Google Scholar]
- 25.Piau J. P., Massot C., Moreau D., et al. Assessing allergic rhinitis in developing countries. The International Journal of Tuberculosis and Lung Disease. 2010;14(4):506–512. [PubMed] [Google Scholar]
- 26.Canonica G. W., Bousquet J., Mullol J., Scadding G. K., Virchow J. C. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007;62(Supplement 85):17–25. doi: 10.1111/j.1398-9995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 27.Maurer M., Zuberbier T. Undertreatment of rhinitis symptoms in Europe: findings from a cross-sectional questionnaire survey. Allergy. 2007;62(9):1057–1063. doi: 10.1111/j.1398-9995.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 28.Abdulrahman H., Hadi U., Tarraf H., et al. Nasal Allergies in the Middle Eastern Population: Results from the “Allergies in Middle East Survey”. American Journal of Rhinology & Allergy. 2012;26(6_Supplement):S3–S23. doi: 10.2500/ajra.2012.26.3836. [DOI] [PubMed] [Google Scholar]
- 29.Musmar S. Prevalence of Allergic Rhinitis & Its Risk Factors Among An-Najah University Students - Nablus/Palestine. Middle East Journal of Family Medicine. 2009;7:16–22. [Google Scholar]
- 30.Salarnia S., Momen T., Jari M. Prevalence and Risk Factors of Allergic Rhinitis in Primary School Students of Isfahan, Iran. Advanced Biomedical Research. 2018;7(1):p. 157. doi: 10.4103/abr.abr_194_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Başak O., Başak S., Gültekin B., Tekin N., Söylemez A. The prevalence of allergic rhinitis in adults in Aydin, Turkey. Rhinology. 2006;44(4):283–287. [PubMed] [Google Scholar]
- 32.Varshney J., Varshney H. Allergic rhinitis: an overview. Indian Journal of Otolaryngology and Head & Neck Surgery. 2015;67(2):143–149. doi: 10.1007/s12070-015-0828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakaje A., Alhalabi M. M., Alyousbashi A., Ghareeb A., Hamid L. Smoking Habits in Syria and the Influence of War on Cigarette and Shisha Smoking. 2020. [DOI] [PMC free article] [PubMed]
- 34.Kakaje A., Alhalabi M. M., Alyousbashi A., Hamid A., Aldeen O. H. Allergic Rhinitis, Asthma and Gastro-Esophageal Reflux Disease: A Cross-Sectional Study on their Reciprocal Relations. 2020. [DOI] [PMC free article] [PubMed]
- 35.Kakaje A., Alhalabi M. M., Alyousbashi A., Hamid A., Mahmoud Y. Laryngopharyngeal reflux disease in war-torn syria and its association with smoking and other risks. 2020. [DOI] [PMC free article] [PubMed]
- 36.Fröhlich M., Pinart M., Keller T., et al. Is there a sex-shift in prevalence of allergic rhinitis and comorbid asthma from childhood to adulthood? A meta-analysis. Clinical and Translational Allergy. 2017;7(1) doi: 10.1186/s13601-017-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinart M., Keller T., Reich A., et al. Sex differences in the prevalence of rhinitis: A systematic review and meta-analysis. 7.2 Paediatric Asthma and Allergy; September 2016; [DOI] [Google Scholar]
- 38.Christensen S. H., Timm S., Janson C., et al. A clear urban–rural gradient of allergic rhinitis in a population-based study in Northern Europe. European Clinical Respiratory Journal. 2016;3(1) doi: 10.3402/ecrj.v3.33463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercer M. J., Joubert G., Ehrlich R. I., et al. Socioeconomic status and prevalence of allergic rhinitis and atopic eczema symptoms in young adolescents. Pediatric Allergy and Immunology. 2004;15(3):234–241. doi: 10.1111/j.1399-3038.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 40.Torfi Y., Bitarafan N., Rajabi M. Impact of socioeconomic and environmental factors on atopic eczema and allergic rhinitis: a cross sectional study. EXCLI Journal. 2015;14:1040–1048. doi: 10.17179/excli2015-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uphoff E., Cabieses B., Pinart M., Valdés M., Antó J. M., Wright J. A systematic review of socioeconomic position in relation to asthma and allergic diseases. European Respiratory Journal. 2015;46(2):364–374. doi: 10.1183/09031936.00114514. [DOI] [PubMed] [Google Scholar]
- 42.Bråbäck L., Hjern A., Rasmussen F. Social class in asthma and allergic rhinitis: a national cohort study over three decades. European Respiratory Journal. 2005;26(6):1064–1068. doi: 10.1183/09031936.05.00022105. [DOI] [PubMed] [Google Scholar]
- 43.Bousquet P. J., Cropet C., Klossek J. M., Allaf B., Neukirch F., Bousquet J. Effect of smoking on symptoms of allergic rhinitis. Annals of Allergy, Asthma & Immunology. 2009;103(3):195–200. doi: 10.1016/S1081-1206(10)60181-0. [DOI] [PubMed] [Google Scholar]
- 44.Shargorodsky J., Garcia-Esquinas E., Galán I., Navas-Acien A., Lin S. Y. Allergic Sensitization, Rhinitis and Tobacco Smoke Exposure in US Adults. PLoS One. 2015;10(7, article e0131957) doi: 10.1371/journal.pone.0131957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canonica G. W., Bousquet J., Casale T., et al. Sub-lingual immunotherapy: world allergy organization position paper 2009. Allergy. 2009;64:1–59. doi: 10.1111/j.1398-9995.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 46.Canonica G. W., Cox L., Pawankar R., et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organization Journal. 2014;7(1):p. 6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kakaje A., Zohbi R. A., Aldeen O. H., Makki L., Alyousbashi A., Alhaffar B. A. Mental disorder and PTSD in Syria during wartime: a national-wide crisis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kakaje A., Alhalabi M. M., Ghareeb A., Karam B., Hamid A., Mansour B. Breastfeeding and acute lymphoblastic leukaemia: potential leukemogenesis in children in developing countries. 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available upon request.


