Abstract
Aims
Tenofovir (TDF) is an antiviral drug with potential risk of kidney injury. The study is aimed at comparing the incidence of acute kidney injury (AKI) between TDF and entecavir (ETV) treatment in hepatitis B virus- (HBV-) related acute on chronic liver failure (ACLF).
Methods
Treatment-naive patients with HBV-related ACLF were included. Propensity score matching was used to balance the baseline characteristics between ETV and TDF groups. The risk of AKI and the efficacy of TDF and ETV were compared.
Results
A total of 95 cases with HBV-related ACLF were included in this study, with 74.74% of male and a mean age of 47.01 ± 14.71 years. The antiviral therapy was initiated within 2 days after admission, with 39 cases on the TDF group and 56 on the ETV group. Patients in the TDF group had higher AST, hemoglobin, and serum sodium levels and lower MELD-Na score. After propensity matching, 39 cases of TDF and 39 of ETV were included in the final analysis. No difference was found in the changes of creatinine and cystatin C from baseline to 4 weeks after treatment between ETV and TDF groups. AKI was developed in 1 (2.56%) patient in the ETV group and 2 (5.13%) in the TDF group within one month (P = 0.556). Survival analysis revealed no significant difference in the 6-month mortality between the two groups (P = 0.813). Cox analysis showed that the type of antiviral drug or the development of AKI was not an independent risk factor for the outcomes.
Conclusions
Compared to ETV, TDF did not increase the risk of AKI nor the mortality in patients with HBV-related ACLF in the short time.
1. Introduction
Hepatitis B virus (HBV) is a major health problem with 3.5% of the population being chronically infected globally [1]. Patients with chronic HBV infection may suffer from various hepatic complications, such as cirrhosis, liver failure, and hepatocellular carcinoma [2]. Acute on chronic liver failure (ACLF) is defined as a precipitating event in a patient with chronic liver disease, leading to jaundice and coagulopathy complicated by clinical ascites and/or encephalopathy [3]. Patients with ACLF due to HBV reactivation (HBV-ACLF) have extremely poor prognosis, with a reported short-term mortality ranging from 29.7% to 40% within 28 days [4–6]. Acute kidney injury (AKI) is common in ACLF and may develop within a very short period and lead to a poor outcome in ACLF [7].
The management of HBV-ACLF includes antiviral therapy, artificial liver support system, alternative therapies, and liver transplantation [8]. The antiviral therapy is the most evident treatment among them. Currently, tenofovir (TDF) and entecavir (ETV) are both recommended as the first-line antiviral agents for their potent antiviral activity and high genetic barrier for drug resistance [9, 10]. However, TDF has also been demonstrated to have potential kidney toxicity by several observational studies and case reports [11–14]. It is unclear whether or not the use of TDF may increase the risk of AKI in ACLF. The aim of this study was to compare the risk of AKI and the mortality between ETV and TDF groups in HBV-ACLF.
2. Patients and Methods
2.1. Patients
We retrospectively reviewed cases of HBV-related ACLF hospitalized in the First Affiliated Hospital of Fujian Medical University between January 2016 and November 2018. Treatment-naive patients who were diagnosed with ACLF and received TDF or ETV therapy after hospitalization were included in this study. The exclusion criteria were as follows: (1) patients with kidney injury on baseline; (2) patients with nucleotide treatment other than ETV or TDF; (3) patients with malignant tumor; (4) patients concomitant with other liver diseases such as alcoholic liver disease, autoimmune hepatitis, drug-induced liver injury, or other viral infections (hepatitis A, C, and E virus or HIV infection); (5) patients with missing data; and (6) patients who died or were lost to follow-up within one week after admission.
The diagnosis of ACLF was based on the definition by the Asian Pacific Association for the Study of the Liver (APASL) [3]: jaundice (a serum bilirubin level of ≥5 mg/dL) and coagulopathy (an international normalized ratio (INR) of ≥1.5 or prothrombin activity of <40%). The definition of AKI was based on the criteria by the International Club of Ascites (ICA), which is an increase in serum creatinine (sCr) ≥ 0.3 mg/dL (≥26.5 μmol/L) within 48 hours or a percentage increase in sCr ≥ 50% from baseline which is known, or presumed, to have occurred within the prior 7 days. A value of sCr obtained in the previous 3 months, when available, can be used as baseline sCr. In patients with more than one value within the previous 3 months, the value closest to the admission time to the hospitalization was used [15].
2.2. Treatments
During hospitalization, all patients received supportive treatments including nutrition support, albumin, and other medications that aimed to protect the liver. In patients with liver failure, plasma exchange was given if necessary. Antiviral therapy with TDF or ETV was started immediately when HBV-DNA was detected.
2.3. Data Collection and Follow-Up
The clinical and laboratory data were collected on admission, including the presence of ascites or hepatic encephalopathy (HE), the presence of underlying cirrhosis, total bilirubin (TBIL), albumin, alanine aminotransferase (ALT), aspartate transaminase (AST), international normalized ratio (INR), serum creatinine (sCr), cystatin C, glomerular filtration rate (GFR), serum sodium (Na), hemoglobin, platelets, white blood cell (WBC), Child-Turcotte-Pugh (CTP) score, model for end-stage liver disease (MELD) score, chronic liver failure-sequential organ failure assessment (CLIF-SOFA), hepatitis B surface antigen (HBsAg) levels, hepatitis B e antigen (HBeAg), and HBV DNA levels. Patients were divided into ETV and TDF groups according to the antiviral treatment.
The renal function was reexamined in all survival patients on 4 weeks after antiviral treatment. The survival status was followed up until 2019. For patients being transferred to local hospital, the survival status was collected upon phone contact. The primary outcome was the incidence of AKI within 1 month; the secondary outcome was death or liver transplantation.
2.4. Statistical Analyses
The continuous variables were reported as mean ± standard deviation or medium (interquartile rage), while categorical variables were reported as percentage. The Student t-test was used for the comparisons of continuous variables, and the chi-squared test was used for the comparison of categorical variables [16]. Propensity score matching (PSM) analysis was performed to minimize the probability of selection bias [17]. The Cox proportional hazard model was used to analyze the risk factors of mortality. The log-rank test was used to compare the risks between groups. All statistical analyses were performed using SPSS software version 24.0 (SPSS Inc., Chicago, USA).
3. Results
3.1. Patient Characteristics
A total of 143 patients were diagnosed with ACLF during the study period, among whom 48 patients were excluded due to various reasons (Figure 1). Ninety-five cases were eligible for the final analysis, including 56 cases with ETV therapy and 39 cases with TDF therapy (Figure 1). The average age was 47.01 ± 14.71 years old, and 71 (74.74%) of them were male. The median follow-up time of the overall population was 531 days (range 14-1207 days). There were 20 patients who died during this time period, with a median survival time of 26 days. The baseline characteristics are shown in Table 1. Patients in the TDF group had higher AST, hemoglobin, and serum sodium levels and lower MELD-Na score. There was no difference in other baseline characteristics, including age, sex, HBV DNA levels, MELD score, and the presence of underlying cirrhosis.
Figure 1.
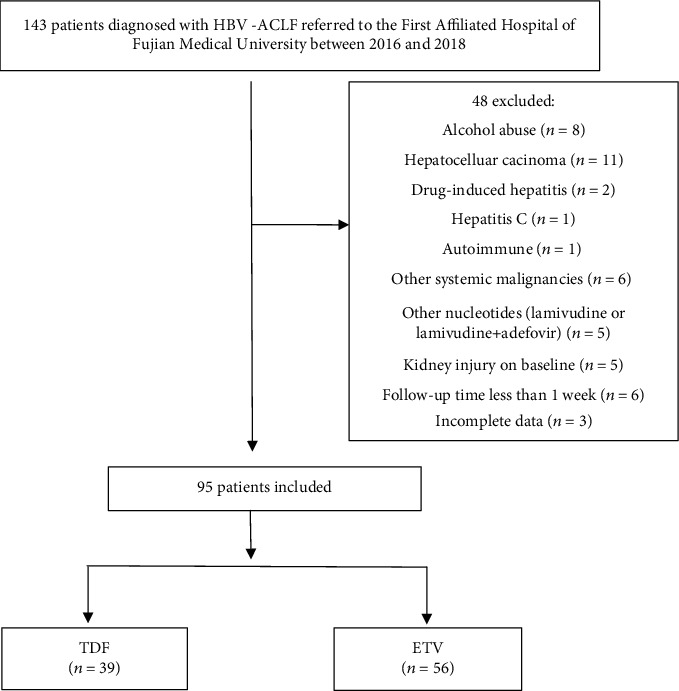
Flow chart of patient selection.
Table 1.
Baseline characteristics of study population.
| Variable | Unmatched | Matched | ||||
|---|---|---|---|---|---|---|
| ETV group (n = 56) | TDF group (n = 39) | P value | ETV group (n = 39) | TDF group (n = 39) | P value | |
| Age (years) | 47.80 ± 14.16 | 44.33 ± 15.87 | 0.266 | 45.97 ± 14.10 | 44.33 ± 15.87 | 0.631 |
| Male, n (%) | 42 (75.00%) | 29 (74.36%) | 0.944 | 30 (76.92%) | 29 (74.36%) | 0.792 |
| Ascites, n (%) | 44 (78.57%) | 31 (79.49%) | 0.914 | 29 (74.36%) | 31 (79.49%) | 0.591 |
| HE, n (%) | 9 (16.07%) | 5 (12.82%) | 0.884 | 7 (17.95%) | 5 (12.82%) | 0.530 |
| Cirrhosis, n (%) | 39 (69.62%) | 29 (74.36%) | 0.787 | 26 (66.67%) | 29 (74.36%) | 0.456 |
| TBIL (mmol/L) | 282.15 ± 131.00 | 259.64 ± 120.26 | 0.396 | 274.60 ± 138.61 | 259.64 ± 120.26 | 0.612 |
| ALT (U/L) | 624.61 ± 571.32 | 861.64 ± 691.44 | 0.071 | 724.79 ± 601.63 | 861.64 ± 691.44 | 0.354 |
| AST (U/L) | 419.04 ± 372.70 | 645.00 ± 629.04 | 0.031 | 490.10 ± 405.92 | 645.00 ± 629.04 | 0.200 |
| Albumin (g/L) | 30.05 (27.85-32.80) | 30.00 (27.90-34.00) | 0.934 | 29.80 (27.40-33.30) | 30.00 (27.90-34.00) | 0.768 |
| INR | 2.14 ± 0.89 | 1.96 ± 0.55 | 0.249 | 1.94 ± 0.59 | 1.96 ± 0.55 | 0.864 |
| BUN (mmol/L) | 4.33 ± 2.00 | 3.58 ± 1.52 | 0.052 | 4.07 ± 1.84 | 3.58 ± 1.52 | 0.203 |
| sCr (μmol/L) | 59.81 ± 12.35 | 57.86 ± 13.87 | 0.474 | 59.23 ± 11.24 | 57.86 ± 13.87 | 0.633 |
| Cystatin C (mg/L) | 1.11 ± 0.41 | 1.00 ± 0.21 | 0.128 | 1.06 ± 0.26 | 1.00 ± 0.21 | 0.301 |
| GFR (mL/min) | 93.00 ± 18.71 | 96.72 ± 23.24 | 0.392 | 94.49 ± 19.79 | 96.72 ± 23.24 | 0.650 |
| HBsAglog10 (ng/mL) | 3.10 ± 1.08 | 2.98 ± 1.07 | 0.595 | 3.28 ± 1.12 | 2.98 ± 1.07 | 0.243 |
| HBeAg-positive, n (%) | 26 (46.43%) | 22 (52.79%) | 0.454 | 19 (48.72%) | 22 (52.79%) | 0.496 |
| HBVDNAlog10 (IU/mL) | 5.11 ± 2.00 | 5.34 ± 1.68 | 0.560 | 5.39 ± 1.95 | 5.34 ± 1.68 | 0.903 |
| Na (mmol/L) | 136.16 ± 3.70 | 138.12 ± 2.93 | 0.007 | 136.62 ± 3.91 | 138.12 ± 2.93 | 0.058 |
| WBC (×109/L) | 6.38 ± 3.24 | 7.21 ± 3.57 | 0.247 | 6.68 ± 3.45 | 7.21 ± 3.57 | 0.510 |
| HGB (g/L) | 119.07 (102.25-136.50) | 132.67 (119.00-147.00) | 0.011 | 124.00 (111.00-143.00) | 132.67 (119.00-147.00) | 0.147 |
| Platelets (×109/L) | 106.95 ± 52.22 | 118.97 ± 60.22 | 0.303 | 116.03 ± 53.48 | 118.97 ± 60.22 | 0.820 |
| CTP score | 10.48 ± 1.87 | 10.36 ± 2.12 | 0.766 | 10.18 ± 1.90 | 10.36 ± 2.12 | 0.694 |
| MELD score | 20.25 ± 6.80 | 18.33 ± 5.20 | 0.139 | 18.22 ± 4.94 | 18.33 ± 5.20 | 0.928 |
| MELD-Na score | 21.68 ± 7.81 | 18.74 ± 5.70 | 0.047 | 19.46 ± 6.15 | 18.74 ± 5.70 | 0.593 |
| CLIF-SOFA score | 7.25 ± 1.73 | 7.05 ± 1.96 | 0.603 | 6.97 ± 1.67 | 7.05 ± 1.96 | 0.852 |
| Diabetes, n (%) | 8 (14.29%) | 3 (7.70%) | 0.508 | 5 (12.82%) | 3 (7.70%) | 0.709 |
| Hypertension, n (%) | 7 (12.50%) | 2 (5.13%) | 0.395 | 4 (10.26%) | 2 (5.13%) | 0.671 |
HE: hepatic encephalopathy; TBIL: total bilirubin; ALT: alanine aminotransferase; AST: aspartate transaminase; INR: international normalized ratio; BUN: blood urea nitrogen; sCr: serum creatinine; GFR: glomerular filtration rate; HBV: hepatitis B virus; HBsAg: hepatitis B surface antigen; HBeAg: hepatitis B e antigen; WBC: white blood cell; HGB: hemoglobin; CTP: Child-Turcotte-Pugh; MELD: model for end-stage liver disease; CLIF-SOFA: chronic liver failure-sequential organ failure assessment.
We performed PSM to balance the baseline factors. After PSM, there were 39 cases with ETV treatment and 39 cases with TDF treatment that were finally included. The baseline characteristics were comparable between the two groups after PSM. There were 15 patients in this PSM cohort who died during this follow-up, with a median survival time of 35 days.
3.2. Virological and Serological Responses in TDF and ETV Groups
Significant reductions in HBV-DNA, bilirubin, and ALT were observed in both TDF and ETV groups after two weeks of treatment, with no difference in the reduction level between the two groups (Table 2). The HBV-DNA undetectable rate after 2 weeks of antiviral therapy was 28.21% (11/39) in the ETV group and 35.90% (14/39) in the TDF group (P = 0.467).
Table 2.
Index changes between ETV and TDF groups after 2-week treatment.
| ETV (n = 39) | TDF (n = 39) | P (ETV vs. TDF) | |
|---|---|---|---|
| HBVDNA | |||
| Before treatment | 5.39 ± 1.95 | 5.34 ± 1.68 | |
| After 2 weeks | 3.36 ± 1.13 | 3.22 ± 1.10 | |
| Reduction | 2.03 ± 1.52 | 2.12 ± 1.01 | P = 0.776 |
| P (baseline vs. 2 weeks) | <0.001 | <0.001 | |
| ALT | |||
| Before treatment | 724.79 ± 601.63 | 861.64 ± 691.44 | |
| After 2 weeks | 130.90 ± 278.18 | 119.51 ± 112.05 | |
| Reduction | 593.90 ± 540.26 | 742.13 ± 689.12 | P = 0.294 |
| P (baseline vs. 2 weeks) | <0.001 | <0.001 | |
| TBIL | |||
| Before treatment | 274.60 ± 138.61 | 259.64 ± 120.26 | |
| After 2 weeks | 239.89 ± 250.38 | 223.54 ± 124.94 | |
| Reduction | 34.71 ± 234.75 | 36.09 ± 105.37 | P = 0.973 |
| P (baseline vs. 2 weeks) | 0.362 | 0.039 |
3.3. The Dynamic Changes of Renal Function in TDF and ETV Groups
Slight increases in sCr were found in both TDF and ETV groups after treatment. However, no significant difference in the change of sCr within 2 weeks or 4 weeks was found within each group or between two groups. Significant difference in the change of cystatin C within 2 weeks or 4 weeks was found within each group, but no significant difference in the dynamic changes of cystatin C between ETV and TDF groups (Table 3). Patients were followed up for 1 month, and AKI was developed in 1 (2.56%) patient in the ETV group and 2 (5.13%) patients in the TDF group. This difference was not statistically significant (P = 0.556). All of these 3 patients with AKI had cirrhotic background and pneumonia on admission. Two of them had diabetes. The patients with AKI in the ETV group died at 8 weeks after admission. The other two patients in the TDF group survived (Table 4).
Table 3.
Comparison changes in serum creatinine and cystatin C between the ETV and TDF group.
| ETV (n = 39) | TDF (n = 39) | P (ETV vs. TDF) | |
|---|---|---|---|
| sCr | |||
| Before treatment | 59.23 ± 11.24 | 57.86 ± 13.87 | |
| After 2 weeks | 61.06 ± 12.69 | 58.82 ± 11.56 | |
| Changes from baseline to 2 weeks | −1.57 ± 5.95 | −0.96 ± 10.32 | 0.748 |
| P (baseline vs. 2 weeks) | 0.080 | 0.565 | |
| After 4 weeks | 61.71 ± 12.14 | 60.92 ± 16.52 | |
| Changes from baseline to 4 weeks | −2.68 ± 8.96 | −2.17 ± 11.81 | 0.837 |
| P (baseline vs. 4 weeks) | 0.072 | 0.285 | |
| Cystatin C | |||
| Before treatment | 1.06 ± 0.26 | 1.00 ± 0.21 | |
| After 2 weeks | 1.18 ± 0.32 | 1.11 ± 0.24 | |
| Changes from baseline to 2 weeks | −0.12 ± 0.31 | −0.11 ± 0.16 | 0.810 |
| P (baseline vs. 2 weeks) | 0.02 | <0.001 | |
| After 4 weeks | 1.15 ± 0.16 | 1.28 ± 0.30 | |
| Changes from baseline to 4 weeks | −0.08 ± 0.39 | −0.25 ± 0.25 | 0.237 |
| P (baseline vs. 4 weeks) | 0.044 | 0.011 |
Table 4.
The clinical features of the AKI patients.
| A | B | C | |
|---|---|---|---|
| Age | 61 | 51 | 46 |
| Sex | Male | Female | Male |
| sCr (baseline) (μmol/L) | 64 | 64 | 67 |
| sCr (after treatment) (μmol/L) | 113 | 104 | 105 |
| Antivirus therapy | ETV | TDF | TDF |
| Cirrhosis | Yes | Yes | Yes |
| Hypertension | Yes | Yes | No |
| Diabetes | Yes | No | No |
| Pneumonia | Yes | Yes | Yes |
| Outcome | Death | Survival | Survival |
3.4. The Mortality in Overall Study Population and Predictors for Mortality
A total of 15/78 (19.23%) patients died within 6 months. Survival analysis revealed no significant difference in the 6-month mortality between two groups (P = 0.813). The results of univariate analysis showed that age, HE, HBeAg positive, MELD score-Na, CTP score, and SOFA score were related to the overall mortality.
Before multivariate analysis, collinearity diagnostics was conducted to assess the sources of collinearity among MELD-Na, CTP, and SOFA scores. The result showed that the tolerance of all variables > 0.1 and the variance inflation factor < 5, indicating limited collinearity among the above variables. As the presence of cirrhosis, HBV DNA, and AKI and gender had been reported to be important predictive factors for the prognosis of ACLF [18–21], those were included in multivariate analysis as well.
The results of multivariate Cox regression analysis showed that the age (HR = 1.103, 95% CI: 1.038-1.172, P = 0.002), CTP score (HR = 1.990, 95% CI: 1.210-3.271, P = 0.007), SOFA score (HR = 3.000, 95% CI: 1.366-3.171, P < 0.001), and cirrhosis (HR = 47.232, 95% CI: 5.538-402.802, P < 0.001) were independent risk factors for mortality (Table 5). The types of antiviral drug and the development of AKI were not independently associated with the outcome (Figure 2 and Table 5).
Table 5.
Cox analysis of risk factors for mortality.
| Variable | Univariate analysis (95% CI) | P value | Multivariate analysis (95% CI) | P value |
|---|---|---|---|---|
| Age | 1.044 (1.010-1.079) | 0.011 | 1.103 (1.038-1.172) | 0.002 |
| Male | 1.276 (0.360-4.522) | 0.706 | 1.200 (0.277-6.340) | 0.830 |
| HE | 3.291 (1.123-9.644) | 0.030 | 7.156 (0.740-69.170) | 0.089 |
| HBeAg-positive | 8.356 (1.884-37.062) | 0.005 | 10.611 (1.314-85.709) | 0.027 |
| Cirrhosis | 1.253 (0.428-3.667) | 0.681 | 47.232 (5.538-402.802) | <0.001 |
| Antivirus therapy | 0.885 (0.321-2.442) | 0.814 | ||
| AKI | 1.617 (0.213-12.302) | 0.642 | 5.394 (0.535-54.420) | 0.153 |
| lgHBsAg (ng/mL) | 0.874 (0.551-1.388) | 0.570 | ||
| lgHBV-DNA (IU/mL) | 0.920 (0.687-1.233) | 0.577 | 0.925 (0.563-1.522) | 0.760 |
| ALT (U/L) | 1.000 (1.000-1.001) | 0.407 | ||
| sCr (μmol/L) | 0.985 (0.879-1.104) | 0.795 | ||
| WBC (1012/L) | 0.993 (0.857-1.151) | 0.929 | ||
| PLT (109/L) | 0.997 (0.988-1.007) | 0.598 | ||
| Meld-Na score | 1.107 (1.020-1.201) | 0.015 | 0.972 (0.837-1.128) | 0.704 |
| CTP score | 1.743 (1.266-2.400) | 0.001 | 1.990 (1.210-3.271) | 0.007 |
| SOFA score | 2.146 (1.528-3.013) | <0.001 | 3.000 (1.621-5.553) | <0.001 |
Figure 2.
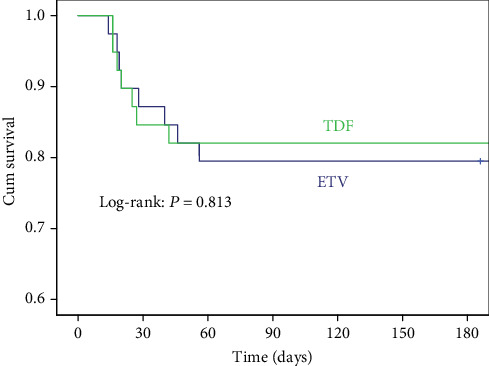
Cumulative survival of ETV and ETV within 6 months.
4. Discussion
This study compared the impact of TDF and ETV in renal function in patients with HBV-ACLF. The results showed that TDF did not increase the risk of AKI nor the mortality in patients with HBV-related ACLF within 6 months.
Both TDF and ETV are currently recommended as the first-line treatment for chronic hepatitis B (CHB) for their high efficacy and low resistance rate [9, 22–24]. Previous studies have demonstrated that TDF and ETV have similar effectiveness in treatment-naive CHB patient [25–27]. However, some reports indicate that TDF might lead to a higher incidence of AKI compared to ETV in CHB patients [28, 29]. As AKI is common in ACLF [30], renal injury associated with TDF use has raised some concerns [31]. However, in this single-center study, we found that the use of TDF did not increase the risk of AKI within one month of treatment. This might be due to the short follow-up period of this study. As reported previously, renal injury associated with TDF use usually develops after at least one year of treatment. A recent real-world study from Korea showed that TDF therapy did decrease overall renal function in CHB patients during the first two years of TDF use [13]. Therefore, long-term follow-up might be helpful to access the renal impairment in ACLF patients with different antiviral therapies.
It is worth noticing that all three patients suffering from AKI had bacterial infection and two of them had comorbidities like diabetes and hypertension. Hypertension and diabetes are both well-known risk factors for chronic kidney injury. The bacterial infection is also a main trigger for AKI in liver failure [32]; thus for patients who had AKI in this cohort, the impact of the other complication/comorbidities might overwhelm the influence of antiviral drugs. Prospective studies with longer follow-up period are greatly needed to reveal the real relationship between AKI and TDF in ACLF patients.
Cystatin C is a sensitive marker for renal impairment [33]. In this study, no significant difference in the change of sCr within 1 month was found in both TDF and ETV groups, while there was significant difference in the change of cystatin C in both groups. Cystatin C levels may be more sensitive for evaluating the renal impairment in ACLF [34]. However, in terms of the impact of different antiviral drugs on renal function, the changes of cystatin C were similar as those of sCr, which further consolidated that TDF had limited influence on renal function in an ACLF population in a short-term period.
The efficacy of different antiviral drugs in ACLF remains controversial. Wan et al. [35] showed that TDF was superior to ETV in the treatment of HBV-ACLF; however, more studies showed no difference between these two groups [27, 36, 37]. The results of our study were in consistence with most studies showing that TDF was not superior to ETV regarding the HBV DNA suppression or mortality.
There are several limitations of this study. Firstly, the data of HBV-DNA levels, liver function, and kidney function is largely missing after 3 months because most survival patients were transferred to a local hospital after recovery; thus, the long-term changes of renal function were unclear. Secondly, the incidence rate of AKI was low and the sample size relatively small, which may easily lead to false-negative results. Further study with larger sample size is needed to guarantee the results.
In summary, our study showed that compared with ETV, TDF did not increase the risk of AKI nor the mortality in patients with HBV-related ACLF within a short-term period.
Acknowledgments
This study was supported by the Chinese National Science and Technology Projects (2017ZX10202201); Fujian provincial health technology project (2018-ZQN-54); Fujian Medical University Sailing Fund Project (2018QH1047); and Fujian Province Health Youth Research Project (2019-1-37).
Data Availability
The data in this study are available from the corresponding author on reasonable request.
Ethical Approval
The study protocol has been approved by the Institutional Ethics Committee of the First Affiliated Hospital of Fujian Medical University. The clinical activities being reported are consistent with the principles of the Declaration of Helsinki.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Kai Zhang, Jiaofeng Huang, and Mingfang Wang collected and analyzed the data. Kai Zhang and Su Lin wrote the primary draft. Yueyong Zhu and Su Lin did the study design and revised the final article for important intellectual content. All authors read and approved the final version of the manuscript.
References
- 1.Yuen M. F., Chen D. S., Dusheiko G. M., et al. Hepatitis B virus infection. Nature Reviews Disease Primers. 2018;4(1, article 18035) doi: 10.1038/nrdp.2018.35. [DOI] [PubMed] [Google Scholar]
- 2.Tada T., Kumada T., Toyoda H., et al. Long-term prognosis of patients with hepatitis B infection: causes of death and utility of nucleos(t)ide analogue therapy. Journal of Gastroenterology. 2015;50(7):795–804. doi: 10.1007/s00535-014-1011-6. [DOI] [PubMed] [Google Scholar]
- 3.Sarin S. K., Kedarisetty C. K., Abbas Z., et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatology International. 2014;8(4):453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 4.Wan Y. M., Li Y. H., Xu Z. Y., et al. Therapeutic plasma exchange versus double plasma molecular absorption system in hepatitis B virus-infected acute-on-chronic liver failure treated by entercavir: a prospective study. Journal of Clinical Apheresis. 2017;32(6):453–461. doi: 10.1002/jca.21535. [DOI] [PubMed] [Google Scholar]
- 5.Lin S., Zhang K., Zhang J., Wang M., Velani B., Zhu Y. Long-term outcomes of patients with hepatitis B virus-related acute on chronic liver failure: an observational cohort study. Liver International. 2019;39(5):854–860. doi: 10.1111/liv.14072. [DOI] [PubMed] [Google Scholar]
- 6.Zeng Y., Li Y., Xu Z., et al. Myeloid-derived suppressor cells expansion is closely associated with disease severity and progression in HBV-related acute-on-chronic liver failure. Journal of Medical Virology. 2019;91(8):1510–1518. doi: 10.1002/jmv.25466. [DOI] [PubMed] [Google Scholar]
- 7.Yuan W., Zhang Y. Y., Zhang Z. G., Zou Y., Lu H. Z., Qian Z. P. Risk factors and outcomes of acute kidney injury in patients with hepatitis B virus-related acute-on-chronic liver failure. The American Journal of the Medical Sciences. 2017;353(5):452–458. doi: 10.1016/j.amjms.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Zhao R.-H., Shi Y., Zhao H., Wu W., Sheng J.-F. Acute-on-chronic liver failure in chronic hepatitis B: an update. Expert Review of Gastroenterology & Hepatology. 2018;12(4):341–350. doi: 10.1080/17474124.2018.1426459. [DOI] [PubMed] [Google Scholar]
- 9.Terrault N. A., Bzowej N. H., Chang K. M., et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Ma K., Han M., et al. Nucleoside analogs prevent disease progression in HBV-related acute-on-chronic liver failure: validation of the TPPM model. Hepatology International. 2014;8(1):64–71. doi: 10.1007/s12072-013-9485-5. [DOI] [PubMed] [Google Scholar]
- 11.Chan L., Asriel B., Eaton E. F., Wyatt C. M. Potential kidney toxicity from the antiviral drug tenofovir: new indications, new formulations, and a new prodrug. Current Opinion in Nephrology and Hypertension. 2018;27(2):102–112. doi: 10.1097/MNH.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kara A. V., Yıldırım Y., Ozcicek F., et al. Effects of entecavir, tenofovir and telbivudine treatment on renal functions in chronic hepatitis B patients. Acta Gastroenterologica Belgica. 2019;82(2):273–277. [PubMed] [Google Scholar]
- 13.Lim T. S., Lee J. S., Kim B. K., et al. An observational study on long-term renal outcome in patients with chronic hepatitis B treated with tenofovir disoproxil fumarate. Journal of Viral Hepatitis. 2019;27(3):316–322. doi: 10.1111/jvh.13222. [DOI] [PubMed] [Google Scholar]
- 14.Viganò M., Loglio A., Labanca S., et al. Effectiveness and safety of switching to entecavir hepatitis B patients developing kidney dysfunction during tenofovir. Liver International. 2019;39(3):484–493. doi: 10.1111/liv.14017. [DOI] [PubMed] [Google Scholar]
- 15.Angeli P., Ginès P., Wong F., et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Journal of Hepatology. 2015;62(4):968–974. doi: 10.1016/j.jhep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Annals of Translational Medicine. 2016;4(5):91–91. doi: 10.21037/atm.2016.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in Medicine. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maiwall R., Sarin S. K., Kumar S., et al. Development of predisposition, injury, response, organ failure model for predicting acute kidney injury in acute on chronic liver failure. Liver International. 2017;37(10):1497–1507. doi: 10.1111/liv.13443. [DOI] [PubMed] [Google Scholar]
- 19.Jeng W.-J., Sheen I.-S., Liaw Y.-F. Hepatitis B virus DNA level predicts hepatic decompensation in patients with acute exacerbation of chronic hepatitis B. Clinical Gastroenterology and Hepatology. 2010;8(6):541–545. doi: 10.1016/j.cgh.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Chen T., Yang Z., Choudhury A. K., et al. Complications constitute a major risk factor for mortality in hepatitis B virus-related acute-on-chronic liver failure patients: a multi-national study from the Asia-Pacific region. Hepatology International. 2019;13(6):695–705. doi: 10.1007/s12072-019-09992-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang S.-H., Chen P.-J., Yeh S.-H. Gender disparity in chronic hepatitis B: mechanisms of sex hormones. Journal of Gastroenterology and Hepatology. 2015;30(8):1237–1245. doi: 10.1111/jgh.12934. [DOI] [PubMed] [Google Scholar]
- 22.Liaw Y. F., Kao J. H., Piratvisuth T., et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatology International. 2012;6(3):531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 23.Lampertico P., Agarwal K., Berg T., et al. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. Journal of Hepatology. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Hsu C. W., Yeh C., Yeh C. T. Maintaining complete virological suppression by reduced doses of nucleos(t)ide analogue in patients with chronic hepatitis B. Journal of Medical Virology. 2019;91(7):1360–1363. doi: 10.1002/jmv.25443. [DOI] [PubMed] [Google Scholar]
- 25.Wu I.-T., Hu T. H., Hung C. H., et al. Comparison of the efficacy and safety of entecavir and tenofovir in nucleos(t)ide analogue-naive chronic hepatitis B patients with high viraemia: a retrospective cohort study. Clinical Microbiology and Infection. 2017;23(7):464–469. doi: 10.1016/j.cmi.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Kim S. U., Seo Y. S., Lee H. A., et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. Journal of Hepatology. 2019;71(3):456–464. doi: 10.1016/j.jhep.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Park J. W., Kwak K. M., Kim S. E., et al. Comparison of the long-term efficacy between entecavir and tenofovir in treatment-naïve chronic hepatitis B patients. BMC Gastroenterology. 2017;17(1):p. 39. doi: 10.1186/s12876-017-0596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen T. M., Lin C. C. Letter: tenofovir is associated with higher probability of acute kidney injury compared with entecavir. Alimentary Pharmacology & Therapeutics. 2014;40(4):406–407. doi: 10.1111/apt.12854. [DOI] [PubMed] [Google Scholar]
- 29.Tsai M.-C., Chen C. H., Tseng P. L., et al. Comparison of renal safety and efficacy of telbivudine, entecavir and tenofovir treatment in chronic hepatitis B patients: real world experience. Clinical Microbiology and Infection. 2016;22(1):95.e1–95.e7. doi: 10.1016/j.cmi.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Zang H., Liu F., Liu H., et al. Incidence, risk factors and outcomes of acute kidney injury (AKI) in patients with acute-on-chronic liver failure (ACLF) of underlying cirrhosis. Hepatology International. 2016;10(5):807–818. doi: 10.1007/s12072-016-9756-z. [DOI] [PubMed] [Google Scholar]
- 31.Buti M., Roade L., Riveiro-Barciela M., Esteban R. Optimal management of chronic hepatitis B patients receiving nucleos(t)ide analogues. Liver International. 2020;40(Supplement 1):15–21. doi: 10.1111/liv.14367. [DOI] [PubMed] [Google Scholar]
- 32.Ginès P., Solà E., Angeli P., Wong F., Nadim M. K., Kamath P. S. Hepatorenal syndrome. Nature Reviews Disease Primers. 2018;4(1):p. 23. doi: 10.1038/s41572-018-0022-7. [DOI] [PubMed] [Google Scholar]
- 33.Kar S., Paglialunga S., Islam R. Cystatin C is a more reliable biomarker for determining eGFR to support drug development studies. Journal of Clinical Pharmacology. 2018;58(10):1239–1247. doi: 10.1002/jcph.1132. [DOI] [PubMed] [Google Scholar]
- 34.Wan Z. H., Wang J. J., You S. L., et al. Cystatin C is a biomarker for predicting acute kidney injury in patients with acute-on-chronic liver failure. World Journal of Gastroenterology. 2013;19(48):9432–9438. doi: 10.3748/wjg.v19.i48.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan Y. M., Li Y. H., Xu Z. Y., et al. Tenofovir versus entecavir for the treatment of acute-on-chronic liver failure due to reactivation of chronic hepatitis B with genotypes B and C. Journal of Clinical Gastroenterology. 2019;53(4):e171–e177. doi: 10.1097/MCG.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 36.Han Y., Zeng A., Liao H., Liu Y., Chen Y., Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: a systematic review and meta-analysis. International Immunopharmacology. 2017;42:168–175. doi: 10.1016/j.intimp.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Sriprayoon T., Mahidol C., Ungtrakul T., et al. Efficacy and safety of entecavir versus tenofovir treatment in chronic hepatitis B patients: a randomized controlled trial. Hepatology Research. 2017;47(3):E161–e168. doi: 10.1111/hepr.12743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study are available from the corresponding author on reasonable request.


