Abstract
The burden of several common diseases including obesity, diabetes, hypertension, asthma, and depression is increasing in most world populations. However, the mechanisms underlying the numerous epidemiological and genetic correlations among these disorders remain largely unknown. We investigated whether common polymorphic inversions underlie the shared genetic influence of these disorders. We performed an inversion association analysis including 21 inversions and 25 obesity-related traits on a total of 408,898 Europeans and validated the results in 67,299 independent individuals. Seven inversions were associated with multiple diseases while inversions at 8p23.1, 16p11.2, and 11q13.2 were strongly associated with the co-occurrence of obesity with other common diseases. Transcriptome analysis across numerous tissues revealed strong candidate genes for obesity-related traits. Analyses in human pancreatic islets indicated the potential mechanism of inversions in the susceptibility of diabetes by disrupting the cis-regulatory effect of SNPs from their target genes. Our data underscore the role of inversions as major genetic contributors to the joint susceptibility to common complex diseases.
Keywords: genetic inversions, obesity-related diseases, common diseases, obesity, diabetes, hypertension, asthma, human traits, disease co-occurrence, genomic variation
Introduction
Obesity is a disorder with increasing but non-uniform prevalence in the world population and one of the major public health burdens.1 Obesity (MIM: 615812)-derived morbidity and years of life lost strongly associate to a broad range of highly prevalent diseases, including type 2 diabetes (MIM: 125853), cardiovascular disease (MIM: 608901), asthma (MIM: 600807), and (neuro)psychological disturbance such as depression (MIM: 608516) or intellectual disability, among others.2 While the causes underlying the multiple co-occurrences of obesity are likely complex and diverse, common mechanisms underlying these comorbidities, which are potential targets for preventive or therapeutic intervention, are largely unknown.
One of the possible genetic mechanisms of comorbidity can be through rare copy number variants (CNVs), which are more prevalent in people with some severe forms of obesity3,4 and might confer at least part of the increased risk for obesity via developmental delay.5 Most of these findings have been described in pediatric obesity.6,7
Genomic inversions, copy-neutral changes in the orientation of chromosomal segments with respect to the reference, are (also) excellent candidates for being important contributors to the genetic architecture of common diseases. Inversion polymorphisms can alter the function of the including and neighboring genes by multiple mechanisms, disrupting genes, separating their regulatory elements, affecting chromatin structure, and maintaining a strong linkage of functional variants within an interval that escape recombination. Therefore, by putatively affecting multiple genes in numerous ways, inversions are important sources of shared genomic variation underlying different human diseases and traits. Consequently, human inversions show genetic influences in multiple phenotypes. For instance, the common inversion at 8p23.1 has been independently linked to obesity,8 autism (MIM: 209850),9 neuroticism (MIM: 607834),10 and several risk behavior traits,11 while inversion at 17q21.31 has been associated with Alzheimer (MIM: 607822)12 and Parkinson (MIM: 168600)13 diseases, heart failure,14 and intracranial volume.15 We previously reported a ∼40% of population attributable risk for the co-occurrence of asthma and obesity given by a common inversion polymorphism at 16p11.2.16 In addition, transcriptional effects have been documented in several tissues for inversions at 17q21.3113,17 and 16p11.2.16
It is estimated that each human genome contains about 156 inversions.18 Therefore, inversions constitute a substantial source of genetic variability. Many of those polymorphic inversions show signatures of positive or balancing selection associated with functional effects.19 However, the overall impact of polymorphic inversions on human health remains largely unknown because they are difficult to genotype in large cohorts. We overcame this limitation by recently reporting a subset of 20 inversions that can be genotyped with SNP array data as they are old in origin, low or not recurrent, and frequent in the population.20 We have also included an additional inversion in our catalog, 16p11.2, previously validated and genotyped in diverse populations.16 Three of the inversions are submicroscopic (0.45–4 Mb), flanked by large segmental duplications, and contain multiple genes. Five are small (0.7–5 kb) and intragenic, and 13 are intergenic of variable size (0.7–90 kb) but highly enriched in pleiotropic genomic regions.21 While this is clearly not a comprehensive set of inversions, it is probably the largest set that can be genotyped in publicly available datasets.
In this manuscript, we aimed to study the association of 21 common polymorphic inversions in Europeans with highly prevalent co-morbid disorders and related traits. We particularly aimed to decipher the role of inversions in known epidemiological co-occurrences with obesity such as diabetes, hypertension (MIM: 145500), asthma, and mental diseases like depression, bipolar disorder (MIM: 125480), or neuroticism. For significant associations, we investigated whether causal pathways could be established and the most likely underlying mechanisms.
Material and Methods
Discovery Dataset
The UK Biobank (UKB) is a population-based cohort involving 500,000 individuals aged between 37 and 73 years, recruited across UK in the period 2006–2010. Further details on the quality control and genotyping are described in the study design.22 Phenotypic information is recorded via questionnaires and interviews (e.g., demographics and health status) and SNP genotypes were generated from the Affymetrix Axiom UK Biobank and UKBiLEVE arrays. We based our study on 408,898 individuals from European descent and from whom inversion genotypes were called using SNP array data. Principal components computed by the UK Biobank (data-field 22009) were used in the analyses to control for population stratification.
Replication Datasets
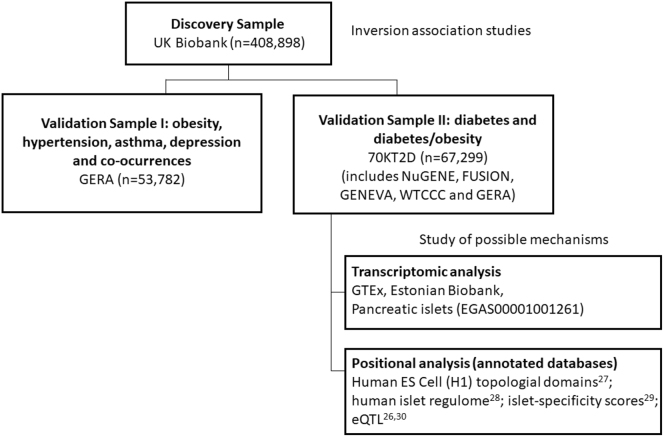
Different public datasets with access grant to the co-authors were used to attempt to replicate our positive findings in the association studies (Figure 1). The next sections describe these resources.
Figure 1.
Discovery and Validation Datasets
The flow chart shows the discovery sample and the validation datasets as well as the datasets used for post-genomic data analyses. Sample size (n) used from each dataset after performing quality control are also shown.
Genetic Epidemiology Research on Aging (GERA)
The GERA cohort (dbGaP: phs000674.v1.p1) consists of more than 100,000 adults from the Northern California Region (USA). Only individuals with reported race (variable phv00196837.v2.p2) equal to white were selected for the analyses (n = 56,638). The resulting studied cohort is 40% male, 60% female, and ranges in age from 18 to more than 100 years old, with an average age of 64 years at the time of the survey (2007). Individuals were genotyped with Affymetrix Axiom_KP_UCSF_EUR. After quality control of the inversion genotyping calling process, a total of 53,782 individuals with information about sex, age, principal components for genetic ancestry, and several diseases including obesity (9,439 cases), diabetes (6,529 cases), hypertension (27,009 cases), asthma (8,716 cases), and depression (6,924 cases) were used in the replication studies.
70KforT2D: Diabetes and Obesity
The 70KforT2D study (70KT2D)23 includes five datasets, two publicly available in EGA (NuGENE and GENEVA) and three availabe in dbGAP (FUSION, WTCCC, and GERA). Notice that 70KforT2D includes case subjects diagnosed with diabetes and obesity from the GERA cohort. We used information about being diabetic or not as described elsewhere.23 The five datasets were used to attempt to replicate the significant findings in the UK Biobank data on diabetes. The WTCCC dataset was removed from the obesity and obesity/diabetes analysis since we did not have access to body mass index (BMI) information for that study. The GERA dataset was split in two (GERA1 and GERA2) to speed up the imputation and inversion calling procedure since it is a large dataset. After performing QC on inversion genotypes, a total of 67,299 individuals were used in the replication step (54,801 control subjects and 12,498 diabetic subject). Data were accessed from the portal cg.bsc.es/70kfort2d.
The obesity variable was created using the body mass index (BMI) variable. We considered control individuals those having BMI between 18.5 and 24.9 and obese people those having BMI > 30.0. For obesity associations, we excluded individuals with diabetes. As a result, a total of 34,316 individuals (23,818 control subjects and 10,498 obese subjects) were used for that purpose. The co-occurrence of obesity and diabetes was studied by comparing individuals with no obesity and no diabetes as the reference category with individuals being obese and diabetic simultaneously. This ended up with a total of 23,818 control and 5,715 obese/diabetic individuals. Next, we further describe the studies included in the 70KT2D dataset along with their accession numbers.
Northwestern NUgene Project: Type 2 Diabetes (NUGENE) (dbGaP: phs000237.v1.p1) contains data from individuals from the Northwestern University Medical Center (USA). For this study, T2D case subjects were included if they had been diagnosed with type 2 diabetes, they took drugs to treat type 2 diabetes, or they presented abnormal diabetes-related blood measures. Control subjects were included if they had not been diagnosed with type 2 diabetes, they did not take drugs to treat type 2 diabetes, they presented normal diabetes-related blood measures, and they did not have any family history of diabetes (either type 1 or type 2). In both groups, subjects with type 1 diabetes were excluded. These individuals were genotyped with Illumina Human1M-Duov3_B.
The Finland-United States Investigation of NIDDM Genetics - GWAS Study (FUSION) (dbGaP: phs000100.v4.p1) aims to investigate the association between genetics and type 2 diabetes in Finish families. For this study, case subjects were included if they had been diagnosed with type 2 diabetes, they took drugs to treat type 2 diabetes, or they presented abnormal diabetes-related blood measures. Control subjects were included if they presented normal diabetes-related blood measures and were frequency matched to the case subjects by age, sex, and birth province. In both groups, individuals with family history of type 1 diabetes were excluded. These individuals were genotyped with Illumina HumanHap300v1.1.
GENEVA Genes and Environment Initiatives in Type 2 Diabetes (Nurses’ Health Study/Health Professionals Follow-up Study) (dbGaP: phs000091.v2.p1) is a nested case-control (2,720 case subjects and 3,180 control subjects) study from two USA female cohorts: the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) with a mean age of 57 ranging from 40 to 78. These individuals were genotyped with Affymetrix AFFY_6.0.
Geographical Variation in Europe
POPRES project (dbGaP: phs000145.v4.p2, access granted to the authors) was used to estimate inversion frequencies in European countries and regions. This project aimed to facilitate exploratory genetic research by assembling a DNA resource from a large number of subjects participating in multiple studies throughout the world. We selected European individuals (variable phv00173964.v2.p2) leading a total of 3,071 samples. A geographic label (North, Center, South) was assigned to each individual using information of variable phv00066613.v2.p2.
Transcriptomic Analyses
GTEx Analysis
We associated the 21 chromosomal inversions to changes in gene expression in GTEx project. We determined inversion genotypes on the GTEx v7 genotype calls from dbGAP (dbGaP: phs000424.v7.p2, accession granted to the authors). We included only samples classified as European with a confidence higher than 90% by peddy.24 Inv3_003 was discarded as the calling was not confident. Gene expression counts from RNA-seq data were downloaded using recount2.25 We computed the association between gene expression and inversions using voom26 and limma.27 The linear model included the inversion coded as additive (0: NN, 1: NI, 2: II) and the same covariates than GTEx (first three genome-wide PCA components, sex, and covariates from PEER). In each tissue, we selected those features having more than 10 counts in at least 10% of the samples. We corrected the association results per tissue for multiple comparisons by using a false discovery rate (FDR) adjusted p value per tissue.
EGCUT Biobank
Estonian Gene Expression Cohort was used to attempt to replicate positive transcriptomic results found in GTEx. The cohort is composed of 1,048 randomly selected samples (mean age 37 ± 16.6 years; 50% females) from the 53,000 samples in the Estonian Genome Center Biobank, University of Tartu. Whole-genome gene-expression levels from whole blood RNA were obtained by Illumina HT12v3 arrays according to manufacturer’s protocols. Low-quality samples were excluded. All probes with primer polymorphisms were discarded, leaving 34,282 probes. Raw gene expression data were Log-Quantile normalized using MixupMapper software. DNA was genotyped with Human370CNV array.
Pancreatic Islets
We analyzed the transcriptomic effect of inversions 8p23.1 and 16q11.2 on 118 pancreatic human islet samples using RNA-sequencing counts and high-density genotyping data.28 DNA genotype data (EGA: EGAS00001001261) was used to call inversion genotypes using scoreInvHap, then the association between gene expression and inversions was assessed using voom26 and limma.27 Only genes in the inversion regions were analyzed and un-corrected p values were reported as a measure of association.
Positional Analyses
For the positional analyses, several annotations were gathered from the following sources: TAD boundaries from the Human ES Cell (H1) topological domains;29 promoters, enhancers, CTCF-peaks, and ATAC-seq open-chromatin regions from the human islet regulome annotation;30 islet-specificity scores were calculated using the gene expression data from Miguel-Escalada et al.;31 and eQTL SNP-gene associations from van de Bunt et al.28 and Fadista et al.32 The chromatin landscape coverage percentage was calculated using a sliding window of 500 kb and 1 Mb for inversions 8p23.1 and 16p11.2, respectively, using steps of 1% of the window size, and calculating the percentage of covered nucleotides by significant signal in each of the categories. For the islet-specific expression analysis, we calculated the non-islet median expression level and difference between the 75 and 50 quartiles, and we considered as islet specific any gene that was expressed in islet >3 quartiles over the median of non-islet expression. Visualization was done in python3 using the matplotlib graphics library.
Statistical Methods
SNP Imputation and Inversion Calling
SNP microarray data were imputed with imputeInversion pipeline prior to inversion calling (see Web Resources). This pipeline was designed to impute only those SNPs inside the inversion region or closer than 500 kb to the inversion breakpoints. This step is recommended before performing inversion calling. imputeInversion uses shapeITv2.r904 to phase,33 Minimac334 to impute, and 1000 Genomes as reference haplotypes. Variants with an imputation R2 < 0.3 were discarded. Genotype probabilities were used to call inversions using scoreInvHap20 which is available at Bioconductor. scoreInvHap computes a similarity score between an individual’s alleles and the reference alleles in each chromosomal status. We used the development version of scoreInvHap, which includes references for 21 inversions. These methods were used to perform inversion calling in the discovery and replication studies as well as in individuals from POPRES.
Inversion Frequencies
Inversion frequencies were estimated in UKB and POPRES studies using SNPassoc package.35 A trend test implemented in the R function prop.trend.test was used to assess whether inversion frequencies in European regions from POPRES (North, Center, South) showed a significant cline. Principal component analysis was used to visualize inversion frequencies across European regions of POPRES dataset.
Obesity and Obesity Co-occurrence Traits
Obesity trait was created using body mass index (BMI) information. First, BMI was categorized in five categories using World Health Organization (WHO) classification which considers the following categories: underweight (BMI below 18.5), normal weight (BMI between 18.5 and 25), pre-obesity (BMI between 25 and 29.9), obesity class I (BMI between 30 and 34.9), and obesity classes II and III (BMI above 35). Obesity was considered as obesity classes I, II, and III and was compared with normal weight category. The analysis of obesity co-occurrence with diabetes, hypertension, asthma, depression, and neuroticism was performed by comparing individuals with normal weight and no presence of the disease with individuals being obese and having the disease of interest.
Inversion Association Analyses
Each inversion was independently associated with all the traits by using generalized linear models implemented in SNPassoc package.35 The models were adjusted for gender, age, and the first four principal components obtained from GWAS data in order to control for population genetic differences. The inversions were analyzed using an additive model. Multiple comparison problem was addressed by correcting for the total number of inversions and the phenotypes analyzed by considering the effective number of tests (18 independent tests) using Li and Ji method36 that accounts for correlation among traits. This ended up with a corrected p value equal to 0.00128.
Causal Inference
Mediation analysis using mediation R package37 was used to evaluate whether inversion 8p23.1 mediates the association between obesity and diabetes. Additive Bayesian network models using abn R package38 were used to determine optimal Bayesian network models to identify statistical dependencies between inversions 8p23.1, 16p11.2, and 11q13.2 and obesity, diabetes, and hypertension in the UKB dataset, and validated in the GERA cohort. The most probable network structure was estimated using exact order-based approach as implemented in the mostprobable function of abn package.
Data Availability
The data used in this work were obtained from publicly available datasets that are accessible through public repositories: UKB study, dbGaP, EGA, GTeX, and GEO. The inversion calling of UKB samples will be available through their platform. The inversion calling for the other samples and the complete transcriptomic summary statistics of the 21 inversions are available in our GitHub repository (see Web Resources).
Results
Frequency and Stratification of Inversions in European Populations
Using scoreInvHap, we first called the inversion status of individuals from the UK Biobank (UKB) with European ancestry (n = 408,898). We confirmed the previously reported frequency in the 1000 Genomes project of the 21 inversions analyzed in this work (Table 1). As inversion frequencies have a strong demographic effect, we also analyzed 12 European countries from the POPRES study (Figure S1). We observed significant clines along north-south latitude for several inversions (Table 1 and Figure S2A) as well as subtle ancestral differences (Figure S2B). Thus, population stratification was considered when performing association analyses as explained in the methods section.
Table 1.
Characteristics of the 21 Genomic Inversions
| Chr. Band | Coordinates | Num. SNPs | Length (Kb) | Inv. Freq.20 | UKB |
European Populations (POPRES) |
Trend p Value | ||
|---|---|---|---|---|---|---|---|---|---|
| North | Center | South | |||||||
| 1p22.1 | chr1:92,131,841-92,132,615 | 6 | 0.77 | 11.23 | 10.1 | 8.9∗ | 9.1∗ | 14.4∗ | 0.0057∗ |
| 1q31.3 | chr1:197,756,784-197,757,982 | 5 | 1.2 | 19.68 | 20.2 | 19.4 | 21.7 | 19.1 | 0.8781 |
| 2p22.3 | chr2:33,764,554-33,765,272 | 6 | 0.72 | 15.11 | 15.5 | 13.8 | 13.5 | 11.7 | 0.3199 |
| 2q22.1 | chr2:139,004,949-139,009,203 | 13 | 4.25 | 71.47 | 75.3 | 76.6∗ | 71.9∗ | 66.4∗ | 0.0003∗ |
| 3q26.1 | chr3:162,545,362-162,547,641 | 6 | 2.28 | 56.16 | 51.1 | 53.4∗ | 55.2∗ | 61.1∗ | 0.0140∗ |
| 6p21.33 | chr6:31,009,222-31,010,095 | 5 | 0.87 | 63.12 | 62 | 61.3∗ | 65.0∗ | 72.8∗ | 0.0001∗ |
| 6q23.1 | chr6:130,848,198-130,852,318 | 12 | 4.12 | 6.56 | 7.6 | 7.3 | 8.7 | 8.1 | 0.6070 |
| 7p14.3 | chr7:31,586,765-31,592,019 | 11 | 5.25 | 23.56 | 23.5 | 22.6 | 23.3 | 26.5 | 0.1605 |
| 7p11.2 | chr7:54,302,450-54,376,389 | 180 | 73.9 | 50.39 | 51 | 52.1 | 51.2 | 54.4 | 0.4715 |
| 7q11.22 | chr7:70,426,185-70,438,879 | 10 | 12.7 | 63.52 | 61.8 | 61.0 | 61.8 | 62.4 | 0.6196 |
| 7q36.1 | chr7:151,010,030-151,012,107 | 5 | 2.08 | 19.88 | 20.7 | 20.1 | 24.0 | 24.7 | 0.0775 |
| 8p23.1 | chr8:8,055,789-11,980,649 | 13,411 | 3,925 | 57.95 | 55.6 | 56.5 | 54.9 | 53.6 | 0.3424 |
| 11p12 | chr11:41,162,296-41,167,044 | 7 | 4.75 | 15.81 | 15.4 | 14.3 | 13.9 | 14.6 | 0.8479 |
| 11q13.2 | chr11:66,018,563-66,019,946 | 5 | 1.38 | 34.39 | 28.5 | 32.4 | 31.3 | 30.5 | 0.5287 |
| 12q13.11 | chr12:47,290,470-47,309,756 | 43 | 19.3 | 7.46 | 6.6 | 6.2∗ | 7.9∗ | 10.9∗ | 0.0085∗ |
| 12q21.2 | chr12:71,532,784-71,533,816 | 4 | 1.03 | 36.98 | 38.8 | 37.4 | 36.5 | 33.3 | 0.1647 |
| 14q23.3 | chr14:65,842,304-65,843,165 | 4 | 0.86 | 29.42 | 25.5 | 26.5 | 26.9 | 26.4 | 0.9823 |
| 16p11.2 | chr16:28,424,774-28,788,943 | 361 | 364.17 | ND | 40.5 | 39.3∗ | 32.0∗ | 29.1∗ | 0.0007∗ |
| 17q21.31 | chr17:43,661,775-44,372,665 | 3,637 | 711 | 23.96 | 22.6 | 15.1∗ | 19.4∗ | 22.1∗ | 0.0035∗ |
| 21q21.3 | chr21:28,020,653-28,021,711 | 11 | 1.06 | 51.29 | 49.2 | 51.6 | 52.1 | 57.4 | 0.0651 |
| Xq13.2 | chrX:72,215,927-72,306,774 | 135 | 90.8 | 13.3 | 13.9 | 12.4∗ | 12.1∗ | 8.5∗ | 0.0400∗ |
The table shows the coordinates, SNP content, size, and inversion frequency obtained from 1000 Genomes as described in Ruiz-Arenas et al.,20 the UKB and European regions (north, center and south) using the regions described in the POPRES dataset (see Material and Methods). The p value corresponds to a trend test to assess north-south linear association (asterisk indicates those significant at 5% level).
Inversions at 8p23.1, 16p11.2 Robustly Associate with Obesity and Obesity-Related Traits
The discovery phase of the study used data from UKB. We performed association analyses between the 21 inversions with obesity and co-morbid diseases and traits (see Material and Methods). These include obesity, diabetes, stroke, hypertension, asthma, chronic obstructive pulmonary disease (COPD), depression, and bipolar disorder, along with related traits or phenotypes classified as morphometric (4 traits), metabolic (5 traits), lipidic (2 traits), respiratory (3 traits), and behavioral (3 traits) (Figure 2). Table S1 shows the total number of case and control subjects used to perform the association analyses on each trait. The significant associations were further validated in the GERA independent dataset that contains information about several diseases. Positive results found in diabetes were validated in the 70KT2D dataset, which includes GERA among others (NUGENE, FUSION, GENEVA, and WTCCC) (see Material and Methods and Figure 1 which describes the comprehensive data analysis performed in the different datasets).
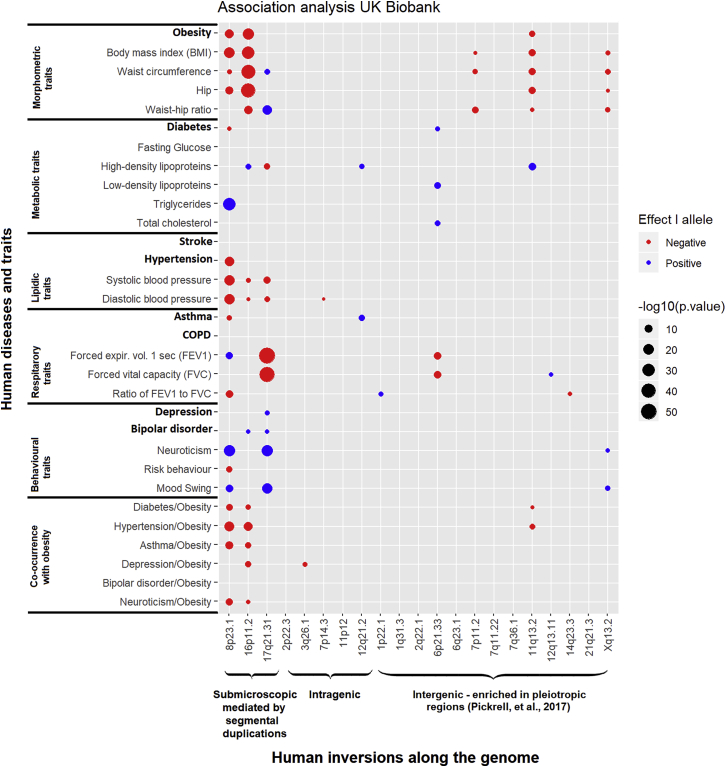
Figure 2.
Association Analyses between 21 Inversions and 8 Diseases (in Bold) and 17 Traits and the Co-occurrence of Obesity with 6 Other Complex Diseases
Circles represent the direction (color) and the two-tailed -log10 p value (size) of the association for different groups of traits (morphometric, metabolic, lipidic, respiratory, and behavioral) and the epidemiological well-established co-occurrence of obesity-related diseases. Inversions are grouped by size and features: (1) submicroscopic are large (0.4–4 Mb) encompassing multiple genes and flanked by segmental duplications; (2) intragenic are located within a gene, either intronic or containing one exon; and (3) intergenic are enriched in pleitropic regions.
The analyses on the UKB revealed several genetic influences of inversions on obesity and related common diseases (Figure 2). We observed a total of 74 significant associations after correcting for the number of inversions analyzed and the effective number of tests to consider the multiple analyzed traits (see Material and Methods). In general, we observed higher numbers of associations and stronger effects for the largest inversions at 8p23.1, 16p11.2, and 17q21.31, consistent with the fact that they encapsulate more genes. Some smaller inversions such as the ones at 11q13.2 and Xq13.2 also showed notable effects such as shared susceptibility and strength, respectively. We found a prominent inflation of association suggesting common genetic influences of the inversions across multiple phenotypes (Figure S3A). Some of the associations found have already been reported, such as those at inversion 8p23.1 with obesity8 and neuroticism10 and the one with inversion 16p11.2 with obesity.16
As a summary of the relevant findings, we observed that inversions at 8p23.1, 16p11.2, and 11q13.2 are all strongly associated with several obesity-related diseases (Figure 2). Remarkably, the non-inverted (N) allele of inversion 8p23.1 (i.e., the risk allele) is independently associated with diabetes (OR = 1.04, p = 1.1 × 10−3), hypertension (OR = 1.04, p = 7.0 × 10−16) and asthma (OR = 1.03, p = 7.0 × 10−5) (Table 2). The association with diabetes was replicated in the 70KT2D study (Figure 3A) (OR = 1.08, p = 1.1 × 10−8) as well as the association with obesity (OR = 1.08, p = 5.6 × 10−6) and the association with hypertension, which was validated in the GERA study (OR = 1.03, p = 0.0183) (Table 2). We also found a significant association between the non-inverted (N) allele of inversion 16p11.2 and obesity (OR = 1.05, p = 3.9 × 10−24) that was replicated in the GERA study (OR = 1.07, p = 1.4 × 10−4). The significant association found in the UKB for the inversion 11q13.2 was not validated in the GERA study (OR = 1.03, p = 0.0712). Consistently, the analysis of UKB study also revealed association of inversions at 8p23.1 and 16p11.2 with different obesity-related traits such as body mass index (BMI), waist circumference, high density lipoprotein (HDL), or systolic and diastolic blood pressure, among others (Figure 2).
Table 2.
Association between Inversions 8p23.1 and 16p1.2 and Different Obesity-Related Traits in UKB and Replication Datasets
| Disease |
Inversion 8p23.1 (Effect of Risk-Haplotype: N-Allele) |
Inversion 16p11.2 (Effect of Risk-Haplotype: N-Allele) |
||||||
|---|---|---|---|---|---|---|---|---|
|
UKB |
Replication |
UKB |
Replication |
|||||
| OR CI95% | p Value | OR CI95% | p Value | OR CI95% | p Value | OR CI95% | p Value | |
| Obesity | 1.04 (1.03–1.05) | 2.4 × 10−13 | 1.08 (1.04–1.11) | 5.6 × 10−6 | 1.05 (1.04–1.06) | 3.9 × 10−24 | 1.07 (1.03–1.10) | 1.4 × 10−4 |
| Diabetes | 1.04 (1.01–1.06) | 1.1 × 10−3 | 1.08 (1.05–1.11) | 1.1 × 10−8 | 1.02 (0.99–1.04) | 0.1450 | 1.07 (1.04–1.11) | 1.2 × 10−6 |
| Hypertension | 1.04 (1.03–1.05) | 7.0 × 10−16 | 1.03 (1.00–1.05) | 0.0183 | 1.01 (1.00–1.02) | 0.0184 | 1.02 (0.99–1.05) | 0.2127 |
| Asthma | 1.03 (1.01–1.04) | 7.0 × 10−5 | 1.02 (0.90–1.05) | 0.2225 | 1.00 (0.99–1.01) | 0.9529 | 1.00 (0.97–1.04) | 0.8074 |
| Depression | 0.98 (0.97–0.99) | 0.0119 | 1.01 (0.97–1.05) | 0.6630 | 0.98 (0.96–1.00) | 0.0184 | 1.01 (0.98–1.05) | 0.5384 |
| Joint Occurrence of Obesity with: | ||||||||
| Diabetes | 1.08 (1.05–1.11) | 3.1 × 10−7 | 1.17 (1.12–1.22) | 1.4 × 10−13 | 1.06 (1.03–1.08) | 7.5 × 10−5 | 1.13 (1.08–1.17) | 1.2 × 10−8 |
| Hypertension | 1.07 (1.05–1.08) | 1.7 × 10−16 | 1.06 (1.02–1.11) | 6.9 × 10−3 | 1.06 (1.05–1.07) | 2.7 × 10−14 | 1.05 (1.00–1.10) | 0.0357 |
| Asthma | 1.08 (1.06–1.10) | 3.0 × 10−11 | 1.09 (1.02–1.16) | 9.7 × 10−3 | 1.05 (1.03–1.07) | 7.4 × 10−6 | 1.08 (1.01–1.15) | 0.0287 |
| Depression | 1.04 (1.02–1.07) | 1.4 × 10−3 | 1.12 (1.04–1.20) | 3.8 × 10−3 | 1.06 (1.03–1.08) | 1.4 × 10−6 | 1.03 (0.95–1.11) | 0.5241 |
The table shows the odds ratios (OR) and their confidence intervals at 95% (CI95%) for the non-inverted allele and different diseases and the joint co-occurrence with obesity at UKB and replication datasets. The p corresponds to the best genetic model depict in the first column of each inversion.
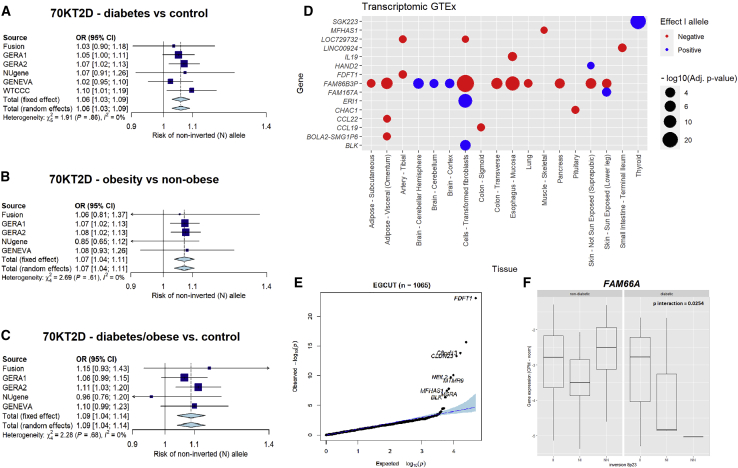
Figure 3.
Validation of Positive Associations between the Inversion 8p23.1 with Diabetes, Obesity, and Their Co-occurrence in the 70KT2D Dataset and Transcriptional Allelic Effects in Samples from EGCUT Biobank and GTEx Tissues
(A–C) 95% confidence intervals and meta-analysis of datasets belonging to 70KT2D for the association of inversion 8p23.1 with diabetes (A), obesity (B), and obese and diabetic individuals (C).
(D) Differential expressed genes at inversion genotypes (at 5% FDR) in different tissues from GTEx, showing effect of the I allele (color) and the two-tailed -log10 p value (size) of the association.
(E) Differentially expressed genes at inversion genotypes (at 5% FDR) in blood samples from EGCUT Biobank.
(F) FAM66A gene expression interaction between diabetic status and inversion 8p23.1 in pancreatic islets samples (p = 0.0254). The boxplots indicate the interquantile range and median of gene expression levels.
Some interesting associations in the discovery sample included those of inversion 17q21.31 with HDL, waist circumference, waist-hip ratio, and systolic and diastolic blood pressure (Figure 2). Interestingly, this inversion also showed a significant role in behavioral traits such as mood swing, depression, and bipolar disorder, which would need further validation. While we also found significant association of the inversion 6p21.33 with asthma (OR = 1.02, p = 0.0215) and different respiratory capacity traits (FEV1, p = 3.4 × 10−9 and FVC, p = 3.2 × 10−9) the association with asthma was not replicated in the GERA study. The inversion 7q11.2 was associated with different morphometric traits (BMI, waist circumference, and waist-to-hip ratio) and will require further validation studies.
Inversions at 8p23.1, 16p11.2, and 11q13.2 Are More Strongly Associated with the Co-occurrence of Diseases than with Single Diseases
Remarkably, the N-allele of the inversion 8p23.1 was significantly associated with the co-occurrence of obesity with diabetes (OR = 1.08, p = 3.1 × 10−7), hypertension (OR = 1.07, p = 1.7 × 10−16), or asthma (OR = 1.08, p = 3.0 × 10−11). These results were validated in the GERA and 70KT2D (Table 2). For obesity/diabetes, we observed an OR = 1.17 (p = 1.4 × 10−13) (Table 2 and Figure 3C) and none of the SNPs located within the inverted region were significantly associated at a genome-wide level (minimum p = 3.8 × 10−5) (Figure S4A). Finally, we also found a significant association of the N-allele of inversion 11q13.2 with the co-occurrence of obesity with diabetes (OR = 1.05, p = 0.0011) and hypertension (OR = 1.03, p = 2.9 × 10−5) (Figure 2), which was not validated in the GERA study.
The study of inversion 16p11.2 also revealed some new significant associations between the inversion and the co-occurrence of obesity with several diseases (Figure 2). The co-occurrence with diabetes at UKB (OR = 1.06, p = 7.5 × 10−5) was independently replicated in the 70KT2D study (OR = 1.13, p = 1.2 × 10−8), where none of the SNPs located within the inverted region were significantly associated at a genome-wide level (minimum p: 0.0214) (Figure S4B). In addition, the significant co-occurrence with hypertension observed in the UKB study (OR = 1.06, 2.7 × 10−14) was validated in the GERA study (OR = 1.05, p = 0.0357) further confirming the robustness of these findings (see Table 2 reporting the effect of the risk allele N).
In order to further illustrate that the association of the inversion is not driven by single variants, we downloaded data from the GWAS catalog and checked whether the GWAS signals for the analyzed traits are associated (i.e., tags) with the inversions. No tag-SNPs for any of these traits were found. In particular, the results for the three inversions associated with the co-occurrence of obesity with other traits showed the following results: the median R2 between SNPs in the 8p23.1 region and the inversion was 0.36 (IQR: 0.17–0.46), 0.71 (IQR: 0.62–0.89) for the inversion 16p11.2, and all the SNPs are not associated (i.e., linkage equilibrium) (R2 < 0.06) for the inversion 11q13.2.
Regulatory Region and Gene Disruption Are the Mechanisms Underlying the Effect of Inversions on Obesity and Diabetes
To investigate the possible mechanisms underlying the shared genetic influences of the inversions with obesity and its co-morbidities, we analyzed the transcriptional effects of the 21 inversions on different tissues from the GTEx project (see Material and Methods). As a result of these analyses, we found that inversion 8p23.1 modulated the transcription in brain, pancreas, and adipose tissue of the pseudogene FAM86B3P (HGNC: 44371), as well as the genes MFHAS1 (MIM: 605352), IL19 (MIM: 605687), HAND2 (MIM: 602407), FDFT1 (MIM: 184420), FAM167A (MIM: 610085), ERI1 (MIM: 608739), CHAC1 (MIM: 614587), CCL22 (MIM: 602957), CCL19 (MIM: CCL19), and BLK (MIM: 191305) in other tissues (Figure 3D). Genes FDFT1 (MIM: 184420), C8orf13 (MIM: 610085), CLDN23 (MIM: 609203), NEIL2 (MIM: 608933), MTMR9 (MIM: 606260), MSRA (MIM: 606260), and BLK (MIM: 191305) and were also differentially expressed in blood samples from the validation study we performed in the independent general population cohort belonging to EGCUT Biobank (Figure 3E). For the inversion 16p11.2, we found a total of 30 genes differentially expressed at 5% FDR level in blood, brain, pancreas, or adipose tissue including TUFM (MIM: 602389), SULT1A2 (MIM: 601292), SPNS1 (MIM: 612583), EIF3CL (MIM: 603916), and FOXO1 (MIM: 136533) among many others (Table S2). These results were also observed in the blood samples of the validation cohort from EGCUT Biobank (Figure S5). The genes affected by the other inversions and the different tissues can be found in Table S2.
Inversions 8p23.1 and 16p11.2 Affect Key Genes Associated with Diabetes in Pancreatic Islets
We conducted a more detailed analysis of gene expression on a relevant tissue to support the association on diabetic/obese individuals. We first genotyped the inversions and analyzed RNA sequencing in human pancreatic islets from 89 deceased donors (see Material and Methods). This revealed a significant association between inversion 8p23.1 and the expression levels of CLDN23 (p = 1.3 × 10−3) and ERI1 (p = 0.0356). We observed a nominally significant interaction of inversion 8p23.1 with obese/diabetic status associated with the expression of lncRNA FAM66A (HGNC: 30444) (p = 0.0254), where individuals carrying the risk allele for obesity and diabetes also present FAM66A downregulation (Figure 3F). In addition, results with inversion at 16p11.2 also revealed a significant interaction between the inversion and obese/diabetic status for the expression of NUPR1 (MIM: 614812) (p = 0.0116) and ATXN2L (MIM: 607931) (p = 0.0167) (Figure S6).
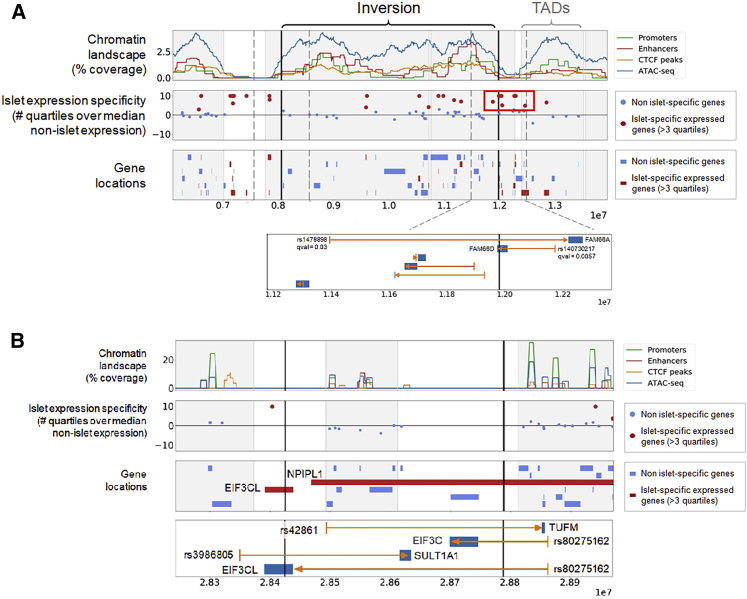
cis-Regulation is Disrupted by Breakpoints of Inversions 8p23.1 and 16p11.2
We also investigated whether the positional effects of the inversions could be associated with diabetes (see Material and Methods). Figure 4A shows the chromatin landscape of the region of the inversion 8p23.1 as well as the location of all genes having a significant alteration of expression, including those that are islet-specifically expressed. A cluster of islet-specific genes is located outside the rightmost boundary of the inversion but inside the inversion’s topologically associated domains (TADs). Therefore, it is likely that the regulatory regions of these genes lie across the inversion’s boundary, and thus their cis-regulatory SNPs being separated from their target genes by the right breakpoint of the inversion 8p23.1 in the case of genes FAM66A and FAM66D (HGNC: 24159) (Figure 4A). Similarly, the analysis of the inversion 16p11.2 also revealed four eQTLs in which the cis-regulatory SNPs were separated from their target genes by the inversion breakpoints: TUFM, SULT1A1 (MIM: 171150), EIF3C (MIM: 603916), and EIF3CL (Figure 4B). EIF3CL is disrupted by the inversion breakpoint providing a different mechanism of action for this gene (Figure 4B).
Figure 4.
Mechanisms Underlying the Inversion Association with Diabetes
(A) Islet-specific expression of inversion 8p23.1 genes. We observed a cluster of islet-specific genes, mainly lncRNAs, next to the distal inversion breakpoint that could be separated from regulatory elements located inside the inverted region. The bottom panel depicts an eQTLs (rs1478898) of FAM66A disrupted by the inversion distal breakpoint.28FAM66D has its gene body split in two by the inversion, and would also have its promoter separated from its eQLT SNP (rs140730217) by the inversion. This could be the most likely causal candidate.
(B) Same information for the inversion 16p11.2. TUFM and EIF3C have their lead eQTL SNP separated by the inversion breakpoint. There is no evidence in the centiSNP database39 for SNP rs42861 to be causal, suggesting that it should be in LD with the causal variant. This promoter region SNP is located in a segmental duplication block that is closer to TUFM in the inverted haplotypes. Therefore, positional changes made by the inversion can affect TUFM expression by separating the gene from regulatory sequences and subsequently increasing obesity risk.
Obesity Mediates the Association of Inversions with Diabetes and Hypertension
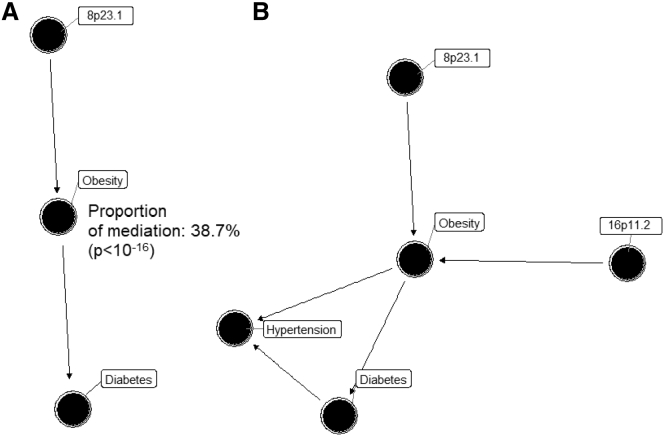
We first aimed to disentangle the shared genetic influence of the inversion 8p23.1 in obesity and diabetes. To this end, a Bayesian network analysis was performed on the discovery study (see Material and Methods). Based on the BIC, the most likely model was for the sequence inv8p23.1 -> obesity -> diabetes, suggesting a mediatory effect of obesity on the association between the inversion and diabetes (Figure 5A). The same network was obtained in the GERA cohort. This was consistent with mediation analyses showing that 38.7% (CI95%: 25.2%–59.0%) of the diabetes risk variance explained by the inversion 8p23.1 was mediated by obesity (p < 10−16). Then, we also investigated whether inversion 8p23.1, 16p11.2, and 11q13.2 act jointly or not on obesity, diabetes, and hypertension. The Bayesian network analysis including the three inversions in the model revealed that the inversions 8p23.1 and 16p11.2 independently associated with diabetes and hypertension being mediated by obesity (Figure 5B).
Figure 5.
Mediation Effect of Obesity in the Causal Link between Inversions and Diabetes and Hypertension
(A) Mediation analysis of obesity in the association between inversion 8p23.1 and diabetes, showing a proportion of the mediation of 38% (p value < 10e−6), which is the Best Bayesian Network when analyzing these three variables. Significant test for the proportion of the median showed a p value < 10−16.
(B) Best Bayesian Network based on AIC obtained after including obesity, hypertension, diabetes, and inversions 8p23.1, 16p11.2, and 11q13.2. Results are obtained from UKB data.
Discussion
Epidemiological studies largely support the co-occurrence of obesity with numerous traits and diseases such as diabetes, hypertension, asthma, and psychiatric disorders among others.40,41 The extent to which obesity is a cause, a consequence, or shares common causes with these traits is subject of intense research.42, 43, 44 Here, we show that at least two common polymorphic inversions at 8p23.1 and 16q11.2 offer a genetic substrate to some widely observed co-morbidities of obesity, such as those with diabetes, hypertension, asthma, and depression.
The analysis of UKB dataset validated the estimated inversion allele frequencies in European populations reported in our recent analyses.20 The observed differences of some inversion allele frequencies among major populations could explain part of the existing geographic variability in disease incidence.45 In particular, the reported cline of the inversion at 8p23.1 and 16p11.2 could capture a proportion of the observed North-South European differences in obesity,46 diabetes, and hypertension47 incidence.
The analysis of our discovery sample also confirmed previous reported associations of inversions with phenotypes, such as neuroticisms for the inversions 17q21.31 and 8p23.1,10 obesity for inversion 8p23.1,8 and the co-occurrence of asthma and obesity with the inversion 16p11.2.16 In addition, we discovered and robustly validated new associations of the inversion 8p23.1 with diabetes and hypertension as well as the co-occurrences of obesity with diabetes, hypertension, and asthma. These results suggest a relevant role of the inversion 8p23.1 in this metabolic syndrome.48
Our data suggest a causal path in which obesity mediates the observed association between inversions and several complex diseases. In particular, obesity mediates the independent effect of inversions at 8p23.1 and 16p11.2 on diabetes. Transcriptome analyses have revealed candidate genes to mediate this effect, such as BLK, involved in pancreatic β-cell insulin metabolism whose rare mutations are associated with young age of onset diabetes,49 or FDFT1, linked to C-reactive protein (CRP) and lipids levels50 and one of the strong candidates for obesity in gene expression networks derived from mouse intercrosses.51 A more specific analysis of transcriptome and eQTLs on pancreatic islets leads to another interesting gene: FAM66A. FAM66 is a multiple copy non-coding gene located in the flanking segmental duplications of the 8p23.1 inversion breakpoint highly expressed in brain and with low-level expression in pancreas. Diabetic individuals carrying the N-allele have lower gene expression, while no differential expression across inversion genotypes is observed in control individuals. Consistently, allele-specific expression analysis of this gene shows clear differences in expression in pancreatic cells of already symptomatic diabetic subjects. Remarkably, a copy-number gain variant including FAM66 gene has been associated with increased risk of diabetes.52 Our positional analyses also pointed out at FAM66D (8p23.1) as a candidate since the gene body was split in two by the inversion breakpoint.
We have also shown that inversion at 16p11.2 affects the joint effect of obesity with diabetes and hypertension and that this effect is independent of the effect found for inversion 8p23.1. Moreover, the odds ratios found for these associations are stronger than those observed when analyzing those diseases independently. The functional consequences of this inversion were previously reported to be mediated by deregulation of TUFM, SULT1A1, SULT1A2, SH2B1 (MIM: 608937), APOB48R (MIM: 605220), and EIF3C in blood.16 Position transcriptional analysis in pancreatic islets revealed that TUFM and EIF3C have their lead eQTL SNPs separated in the inverted allele. Remarkably, the eQTL SNP rs42861 of TUFM does not seem to be causal in the centiSNP database,39 suggesting that it is in linkage disequilibrium with the causal variant. This SNP is located in the promoter region that is closer to TUFM in the inverted haplotypes. This supports the hypothesis that the positional changes made by the inversion can affect TUFM gene expression and subsequently have an effect increasing the risk for obesity/diabetes. Positional analyses also pointed out EIF3CL, a gene also split in two by the inversion breakpoint, and with some isoforms preferentially expressed in human pancreatic islets.31
The inversions at 8p23.1 and 16p11.2 were also associated with the joint occurrence of obesity with behavioral traits, in particular with depression. These data further support our hypothesis that polymorphic inversions are strong candidates for the joint genetic susceptibility to co-occurring diseases by simultaneously affecting multiple genes. The observation that some SNPs located in both inversion regions are not or weakly associated with the analyzed traits, while inversion haplotypes are associated even at genome-wide significant level for GWAS with the strongest association found in people having more than one disease, also indicate that inversions are main contributors to the shared genetic susceptibility of co-occurring diseases. The fact that inverted alleles do not recombine preserving haplotypes in strong linkage disequilibrium highly suggest that the underlying evolutionary genetic event that has maintained or selected functional eQTLs in cis in these haplotypes is the inversion. Functional analyses in the appropriate tissue in case and control subjects, as the one we performed for obesity and diabetes, will shed light into the genes and mechanisms involved in behavioral or psychiatric traits.
Our hypothesis that inversions underlie the shared genetic susceptibility to common diseases is particularly supported by our findings in large inversions. These inversions encapsulate multiple genes and their associations with phenotypes were highly significant and could be replicated. Smaller inversions showed significant effects for numerous traits in the discovery study but only one result could be confirmed, namely the correlation of inversion at 11q13.2 with obesity and related traits and also with the co-occurrence of obesity, hypertension, and diabetes. Similarly, this study opens the door to further association studies of these and other inversions with traits and disorders not studied in this work. Additionally, the large number of significant genes associated with different tissues as well as the significant associations found for some traits also provides good candidate genes for some human diseases that are likely under the influence of inversions. These include, among others, autism, Alzheimer disease, and Parkinson disease.
In conclusion, we report the largest association study of genomic inversions and human traits that represents a breakthrough for genomic association of comorbid disorders, in which polymorphic inversions were often previously disregarded. Our results underscore the role of some inversions as major genetic contribution to the joint susceptibility to common diseases. The results in obesity and diabetes reveal a mechanism in which cis-regulatory SNPs are separated from their target genes by inversion breakpoints. Our findings set a new framework for future studies which are now accessible to the research community thanks to inversion genotyping tools such as our scoreInvHap method.20
Declaration of Interests
L.A.P.-J. is a founding partner and scientific advisor of qGenomics Laboratory. All other authors declare no conflict of interest.
Acknowledgments
This research has received funding from Ministerio de Ciencia, Innovación y Universidades (MICIU), Agencia Estatal de Investigación (AEI), and Fondo Europeo de Desarrollo Regional, UE (RTI2018-100789-B-I00) also through the “Centro de Excelencia Severo Ochoa 2019-2023” Program (CEX2018-000806-S); and the Catalan Government (SGR2017/801 and #016FI_B 00272 to C.R.-A.) through the CERCA Program. J.G. is funded by the European Commission (H2020-ERC-2014-CoG-647900) and the MINECO/AEI/FEDER, EU (BFU2017-82937-P). The L.A.P.-J. lab was funded by the Spanish Ministry of Science and Innovation (ISCIII-FEDER P13/02481), the Catalan Department of Economy and Knowledge (SGR2014/1468, SGR2017/1974, and ICREA Acadèmia), and also acknowledges support from the Spanish Ministry of Economy and Competiveness“Programa de Excelencia María de Maeztu” (MDM-2014-0370). This research was conducted using the UK Biobank Resource under Application Number 43983. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS.
Published: May 28, 2020
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2020.04.017.
Web Resources
European Genome-phenome Archive (EGA), https://www.ebi.ac.uk/ega
GWAS Catalog, https://www.ebi.ac.uk/gwas/
HUGO Gene Nomenclature Committee (HGNC), https://www.genenames.org/
imputeInversion, https://github.com/isglobal-brge/imputeinversion
Inversion Associations, https://github.com/isglobal-brge/inversion_analyses
OMIM, https://www.omim.org/
Supplemental Data
References
- 1.GBD 2015 Obesity Collaborators. Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., Lee A., Marczak L., Mokdad A.H., Moradi-Lakeh M. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon J.B. The effect of obesity on health outcomes. Mol. Cell. Endocrinol. 2010;316:104–108. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Locke A.E., Kahali B., Berndt S.I., Justice A.E., Pers T.H., Day F.R., Powell C., Vedantam S., Buchkovich M.L., Yang J., LifeLines Cohort Study. ADIPOGen Consortium. AGEN-BMI Working Group. CARDIOGRAMplusC4D Consortium. CKDGen Consortium. GLGC. ICBP. MAGIC Investigators. MuTHER Consortium. MIGen Consortium. PAGE Consortium. ReproGen Consortium. GENIE Consortium. International Endogene Consortium Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serra-Juhé C., Martos-Moreno G.Á., Bou de Pieri F., Flores R., González J.R., Rodríguez-Santiago B., Argente J., Pérez-Jurado L.A. Novel genes involved in severe early-onset obesity revealed by rare copy number and sequence variants. PLoS Genet. 2017;13:e1006657. doi: 10.1371/journal.pgen.1006657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaminsky E.B., Kaul V., Paschall J., Church D.M., Bunke B., Kunig D., Moreno-De-Luca D., Moreno-De-Luca A., Mulle J.G., Warren S.T. An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet. Med. 2011;13:777–784. doi: 10.1097/GIM.0b013e31822c79f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvanayagam T., Walker S., Gazzellone M.J., Kellam B., Cytrynbaum C., Stavropoulos D.J., Li P., Birken C.S., Hamilton J., Weksberg R., Scherer S.W. Genome-wide copy number variation analysis identifies novel candidate loci associated with pediatric obesity. Eur. J. Hum. Genet. 2018;26:1588–1596. doi: 10.1038/s41431-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vuillaume M.L., Naudion S., Banneau G., Diene G., Cartault A., Cailley D., Bouron J., Toutain J., Bourrouillou G., Vigouroux A. New candidate loci identified by array-CGH in a cohort of 100 children presenting with syndromic obesity. Am. J. Med. Genet. A. 2014;164A:1965–1975. doi: 10.1002/ajmg.a.36587. [DOI] [PubMed] [Google Scholar]
- 8.Cáceres A., González J.R. Following the footprints of polymorphic inversions on SNP data: from detection to association tests. Nucleic Acids Res. 2015;43:e53. doi: 10.1093/nar/gkv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutiérrez Arumi A. Univ. Pompeu Fabra; 2015. Ancestral genomic submicroscopic inversions of human genome and their relation with multifactorial human diseases. [Google Scholar]
- 10.Okbay A., Baselmans B.M., De Neve J.-E., Turley P., Nivard M.G., Fontana M.A., Meddens S.F., Linnér R.K., Rietveld C.A., Derringer J., LifeLines Cohort Study Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat. Genet. 2016;48:624–633. doi: 10.1038/ng.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson Linnér R., Biroli P., Kong E., Meddens S.F.W., Wedow R., Fontana M.A., Lebreton M., Tino S.P., Abdellaoui A., Hammerschlag A.R., 23and Me Research Team. eQTLgen Consortium. International Cannabis Consortium. Social Science Genetic Association Consortium Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat. Genet. 2019;51:245–257. doi: 10.1038/s41588-018-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laws S.M., Friedrich P., Diehl-Schmid J., Müller J., Eisele T., Bäuml J., Förstl H., Kurz A., Riemenschneider M. Fine mapping of the MAPT locus using quantitative trait analysis identifies possible causal variants in Alzheimer’s disease. Mol. Psychiatry. 2007;12:510–517. doi: 10.1038/sj.mp.4001935. [DOI] [PubMed] [Google Scholar]
- 13.Zabetian C.P., Hutter C.M., Factor S.A., Nutt J.G., Higgins D.S., Griffith A., Roberts J.W., Leis B.C., Kay D.M., Yearout D. Association analysis of MAPT H1 haplotype and subhaplotypes in Parkinson’s disease. Ann. Neurol. 2007;62:137–144. doi: 10.1002/ana.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilbrow A.P., Lewis K.A., Perrin M.H., Sweet W.E., Moravec C.S., Tang W.H.W., Huising M.O., Troughton R.W., Cameron V.A. Cardiac CRFR1 Expression Is Elevated in Human Heart Failure and Modulated by Genetic Variation and Alternative Splicing. Endocrinology. 2016;157:4865–4874. doi: 10.1210/en.2016-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikram M.A., Fornage M., Smith A.V., Seshadri S., Schmidt R., Debette S., Vrooman H.A., Sigurdsson S., Ropele S., Taal H.R., Early Growth Genetics Consortium. Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium Common variants at 6q22 and 17q21 are associated with intracranial volume. Nat. Genet. 2012;44:539–544. doi: 10.1038/ng.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González J.R., Cáceres A., Esko T., Cuscó I., Puig M., Esnaola M., Reina J., Siroux V., Bouzigon E., Nadif R. A common 16p11.2 inversion underlies the joint susceptibility to asthma and obesity. Am. J. Hum. Genet. 2014;94:361–372. doi: 10.1016/j.ajhg.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong S., Chepelev I., Janson E., Strengman E., van den Berg L.H., Veldink J.H., Ophoff R.A. Common inversion polymorphism at 17q21.31 affects expression of multiple genes in tissue-specific manner. BMC Genomics. 2012;13:458. doi: 10.1186/1471-2164-13-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaisson M.J.P., Sanders A.D., Zhao X., Malhotra A., Porubsky D., Rausch T., Gardner E.J., Rodriguez O.L., Guo L., Collins R.L. Multi-platform discovery of haplotype-resolved structural variation in human genomes. Nat. Commun. 2019;10:1784. doi: 10.1038/s41467-018-08148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giner-Delgado C., Villatoro S., Lerga-Jaso J., Gayà-Vidal M., Oliva M., Castellano D., Pantano L., Bitarello B.D., Izquierdo D., Noguera I. Evolutionary and functional impact of common polymorphic inversions in the human genome. Nat. Commun. 2019;10:4222. doi: 10.1038/s41467-019-12173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Arenas C., Cáceres A., López-Sánchez M., Tolosana I., Pérez-Jurado L., González J.R. scoreInvHap: Inversion genotyping for genome-wide association studies. PLoS Genet. 2019;15:e1008203. doi: 10.1371/journal.pgen.1008203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickrell J.K., Berisa T., Liu J.Z., Ségurel L., Tung J.Y., Hinds D.A. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 2016;48:709–717. doi: 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., Downey P., Elliott P., Green J., Landray M. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonàs-Guarch S., Guindo-Martínez M., Miguel-Escalada I., Grarup N., Sebastian D., Rodriguez-Fos E., Sánchez F., Planas-Fèlix M., Cortes-Sánchez P., González S. Re-analysis of public genetic data reveals a rare X-chromosomal variant associated with type 2 diabetes. Nat. Commun. 2018;9:321. doi: 10.1038/s41467-017-02380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen B.S., Quinlan A.R. Who’s Who? Detecting and Resolving Sample Anomalies in Human DNA Sequencing Studies with Peddy. Am. J. Hum. Genet. 2017;100:406–413. doi: 10.1016/j.ajhg.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collado-Torres L., Nellore A., Kammers K., Ellis S.E., Taub M.A., Hansen K.D., Jaffe A.E., Langmead B., Leek J.T. Reproducible RNA-seq analysis using recount2. Nat. Biotechnol. 2017;35:319–321. doi: 10.1038/nbt.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law C.W., Chen Y., Shi W., Smyth G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Bunt M., Manning Fox J.E., Dai X., Barrett A., Grey C., Li L., Bennett A.J., Johnson P.R., Rajotte R.V., Gaulton K.J. Transcript Expression Data from Human Islets Links Regulatory Signals from Genome-Wide Association Studies for Type 2 Diabetes and Glycemic Traits to Their Downstream Effectors. PLoS Genet. 2015;11:e1005694. doi: 10.1371/journal.pgen.1005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon J.R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., Hu M., Liu J.S., Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasquali L., Gaulton K.J., Rodríguez-Seguí S.A., Mularoni L., Miguel-Escalada I., Akerman İ., Tena J.J., Morán I., Gómez-Marín C., van de Bunt M. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat. Genet. 2014;46:136–143. doi: 10.1038/ng.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miguel-Escalada I., Bonàs-Guarch S., Cebola I., Ponsa-Cobas J., Mendieta-Esteban J., Atla G., Javierre B.M., Rolando D.M.Y., Farabella I., Morgan C.C. Human pancreatic islet three-dimensional chromatin architecture provides insights into the genetics of type 2 diabetes. Nat. Genet. 2019;51:1137–1148. doi: 10.1038/s41588-019-0457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fadista J., Vikman P., Laakso E.O., Mollet I.G., Esguerra J.L., Taneera J., Storm P., Osmark P., Ladenvall C., Prasad R.B. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc. Natl. Acad. Sci. USA. 2014;111:13924–13929. doi: 10.1073/pnas.1402665111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delaneau O., Zagury J.-F., Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat. Methods. 2013;10:5–6. doi: 10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- 34.Das S., Forer L., Schönherr S., Sidore C., Locke A.E., Kwong A., Vrieze S.I., Chew E.Y., Levy S., McGue M. Next-generation genotype imputation service and methods. Nat. Genet. 2016;48:1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González J.R., Armengol L., Solé X., Guinó E., Mercader J.M., Estivill X., Moreno V. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. 2007;23:644–645. doi: 10.1093/bioinformatics/btm025. [DOI] [PubMed] [Google Scholar]
- 36.Li J., Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- 37.Tingley D., Yamamoto T., Hirose K., Keele L., Imai K. mediation : R Package for Causal Mediation Analysis. J. Stat. Softw. 2014;59:1–38. [Google Scholar]
- 38.Lewis F.I., Ward M.P. Improving epidemiologic data analyses through multivariate regression modelling. Emerg. Themes Epidemiol. 2013;10:4. doi: 10.1186/1742-7622-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moyerbrailean G.A., Kalita C.A., Harvey C.T., Wen X., Luca F., Pique-Regi R. Which Genetics Variants in DNase-Seq Footprints Are More Likely to Alter Binding? PLoS Genet. 2016;12:e1005875. doi: 10.1371/journal.pgen.1005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banks J., Marmot M., Oldfield Z., Smith J.P. Disease and disadvantage in the United States and in England. JAMA. 2006;295:2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- 41.Stunkard A.J., Faith M.S., Allison K.C. Depression and obesity. Biol. Psychiatry. 2003;54:330–337. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- 42.Martins-Silva T., Vaz J.D.S., Hutz M.H., Salatino-Oliveira A., Genro J.P., Hartwig F.P., Moreira-Maia C.R., Rohde L.A., Borges M.C., Tovo-Rodrigues L. Assessing causality in the association between attention-deficit/hyperactivity disorder and obesity: a Mendelian randomization study. Int. J. Obes. 2019;43:2500–2508. doi: 10.1038/s41366-019-0346-8. [DOI] [PubMed] [Google Scholar]
- 43.Xu S., Gilliland F.D., Conti D.V. Elucidation of causal direction between asthma and obesity: a bi-directional Mendelian randomization study. Int. J. Epidemiol. 2019;48:899–907. doi: 10.1093/ije/dyz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Millard L.A.C., Davies N.M., Tilling K., Gaunt T.R., Davey Smith G. Searching for the causal effects of body mass index in over 300 000 participants in UK Biobank, using Mendelian randomization. PLoS Genet. 2019;15:e1007951. doi: 10.1371/journal.pgen.1007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puig M., Casillas S., Villatoro S., Cáceres M. Human inversions and their functional consequences. Brief. Funct. Genomics. 2015;14:369–379. doi: 10.1093/bfgp/elv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berghöfer A., Pischon T., Reinhold T., Apovian C.M., Sharma A.M., Willich S.N. Obesity prevalence from a European perspective: a systematic review. BMC Public Health. 2008;8:200. doi: 10.1186/1471-2458-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf-Maier K., Cooper R.S., Banegas J.R., Giampaoli S., Hense H.-W., Joffres M., Kastarinen M., Poulter N., Primatesta P., Rodríguez-Artalejo F. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 48.Povel C.M., Boer J.M.A., Reiling E., Feskens E.J.M. Genetic variants and the metabolic syndrome: a systematic review. Obes. Rev. 2011;12:952–967. doi: 10.1111/j.1467-789X.2011.00907.x. [DOI] [PubMed] [Google Scholar]
- 49.Borowiec M., Liew C.W., Thompson R., Boonyasrisawat W., Hu J., Mlynarski W.M., El Khattabi I., Kim S.-H., Marselli L., Rich S.S. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proc. Natl. Acad. Sci. USA. 2009;106:14460–14465. doi: 10.1073/pnas.0906474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ligthart S., Vaez A., Hsu Y.-H., Stolk R., Uitterlinden A.G., Hofman A., Alizadeh B.Z., Franco O.H., Dehghan A., Inflammation Working Group of the CHARGE Consortium. PMI-WG-XCP. LifeLines Cohort Study Bivariate genome-wide association study identifies novel pleiotropic loci for lipids and inflammation. BMC Genomics. 2016;17:443. doi: 10.1186/s12864-016-2712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Logsdon B.A., Hoffman G.E., Mezey J.G. Mouse obesity network reconstruction with a variational Bayes algorithm to employ aggressive false positive control. BMC Bioinformatics. 2012;13:53. doi: 10.1186/1471-2105-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailey J.N.C., Lu L., Chou J.W., Xu J., McWilliams D.R., Howard T.D., Freedman B.I., Bowden D.W., Langefeld C.D., Palmer N.D. The Role of Copy Number Variation in African Americans with Type 2 Diabetes-Associated End Stage Renal Disease. J. Mol. Genet. Med. 2013;7:61. doi: 10.4172/1747-0862.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this work were obtained from publicly available datasets that are accessible through public repositories: UKB study, dbGaP, EGA, GTeX, and GEO. The inversion calling of UKB samples will be available through their platform. The inversion calling for the other samples and the complete transcriptomic summary statistics of the 21 inversions are available in our GitHub repository (see Web Resources).