Abstract
Naturally occurring flavonoids, such as acacetin and pinostrobin, disrupt a wide range of processes during tumor progression, such as cell proliferation, apoptosis, and angiogenesis. Although the antiproliferative and antiapoptotic effects of acacetin and pinostrobin have been studied using various cell lines, relatively little is known about the effects of acacetin and pinostrobin on cancer cell migration and metastasis. For instance, it is unclear whether acacetin or pinostrobin have any effect on breast cancer cell migration or adhesion. In this study, we assessed the effects of acacetin and pinostrobin on malignant MDA-MB-231 and T47D breast epithelial cells and non-tumorigenic MCF10A breast epithelial cells. Our results demonstrate that both acacetin and pinostrobin selectively inhibit the migration of both MDA-MB-231 and T47D cells in a dose-dependent manner while exhibiting blunted effects on MCF10A cells. Interestingly, neither compound had an effect on cell proliferation in any of the 3 cell lines. Furthermore, both acacetin and pinostrobin inhibit MDA-MB-231 and T47D cell adhesion, cell spreading, and focal adhesion formation, but have no significant effect on MCF10A cells. Collectively, these results suggest that both acacetin and pinostrobin selectively inhibit malignant breast epithelial cell migration through attenuation of cell adhesion and focal adhesion formation. These findings indicate that both acacetin and pinostrobin may serve as potential therapeutic options to target breast tumor cell migration during late-stage tumor progression.
Keywords: acacetin, pinostrobin, flavonoids, migration, adhesion, focal adhesion
Introduction
Breast cancer is the most prevalent and deadliest cancer among women, with ~2.1 million women worldwide being afflicted each year.1 Approximately 1 in 8 women in the United States will develop breast cancer in their lifetime.2 Breast cancer is so deadly, in part, because of late-stage tumor metastasis, a process characterized by migration of cancer cells from the primary tumor to other areas of the body where they invade and proliferate, thereby impairing the function of vital organs. Cancer cell metastasis accounts for approximately 90% of all cancer-related deaths.3 While mechanisms of cell motility have been extensively studied, current approaches used to treat invasive breast cancer remain largely ineffective, thereby highlighting the need for treatments targeting breast cancer cell motility.
Cell motility is facilitated by a series of processes involving changes in cytoskeletal dynamics and cell-substratum adhesive interactions.4,5 Cell-substratum adhesive interactions are dependent on the expression of integrins, which are transmembrane receptor proteins chiefly involved in chemical- and mechanical-sensing and forming adhesive linkages to the extracellular matrix (ECM).4,6 These linkages, known as focal adhesions, are composed of a vast array of signaling and scaffolding proteins such as FAK, talin, and vinculin, which act to promote downstream signal transduction for tumorigenic processes such as cell proliferation and cell motility.6-8 Previous studies have shown that disrupting focal adhesions through reductions in scaffold-protein signaling can attenuate cell migration and tumor progression.9,10 Therefore, disruption of focal adhesion formation and subsequent downstream signaling may prove an effective therapeutic action against tumor metastasis.
For the past 30 years, researchers have demonstrated that natural compounds are a viable source for anticancer drugs. Even today, natural compounds continue to be one of the primary sources for drug development, and much of the world’s phytochemicals have yet to be discovered or investigated pharmacologically.11 Found in various fruits and vegetables, flavonoids are phenolic substances with a diverse array of biological activities such as signal transduction, stress tolerance, and protection against pathogens.12,13 Recent phytochemical inquiries have pointed to flavonoids as promising candidates for targeting tumorigenesis, angiogenesis, and metastasis.13-16 While current chemotherapies have proven toxic to malignant tissue, many are accompanied by a variety of side effects including toxicity to healthy tissues as well.17,18 Chemotherapy-induced toxicity to important organ systems is a major concern in cancer patients. Many natural compounds, including flavonoids, have displayed selective targeting of cancer cells with minimal toxicity to normal healthy tissues.19-22 Therefore, focus on flavonoids as alternative cancer treatments could prove useful in selectively targeting cancer cells while having limited effects on normal cells.
Flavonoids have been widely studied for their antioxidative and anti-inflammatory properties, and many have demonstrated anti-tumorigenic effects in breast cancer.16,23-25 Previous studies using the flavonoids acacetin (5,7-dihydroxy-4′-methoxyflavone; Figure 1A) and pinostrobin (5-hydroxy,7-methoxyflavanone; Figure 1B) have investigated their antiproliferative effects in breast cancer cells as well as in many other cancer cell types.26-35 Also, pinostrobin and acacetin have been shown to inhibit angiogenesis.36,37 Acacetin has been shown to inhibit invasion and migration in lung and prostate cancer cell lines.38,39 However, the effects of pinostrobin and acacetin on breast cancer cell migration and metastasis are virtually unknown.
Figure 1.
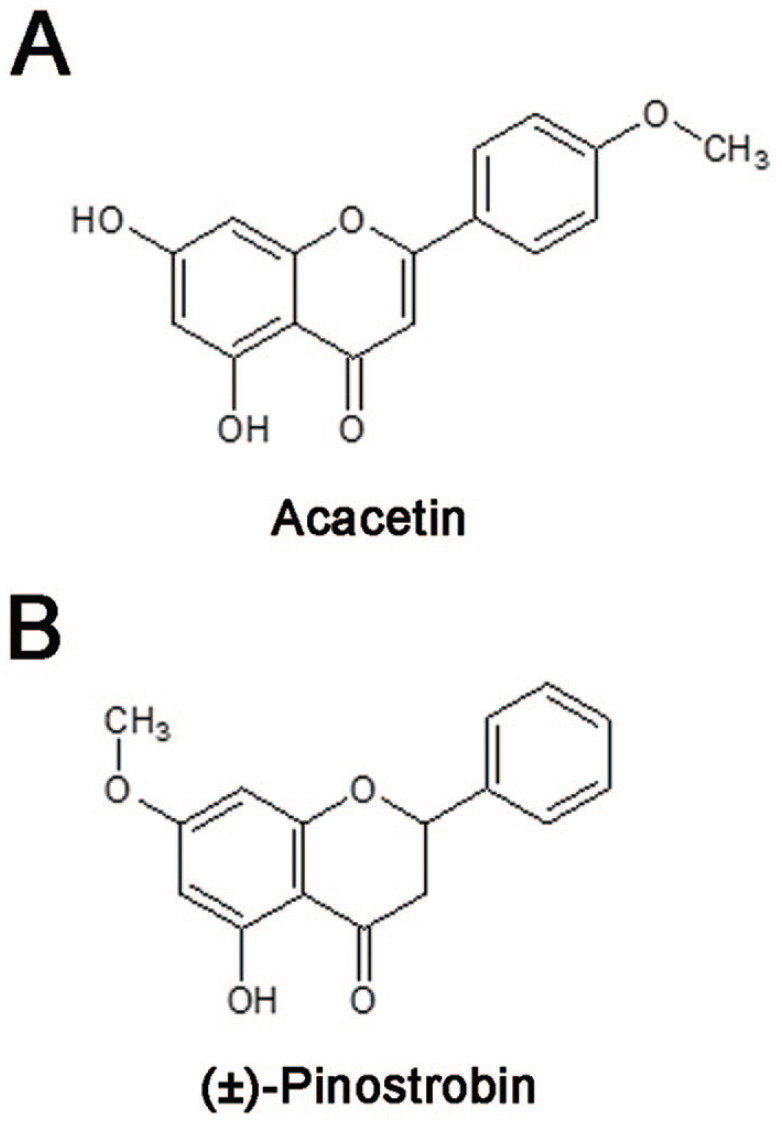
Chemical structure of acacetin (A) and (±)-pinostrobin (B).
In this study, we investigated the role of acacetin and pinostrobin on breast cancer cell adhesion and migration. Both compounds were assessed using MDA-MB-231 and T47D malignant breast epithelial cells. MDA-MB-231 cells are highly metastatic, basal-like cells that lack estrogen receptors (ERs), while T47D cells are luminal cells that are ER-positive. In addition, the effects of acacetin and pinostrobin were measured using MCF10A non-tumorigenic breast epithelial cells. Our findings demonstrate that both acacetin and pinostrobin inhibit MDA-MB-231 and T47D malignant breast epithelial cell migration but exhibit blunted effects on non-tumorigenic MCF10A breast epithelial cells. In addition, both acacetin and pinostrobin reduce cell adhesion, cell spreading, and focal adhesion formation in the malignant, but not the normal, breast cell lines. Interestingly, neither flavonoid have any demonstrable effects on proliferation of MDA-MB-231, T47D, or MCF10A cells. Notably, these cell lines have not been tested with these compounds in previous studies. These findings indicate that both acacetin and pinostrobin selectively target malignant breast epithelial cells through inhibition of cell adhesion and migration. Taken together, these observations have therapeutic considerations for acacetin and pinostrobin as potential compounds to target tumor metastasis during late-stage tumor progression.
Methods
Reagents
Rat tail collagen type I was obtained from BD Biosciences. Characterized fetal bovine serum (FBS) and penicillin-streptomycin were obtained from Fisher Scientific. All culture media was from Corning. Mouse antihuman vinculin monoclonal antibody and phalloidin-Tetramethylrhodamine B isothiocyanate were purchased from Sigma Aldrich. Alexa488 goat anti-mouse IgG was from Jackson ImmunoResearch Laboratories Inc. CellTiter 96 AQueous One Solution was obtained from Promega Corporation. Acacetin (5,7-dihydroxy-4′-methoxyflavone) and (±)pinostrobin (5-hydroxy,7-methoxyflavanone) were purchased from Sigma Aldrich.
Cell Lines and Cell Culture
MDA-MB-231 and T47D breast carcinoma cells, as well as MCF10A normal breast epithelial cells were generously donated by Dr Patricia J. Keely (University of Wisconsin–Madison). MDA-MB-231 cells were cultured in DMEM containing 10% FBS plus penicillin-streptomycin. T47D breast epithelial cells were maintained in RPMI containing 10% FBS and 8 µg/mL insulin. MCF10A cells were cultured in DMEM/F12 media supplemented with 5% horse serum, 20 ng/mL epidermal growth factor, 10 µg/mL insulin, and 0.5 µg/mL hydrocortisone. All cell lines were maintained at 37 °C/5% CO2 in air.
Cell Proliferation and Viability Assays
Ninety-six–well cell culture plates were coated with 100 µg/mL collagen 16 hours at 4 °C before seeding cells. Prior to seeding cells, all wells of the plates were blocked with 10 mg/mL fatty acid–free bovine serum albumin (FA-BSA) in phosphate-buffered saline (PBS) for 30 minutes at room temperature. 100 µL of cells (50 000 cells/mL) suspended in growth media (containing serum) were added to each well and incubated overnight at 37 °C/5% CO2 in air. After 24 hours, the growth media was removed and replaced with 100 µL of serum-free media containing either acacetin or pinostrobin. Following the 24-hour incubation in the presence of the compounds, 20 µL of CellTiter 96 AQueous One Solution Reagent (Promega) was added to each well, including media-only background controls. The plates were incubated for 2 hours (MDA-MB-231 and MCF10A cells) or 4 hours (T47D cells) at 37 °C/5% CO2 in air. Absorbance was measured at 490 nm using a VersaMax microplate reader (Molecular Devices). Corrected absorbance was determined by subtracting background absorbance from all experimental wells.
Scratch Motility Assays
Scratch assays were performed as previously described.5 Briefly, wells of a 12-well tissue culture plate (Corning) were coated with 100 µg/mL collagen for 16 hours at 4 °C. All wells were blocked with 10 mg/mL FA-BSA in PBS for 30 minutes at room temperature. Following PBS rinse, 800 µL of cells (MDA-MB-231, 190 000 cells/well; T47D, 400 000 cells/well; and MCF10A, 220 000 cells/well) suspended in growth media were added to wells. After 24 hours, cells were rinsed and serum-starved with assay media for 18 hours. Once cells reached confluency, a scratch was produced using a pipet tip. The cell monolayer was rinsed and fresh assay media containing different concentrations of either acacetin or pinostrobin was added to the cells. Cell migration proceeded for 16 to 24 hours at 37 °C/5% CO2 in air. Images of the scratches were captured using a 10× objective on an Olympus IX-51 inverted microscope equipped with a QImaging charged coupled device camera. Images were acquired using QImaging Q-Capture Pro. Cell migration (% area closure) was quantified by measuring the area of the cell-free region immediately following scratch formation and after 16 to 24 hours using ImageJ analysis software (https://imagej.nih.gov/ij).
Transwell Motility Assays
Transwell motility assays were performed as previously described.40 The underside of the transwell membrane (Costar 3422) was coated with 10 µg/mL collagen for 18 hours at 37 °C/5% CO2 in air. The bottom chamber of the transwell was rinsed with assay media, followed by the addition of 300 µL of assay media containing either dimethyl sulfoxide (DMSO) control, acacetin, or pinostrobin. Serum-starved cells were resuspended in assay media containing 5 µg/mL FA-BSA and pretreated with DMSO, acacetin, or pinostrobin for 30 minutes at 37 °C/5% CO2 in air prior to plating. Exactly 250 µL of cell suspension was added to the top chamber of the transwell, and cells were permitted to migrate for 24 hours at 37 °C/5% CO2 in air. After 24 hours, cells were fixed with 0.25% glutaraldehyde and then stained with 0.5% crystal violet. Cell motility was quantified by counting the number of cells per field from 5 random fields with a 20× objective using a Nikon E400 bright field microscope.
Adhesion Assays
Cell adhesion assays were performed as previously described.40 Briefly, 96-well cell culture plates were coated with 100 µg/mL collagen 16 hours at 4 °C before seeding cells. Following coating, wells were rinsed with PBS and then blocked using 10 mg/mL FA-BSA (in PBS) for 30 minutes at room temperature. Cells were detached using versene (0.5 mM EDTA [ethylenediaminetetraacetic acid] in Ca2+/Mg2+-free PBS), counted, and resuspended in serum-free media containing 5 mg/mL FA-BSA. Acacetin and pinostrobin were added to a cell suspension of 300 000 cells/mL, and 100 µL cells were added to each well. Cells were permitted to attach for 30 minutes at 37 °C/5% CO2 in air. Plates were gently washed with PBS to remove nonadherent cells, and then attached cells were fixed with 0.25% glutaraldehyde (in PBS) for 10 minutes at room temperature. Following PBS rinse, wells were incubated with 0.5% crystal violet for 30 minutes at room temperature. Wells were rinsed with distilled H2O and permitted to dry. One percent sodium dodecyl sulfate (SDS) (in PBS) was added to wells and allowed to incubate for 30 minutes at room temperature. Cell adhesion was quantified by measuring the absorbance at 590 nm using a VersaMax microplate reader (Molecular Devices). Corrected absorbance was established by subtracting the background absorbance from all experimental treatment wells.
Cell Area/Cell Shape Analysis
Glass coverslips (22 × 22) were acid washed and coated with 100 µg/mL collagen for 18 hours at 4 °C. Coverslips were rinsed with PBS and then plated with 500 µL serum-containing media comprising 20 000 cells (MDA-MB-231) or 30 000 cells (T47D and MCF10A). Cells were incubated with appropriate concentrations of acacetin or pinostrobin for 15 minutes at 37 °C/5% CO2 in air prior to plating onto coverslips. Cells were permitted to attach for 18 hours at 37 °C/5% CO2 in air. Cells were fixed with cold 4% paraformaldehyde for 10 minutes at room temperature. After PBS rinse, 0.1% (MDA-MB-231 and T47D) or 0.5% (MCF10A) TX-100 was added to coverslips and incubated for 10 minutes or 3 minutes, respectively. Following PBS rinse, cells were blocked with 10% FBS (in PBS) for 1 hour at room temperature. Once block was removed, 0.5 µM TRITC-phalloidin was added and coverslips were incubated for 45 minutes at room temperature. Coverslips were rinsed 3 times with PBS and then mounted with ProLong Antifade (Molecular Probes). Images were captured using a 100× objective on a Zeiss Axiovert fluorescent microscope fitted with an AxioCam MRm camera. Cell area and cell shape parameters were quantified using ImageJ. Cell circularity and aspect ratio were used to measure cell shape as previously described.5 Circularity was determined by (4π × cell area/cell perimeter2), while aspect ratio was determined in ImageJ by dividing the length of the major axis by the length of the minor axis (major axis/minor axis).
Immunofluorescence
Glass coverslips (22 × 22) were acid washed and coated with 100 µg/mL collagen for 18 hours at 4 °C. Following PBS rinse, 20 000 cells (MDA-MB-231) or 30 000 cells (T47D and MCF10A) suspended in serum-containing media were added to coverslips. Either DMSO, acacetin, or pinostrobin were added at this time and cells were permitted to incubate for 18 hours at 37 °C/5% CO2 in air. Cells were fixed with ice-cold 4% paraformaldehyde for 10 minutes at room temperature. After rinsing, cells were extracted with 0.1% TX-100 (MDA-MB-231 and T47D) or 0.5% TX-100 (MCF10A) for 10 minutes or 3 minutes, respectively. After PBS rinse, cells were blocked with 10% FBS in PBS for 1 hour at room temperature. Cells were incubated with 1:400 mouse anti-human vinculin antibody in 10% FBS in a humidified chamber overnight at 4 °C. Following PBS rinse, cells were incubated with 1:800 Alexa488 goat anti-mouse IgG plus 0.5 µM TRITC-phalloidin for 1 hour at room temperature. Following thorough rinsing, coverslips were mounted with ProLong Antifade. Images were analyzed using a 100× objective on a Zeiss Axiovert fluorescence microscope equipped with an AxioCam MRm camera. Images were captured using AxioVision 4.7 software.
Quantification of Vinculin Staining
Focal adhesions were quantified using 2 approaches as previously described.5 Briefly, the average total surface area containing vinculin for each cell was quantified using ImageJ. Binary images were created followed by thresholding of vinculin staining. The total surface area containing vinculin for each cell was measured using the analyze particles function. For relative vinculin area, the average total surface area containing vinculin for each cell was normalized to the total cell area as determined by TRITC-phalloidin.
Production of Digital Images
Digital images were processed and produced using ImageJ and Adobe Photoshop CS5 (Adobe Systems).
Results
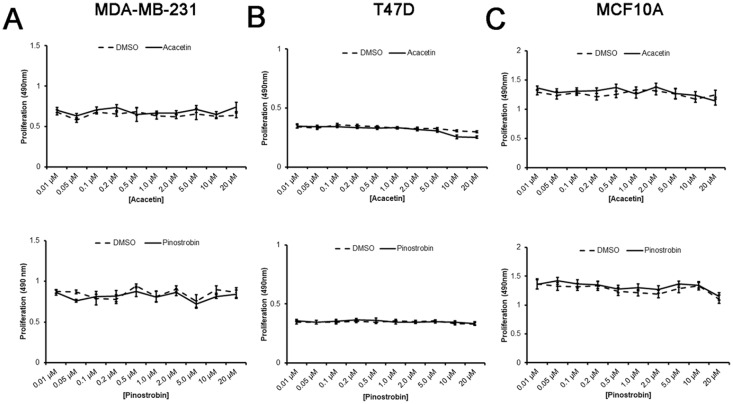
Acacetin and Pinostrobin Do Not Compromise Viability of Breast Epithelial Cells
Currently, no studies have assessed the effects of acacetin and pinostrobin on breast cancer cell motility and metastasis. Therefore, we tested cell migration in breast cancer cells treated with these compounds. In order to assess cell migration, sub-lethal concentrations of acacetin and pinostrobin were first determined for each cell line tested. The effects of both acacetin and pinostrobin on cell viability were assessed on 2 malignant breast epithelial cell lines; basal-like MDA-MB-231 ER-negative cells and luminal T47D ER-positive cells, and MCF10A non-tumorigenic breast epithelial cells. Interestingly, neither acacetin nor pinostrobin reduced cell viability in the malignant cells (Figure 2A and B). Compared with the DMSO vehicle control, cell viability was maintained over the 24-hour treatment period regardless of dosage in both cases. Similarly, MCF10A cells (Figure 2C) maintained viability in the presence of both drugs as well suggesting neither acacetin nor pinostrobin exhibit antiproliferative properties in malignant or normal breast epithelial tissue. In light of these findings, 5 µM, 10 µM, and 20 µM of both compounds were used to assess cell motility and adhesion.
Figure 2.
Both acacetin and pinostrobin have no effect on cell proliferation. MDA-MB-231 (A), T47D (B), and MCF10A cells (C) were cultured for 24 hours in the presence of either pinostrobin or acacetin. Cell proliferation was determined using CellTiter 96 AQueous One Solution Reagent and absorbance was measured at 490 nm. Data are presented as average absorbance ± SEM (standard error of mean) from a minimum of 8 wells. There were no statistically significant differences between DMSO control and treatments for all cell lines.
Acacetin and Pinostrobin Selectively Inhibit Breast Cancer Cell Motility in a Dose-Dependent Manner
We next investigated the antimetastatic potential of these compounds using both transwell and scratch migration assays. For the transwell assay, cells were cultured in collagen-coated transwells, then allowed to migrate for 24 hours in the presence of different concentrations of acacetin or pinostrobin (0, 5, 10, and 20 µM). Treatment with acacetin and pinostrobin inhibited the migration of both MDA-MB-231 and T47D cells in a dose-dependent manner (Figure 3). Treatment with 20 µM acacetin produced a 46% and 71% reduction in MDA-MB-231 and T47D cell migration, respectively (Figure 3A and C), while 20 µM pinostrobin inhibited MDA-MB-231 and T47D migration by 45% and 77%, respectively (Figure 3B and D). Interestingly, MCF10A cells remained relatively unaffected by both acacetin and pinostrobin with the exception of a 13% reduction in motility when treated with 20 µM acacetin (Figure 3C). These findings indicate that malignant breast epithelial cells are more sensitive to acacetin and pinostrobin treatment than MCF10A cells.
Figure 3.
Acacetin and pinostobin inhibit malignant breast epithelial cell migration. Example images of MDA-MB-231, T47D, and MCF10A cells (stained with crystal violet) in a transwell migration assay in response to increasing concentrations of acacetin (A) and pinostrobin (B). Scale bar = 100 µm. (C and D) 20 µM acacetin or pinostrobin inhibited MDA-MB-231 transwell migration by approximately 45% and 70%, while T47D transwell migration was reduced by approximately 46% and 77%, respectively. Only 20 µM acacetin produced a significant inhibition of 13% on non-tumorigenic MCF10A cells. Data in C and D represent the mean ± SEM (standard error of mean) from a minimum of 3 independent experiments performed in duplicate. *P < .05, **P < .01, ***P < .001 indicate statistical significance relative to DMSO control; 2-sample t test.
For the scratch assay, both acacetin and pinostrobin inhibited cell motility in a dose-dependent manner while exhibiting marginal effects on MCF10A cells (Figure 4). At 20 µM, acacetin produced a 40% and 34% reduction in MDA-MB-231 and T47D cell migration, respectively, while MCF10A cell migration was reduced by 20% (Figure 4A and C). Treatment with 20 µM pinostrobin inhibited MDA-MB-231 and T47D cell migration by 30% and 32%, respectively, while MCF10A cell migration was reduced by approximately 11% (Figure 4B and D). These results, along with the results obtained from the transwell assays, demonstrate that both flavonoids selectively inhibit the migration of malignant breast cells. In turn, MCF10A cells are less sensitive to both acacetin and pinostrobin and exhibit marginal inhibition at high concentrations of both compounds.
Figure 4.
Malignant breast epithelial cells are more sensitive, compared with non-tumorigenic cells, to the inhibitory effects of acacetin and pinostrobin on cell migration. (A and B) Example phase-contrast images of cells immediately following scratch formation (0 hour) and following migration for 24 hours (MDA-MB-231), 30 hours (T47D), or 18 hours (MCF10A) in the absence or presence of acacetin (A) or pinostrobin (B). Scale bar = 100 µm. (C and D) Both acacetin and pinostrobin produced a dose-dependent inhibition of malignant cell migration, while only 20 µM acacetin or pinostrobin produced a significant inhibition on MCF10A cells. Data in C and D are presented as mean ± SEM (standard error of mean) from a minimum of 4 independent experiments performed in triplicate. *P < .05, **P < .01, ***P < .001 indicate statistical significance relative to DMSO control; 2-sample t test.
Acacetin and Pinostrobin Selectively Inhibit Breast Cancer Cell Adhesion and Spreading in a Dose-Dependent Manner
Cancer cell invasion and metastasis are mediated by cell-ECM adhesive interactions that promote cytoskeleton organization, motive force generation, and survival.4 Therefore, we examined the effects of acacetin and pinostrobin treatment on cell adhesion (Figure 5). Similar to cell motility, acacetin and pinostrobin treatment inhibited malignant cell adhesion in a dose-dependent manner while exhibiting no significant effects on normal MCF10A cell adhesion. At 20 µM, acacetin inhibited MDA-MB-231 and T47D cell adhesion by 35% and 38%, respectively (Figure 5A), while 20 µM pinostrobin produced a 51% and 40% reduction in MDA-MB-231 and T47D cell adhesion, respectively (Figure 5B). However, there was no statistically significant effect of either compound on MCF10A cells (Figure 5). These findings suggest that the observed reduction in malignant breast cell motility (Figures 3 and 4) is attributed, in part, to a disruption in cell-ECM adhesion dynamics that is limited to MDA-MB-231 and T47D malignant cells.
Figure 5.

Acacetin and pinostrobin produced a dose-dependent inhibition of malignant cell adhesion. Both acacetin (A) and pinostrobin (B) selectively inhibited cell adhesion of MDA-MB-231 and T47D cells but had no measurable effect on MCF10A cells. The data are presented as mean ± SEM (standard error of mean) from a minimum of 3 independent experiments performed in quadruplicate. *P < .05, **P < .01, ***P < .001 indicate statistical significance relative to DMSO control; 2-sample t test.
Maximal cell spreading is an indication of increased integrin-mediated changes in cell adhesion and cytoskeleton reorganization which is a necessary component for cell motility.5 Since both acacetin and pinostrobin reduced the adhesion of malignant breast cells, we investigated whether these flavonoids decrease cell spreading. We determined the average cell area of acacetin- and pinostrobin-treated cells as a measure for cell spreading (Table 1). Both acacetin and pinostrobin significantly reduced MDA-MB-231 and T47D cell area at all tested dosages indicative of altered adhesion-dependent changes in cell morphology. For instance, treatment with 20 µM acacetin decreased MDA-MB-231 and T47D cell area by 30% and 22%, respectively. However, MCF10A cell area was reduced by only 5%. Neither compound influenced shape parameters in any of the tested cell lines despite observing reduced cell spreading in malignant breast epithelial cells (Table 1). Although various concentrations of pinostrobin had a statistically significant effect on cell circularity in MDA-MB-231 and MCF10A cells, this trend was not observed using aspect ratio. In light of these findings, the results indicate that acacetin and pinostrobin display a cell-selective effect on integrin-based cell spreading.
Table 1.
Cell Area and Shape Parameters Following Treatment With Acacetin or Pinostrobina.
| Treatment | Area | Circularity | Aspect ratio |
|---|---|---|---|
| MDA-MB-231 | |||
| Control | 869.27 ± 41.47 | 0.188 ± 0.011 | 2.41 ± 0.15 |
| 5 µM acacetin | 654.04 ± 25.56*** | 0.192 ± 0.011 | 2.39 ± 0.13 |
| 10 µM acacetin | 591.59 ± 21.33*** | 0.204 ± 0.010 | 2.59 ± 0.17 |
| 20 µM acacetin | 611.96 ± 27.56*** | 0.201 ± 0.010 | 2.44 ± 0.18 |
| 5 µM (±) pinostrobin | 660.67 ± 24.94*** | 0.232 ± 0.010* | 2.32 ± 0.15 |
| 10 µM (±) pinostrobin | 594.30 ± 24.33*** | 0.226 ± 0.009* | 2.34 ± 0.15 |
| 20 µM (±) pinostrobin | 597.76 ± 26.95*** | 0.211 ± 0.009 | 2.54 ± 0.16 |
| T47D | |||
| Control | 717.10 ± 39.33 | 0.364 ± 0.016 | 1.61 ± 0.06 |
| 5 µM acacetin | 529.86 ± 31.37*** | 0.344 ± 0.016 | 1.69 ± 0.06 |
| 10 µM acacetin | 573.72 ± 26.53*** | 0.344 ± 0.015 | 1.80 ± 0.13 |
| 20 µM acacetin | 562.29 ± 28.46*** | 0.335 ± 0.013 | 1.82 ± 0.10 |
| 5 µM (±) pinostrobin | 520.90 ± 21.71** | 0.365 ± 0.014 | 1.78 ± 0.08 |
| 10 µM (±) pinostrobin | 484.77 ± 28.46*** | 0.370 ± 0.014 | 1.80 ± 0.09 |
| 20 µM (±) pinostrobin | 481.05 ± 20.75*** | 0.387 ± 0.014 | 1.80 ± 0.09 |
| MCF-10A | |||
| Control | 837.93 ± 44.64 | 0.462 ± 0.011 | 1.78 ± 0.09 |
| 5 µM acacetin | 898.85 ± 52.39 | 0.458 ± 0.010 | 1.73 ± 0.08 |
| 10 µM acacetin | 792.85 ± 35.31 | 0.465 ± 0.011 | 1.69 ± 0.08 |
| 20 µM acacetin | 791.82 ± 45.17* | 0.459 ± 0.012 | 1.91 ± 0.10 |
| 5 µM (±) pinostrobin | 892.88 ± 44.25 | 0.463 ± 0.010 | 1.63 ± 0.07 |
| 10 µM (±) pinostrobin | 841.02 ± 48.16 | 0.500 ± 0.011* | 1.64 ± 0.06 |
| 20 µM (±) pinostrobin | 894.35 ± 49.46 | 0.498 ± 0.010* | 1.61 ± 0.06 |
Both acacetin and pinostrobin reduced cell area but had no substantial effect on cell shape parameters in malignant breast cells. Treatment with acacetin or pinostrobin reduced cell spreading, as measured by total cell area determined by TRITC-phalloidin, in MDA-MB-231, and T47D cells. 20 µM acacetin reduced cell area in MCF10A cells, but the level of inhibition was substantially less than in the other cell lines. Neither acacetin nor pinostrobin produced a consistent effect on cell shape parameters in any of the cell lines, as measured by circularity and aspect ratio. Data are presented as mean ± standard error of mean (mean ± SEM) from a minimum of 72 cells for each condition.
P < .05, **P < .01, and *** P < .001 represent statistical significance relative to DMSO control; 2-sample t test.
Acacetin and Pinostrobin Selectively Inhibit Focal Adhesion Formation in Malignant, but Not Normal, Breast Epithelial Cells
Facilitation of integrin-mediated signal transduction and subsequent cell locomotion are dependent on the recruitment of scaffolding proteins to form focal adhesion complexes at the intracellular domain of integrins.4,6,10 Consequently, inhibition in cell motility may occur through blockage of focal adhesion formation and its downstream effects. Since acacetin and pinostrobin inhibit malignant breast epithelial cell adhesion and spreading, we assessed whether these flavonoids have an effect on focal adhesion formation. The protein vinculin was used as a measure for focal adhesion formation. We found that both acacetin and pinostrobin reduced focal adhesion formation in a dose-dependent manner, as measured by average vinculin area, in both MDA-MB-231 and T47D cells while having no significant effect on MCF10A cells (Figure 6A-E). To rule out the possibility that reductions in vinculin area were due to differences in overall cell area, relative vinculin area was measured by normalizing the average vinculin area to the average cell area (measured using TRITC phalloidin) for each cell. Indeed, treatment with acacetin and pinostrobin produced a dose-dependent reduction in relative vinculin area in the malignant breast cells but not MCF10A cells (Figure 6F and G). Overall, both acacetin and pinostrobin reduced cell adhesion, cell spreading, and focal adhesion formation in a dose-dependent manner while exerting no significant effects on normal MCF10A cells. These findings indicate that acacetin- and pinostrobin-mediated inhibition of cell motility in malignant breast epithelial cells is likely attributed to changes in cell-ECM adhesion dynamics.
Figure 6.
Acacetin and pinostrobin reduced focal adhesion formation in malignant breast epithelial cells. (A-C) Representative fluorescence images of MDA-MB-231 (A), T47D (B), and MCF10A (C) cells treated with DMSO control, 20 µM acacetin, or pinostrobin. Indirect immunofluorescence of focal adhesions was assessed with a vinculin antibody and counterstained with TRITC-phalloidin. Scale bar = 10 µm. (D and E) Both acacetin and pinostrobin produced a dose-dependent reduction in average vinculin area in both MDA-MB-231 cells and T47D cells. Treatment with 20 µM acacetin reduced average vinculin area by 57% and 76% in MDA-MB-231 cells and T47D cells, respectively, while 20 µM pinostrobin decreased average vinculin area by 59% and 73% in MDA-MB-231 and T47D cells, respectively. Acacetin and pinostrobin produced no statistically significant effect in average vinculin area in MCF10A cells. (F and G) Relative vinculin area was examined by normalizing the average total surface area containing vinculin to the total cell area as assessed by TRITC-phalloidin. Data are presented as average ± SEM (standard error of mean) from a minimum of 72 cells for each condition. *P < .05, **P < .01, ***P < .001 indicate statistical significance relative to DMSO control; 2-sample t test.
Discussion
Cancer cell metastasis accounts for 90% of all cancer-related deaths.3 There has been a push in the field to identify new anticancer agents derived from natural compounds. Furthermore, identifying compounds with low toxicity that may be effective for prevention and treatment of cancer are needed. In fact, numerous studies have demonstrated that natural compounds can selectively target cancer cells with minimal toxicity to healthy tissues.19-22 Flavonoids, such as acacetin and pinostrobin, have been reported to target wide ranging mechanisms of tumor progression, such as cell proliferation and angiogenesis. Although the antiproliferative and pro-apoptotic effects of acacetin and pinostrobin have been studied using various cancer cells, little is known of the effects of acacetin and pinostrobin on cell migration and metastasis. Moreover, it is not known whether acacetin or pinostrobin exert inhibitory effects on malignant breast epithelial cell migration and adhesion.
In this study, we demonstrated that both acacetin and pinostrobin selectively inhibit MDA-MB-231 and T47D malignant breast epithelial cell migration and adhesion at sublethal concentrations in vitro. Studies have shown that acacetin exerts cytotoxic effects on various cell types, including prostate, hepatocellular carcinoma, lung, breast, and gastric cancer cells.27,31-35 Although pinostrobin has been shown to produce inhibitory effects on cervical, hepatocellular carcinoma, and leukemia cells, it has no inhibitory effect on MCF-7 breast epithelial cells.26,28-30 In this study, we used a maximum concentration of 20 µM to test acacetin and pinostrobin. Given the solubility limitations of both acacetin and pinostrobin in the cell media used for this study, higher concentrations were not tested. This may be due to the hydrophobic nature of phenolic compounds. Given that both flavonoids inhibit motility and adhesion in a dose-dependent manner, higher dosages would be expected to enhance their inhibitory effects on the tested malignant breast epithelial cells, likely at the expense of cell viability. For instance, Shim et al27 reported a reduction in proliferation for acacetin-treated MCF-7 breast cancer cells, albeit at concentrations that exceeded those tested in this study. Given the results of this study, it is possible that the effects of acacetin and pinostrobin on MCF10A cells would be more pronounced if treated with higher concentrations. Therefore, further investigation into the vehicle delivery of acacetin and pinostrobin is warranted. However, it is worth noting that concentrations below cytotoxic levels for both acacetin and pinostrobin effectively inhibited MDA-MB-231 and T47D malignant breast cell migration and adhesion through a reduction in focal adhesion formation.
The mechanisms by which flavonoids, in particular acacetin and pinostrobin, regulate breast epithelial cell migration and adhesion are not well understood. Cell migration is regulated by integrin-based adhesions that link the ECM to the underlying cytoskeleton. The strength of cell-substratum adhesions is dependent on many variables, including cell-substratum interactions, levels of integrins, integrin affinity, and integrin-cytoskeletal interactions.41-43 Furthermore, integrins relay signals from the ECM to influence cell migration and cell shape.4 Research has shown that decreasing the expression of integrins or the affinity of integrins for their respective ECM disrupts cell migration and adhesion.42,44 Findings from this study demonstrate that treatment with acacetin or pinostrobin decreased adhesion and cell area of MDA-MB-231 and T47D cells but had no effect on MCF10A cells (Figure 5 and Table 1). In addition, acacetin and pinostrobin reduced integrin-mediated focal adhesion formation of malignant breast epithelial cells with no measurable effect on non-tumorigenic MCF10A cells (Figure 6). These results suggest that the flavonoids acacetin and pinostrobin downregulate integrin signaling to modulate cell adhesion and focal adhesion formation, resulting in altered migration of malignant breast epithelial cells. In support of this notion, glabridin, another flavonoid, decreases integrin expression in MDA-MB-231 cells by increasing integrin degradation.16 It would be beneficial to examine the effects of acacetin and pinostrobin on integrin expression, activation, and signaling in order to better understand the attenuated breast cancer cell migration and adhesion in response to these flavonoids.
To our knowledge, this is the first study demonstrating the inhibitory effects of acacetin and pinostrobin on breast cancer cell adhesion and migration. RhoA/ROCK signaling contributes substantially to cell migration by triggering actin-myosin contractility, stress fiber formation, and membrane protrusion.45 Studies have demonstrated that the flavonoid glabridin inhibits the migration of breast and lung cancer cells through downregulation of integrins as well as inhibition of FAK and RhoA signaling.16,46 While we do not directly investigate the roles of FAK and RhoA in the present study, our results suggest that acacetin and pinostrobin disrupt focal adhesion formation, potentially through regulation of RhoA signaling. Both FAK and vinculin are recruited to integrin-activated focal adhesion complexes to promote cell migration.7 Given that acacetin and pinostrobin were shown to reduce vinculin-containing focal adhesions in malignant breast cells (Figure 6), it is possible that acacetin and pinostrobin may block focal adhesion formation by attenuating FAK and RhoA signaling in these cell lines, which could, in turn, inhibit cell motility. Acacetin has been shown to inhibit migration in other cancer types such as lung and prostate through p38 MAPK downregulation of MMP-2 and MMP-9.38,39 Other flavonoids have been shown to inhibit cell migration and MMP-2/9 expression in MDA-MB-231 cells by blocking MAPK or PI3K/AKT signaling.23,47,48 Further investigation is needed to determine whether acacetin and pinostrobin target these various mechanisms to regulate breast cancer cell motility and adhesion.
Traditional treatments for metastatic breast cancer utilize cytotoxic drugs often with limited success. However, adverse side effects, such as genotoxicity, can occur due to a lack of selectivity. Therefore, identifying additional approaches that have selective effects on breast cancer cells with limited cytotoxic effects on healthy cells is desired. Many natural compounds, including flavonoids, have displayed selective targeting of cancer cells with minimal toxicity to normal healthy tissues. For instance, the flavonoid quercetin and hibiscus flower extract selectively induce apoptosis in prostate and breast cancer cells, respectively.21,49 The flavonoids xanthohumol and α,β-dihydroxanthohumol, as well as analogues of allicin (found in garlic [Allium sativum]), selectively inhibit proliferation of breast cancer cells while having limited cytotoxic effects on non-tumorigenic cells.50,51 In the current study, the flavonoids acacetin and pinostrobin selectively inhibited cell adhesion and focal adhesion formation in malignant breast epithelial cell migration. Interestingly, neither acacetin nor pinostrobin demonstrated any effects on malignant or non-tumorigenic cell viability at the tested concentrations in this study. This suggests acacetin and pinostrobin may be effective in targeting breast cancer cell migration and metastasis with limited cytotoxic effects.
Conclusions
Many chemotherapy drugs have been discovered by investigating organic compounds derived from natural sources. As such, studying the effects of natural compounds on tumor progression may inform development of novel therapeutic strategies for prevention and treatment. Furthermore, natural compounds that have anti-tumorigenic effects may serve as a template for the synthesis of novel therapeutic drugs. Although the flavonoids acacetin and pinostrobin have been shown to inhibit proliferation and induce apoptosis in a variety of cancer cell types, their role in cancer cell migration and metastasis is not clear. In this study, we show that acacetin and pinostrobin selectively inhibit malignant breast cell motility in a dose-dependent manner. Notably, both flavonoids exert their effects on cell motility at noncytotoxic levels. Additionally, acacetin and pinostrobin produce a dose-dependent inhibition on cell adhesion, cell spreading, and focal adhesion formation that is selective for malignant breast cells. To our knowledge, this is the first study by which acacetin and pinostrobin have been shown to regulate breast cancer cell adhesion and motility. Together, these findings position both acacetin and pinostrobin as potential therapeutic agents for preventing and treating late-stage breast tumor progression through regulation of cell-ECM adhesive interactions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Augustana Faculty Research Grant (S.G.), Larry P. Jones Faculty Fellowship (S.G.) and Augustana Undergraduate Research Fellowship (A.A.J).
ORCID iD: Scott Gehler  https://orcid.org/0000-0002-5381-3967
https://orcid.org/0000-0002-5381-3967
References
- 1. World Health Organization. Breast cancer: early diagnosis and screening. Accessed May 23, 2019 https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/
- 2. DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52-62. [DOI] [PubMed] [Google Scholar]
- 3. Christofori G. New Signals from the invasive front. Nature. 2006;441:444-450. [DOI] [PubMed] [Google Scholar]
- 4. Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Center. 2002;2:91-100. [DOI] [PubMed] [Google Scholar]
- 5. Gehler S, Compere FV, Miller AM. Semaphorin 3A increases FAK phosphorylation at focal adhesions to modulate MDA-MB-231 cell migration and spreading on different substratum concentrations. Int J Breast Cancer. 2017;2017:9619734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21-33. [DOI] [PubMed] [Google Scholar]
- 7. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-687. [DOI] [PubMed] [Google Scholar]
- 8. Gehler S, Ponik SM, Riching KM, Keely PJ. Bi-directional signaling: extracellular matrix and integrin regulation of breast tumor progression. Crit Rev Eukaryot Gene Expr. 2013;23:139-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin TY, Hsu HY. Ling Zhi-8 reduces lung cancer mobility and metastasis through disruption of focal adhesion and induction of MDM2-mediated Slug degradation. Cancer Lett. 2016;375:340-348. [DOI] [PubMed] [Google Scholar]
- 10. Hensley PJ, Desiniotis A, Wang C, Stromberg A, Chen CS, Kyprianou N. Novel pharmacologic targeting of tight junctions and focal adhesions in prostate cancer cells. PLoS One. 2014;9:e86238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830:3670-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;2013:162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tian SS, Jiang FS, Zhang K, et al. Flavonoids from the leaves of Carya cathayensis Sarg. inhibit vascular endothelial growth factor-induced angiogenesis. Fitoterapia. 2014;92:34-40. [DOI] [PubMed] [Google Scholar]
- 15. Wang H, Khor TO, Shu L, et al. Plants against cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12:1281-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu YL, Wu LY, Hou MF, et al. Glabridin, an isoflavan from licorice root, inhibits migration, invasion and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. Mol Nutr Food Res. 2011;55:318-327. [DOI] [PubMed] [Google Scholar]
- 17. Livshits Z, Rao RB, Smith SW. An approach to chemotherapy-associated toxicity. Emerg Med Clin North Am. 2014;32:167-203. [DOI] [PubMed] [Google Scholar]
- 18. Yarana C, St Clair DK. Chemotherapy-induced tissue injury: an insight into the role of extracellular vesicles-mediated oxidative stress responses. Antioxidants (Basel). 2017;6:E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun B, Wang G, Liu H, et al. Oridonin inhibits aberrant AKT activation in breast cancer. Oncotarget. 2018;9:23878-23889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pignanelli C, Ma D, Noel M, et al. Selective targeting of cancer cells by oxidative vulnerabilities with novel curcumin analogs. Sci Rep. 2017;7:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aalinkeel R, Bindukumar B, Reynolds JL, et al. The dietary bioflavonoid, quercetin, selectively induces apoptosis of prostate cancer cells by down-regulating the expression of heat shock protein 90. Prostate. 2008;68:1773-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ranzato E, Martinotti S, Magnelli V, et al. Epigallocatechin-3-gallate induces mesothelioma cell death via H2O2-dependent T-type Ca2+ channel opening. J Cell Mol Med. 2012;16:2667-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou R, Xu L, Ye M, Liao M, Du H, Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm Metab Res. 2014;46:753-760. [DOI] [PubMed] [Google Scholar]
- 24. Singhal RL, Yeh YA, Prajda N, Olah E, Sledge GW, Jr, Weber G. Quercetin down-regulates signal transduction in human breast carcinoma cells. Biochem Biophys Res Commun. 1995;208:425-431. [DOI] [PubMed] [Google Scholar]
- 25. Ferry DR, Smith A, Malkhandi J. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996;2:659-668. [PubMed] [Google Scholar]
- 26. Le Bail JC, Aubourg L, Habrioux G. Effects of pinostrobin on estrogen metabolism and estrogen receptor transactivation. Cancer Lett. 2000;156:37-44. [DOI] [PubMed] [Google Scholar]
- 27. Shim HY, Park JH, Paik HD, Nah SY, Kim DS, Han YS. Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. Mol Cells. 2007;24:95-104. [PubMed] [Google Scholar]
- 28. Jaudan A, Sharma S, Malek SNA, Dixit A. Induction of apoptosis by pinostrobin in human cervical cancer cells: possible mechanism of action. PLoS One. 2018;13:e0191523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao XD, Ding ZS, Jiang FS, et al. Antitumor constituents from the leaves of Carya cathayensis. Nat Prod Res. 2012;26:2089-2094. [DOI] [PubMed] [Google Scholar]
- 30. Smolarz HD, Mendyk E, Bogucka-Kocka A, Kocki J. Pinostrobin—an anti-leukemic flavonoid from Polygonum lapathifolium L. ssp. nodosum (Pers.) Dans. Z Naturforsch C J Biosci. 2006;61:64-68. [DOI] [PubMed] [Google Scholar]
- 31. Hsu YL, Kuo PL, Lin CC. Acacetin inhibits the proliferation of Hep G2 by blocking cell cycle progression and inducing apoptosis. Biochem Pharmacol. 2004;67:823-829. [DOI] [PubMed] [Google Scholar]
- 32. Hsu YL, Kuo PL, Liu CF, Lin CC. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett. 2004;212:53-60. [DOI] [PubMed] [Google Scholar]
- 33. Singh RP, Agrawal P, Yim D, Agarwal C, Agarwal R. Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: structure-activity relationship with linarin and linarin acetate. Carcinogenesis. 2005;26:845-854. [DOI] [PubMed] [Google Scholar]
- 34. Kim HR, Park CG, Jung JY. Acacetin (5,7-dihydroxy-4′-methoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-κB/Akt signaling in prostate cancer cells. Int J Mol Med. 2014;33:317-324. [DOI] [PubMed] [Google Scholar]
- 35. Pan MH, Lai CS, Hsu PC, Wang YL. Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J Agric Food Chem. 2005;53:620-630. [DOI] [PubMed] [Google Scholar]
- 36. Siekmann TRL, Burgazli KM, Bobrich MA, Noll G, Erdogan A. The antiproliferative effect of pinostrobin on human umbilical vein endothelial cells (HUVEC). Eur Rev Med Pharmacol Sci. 2013;17:668-672. [PubMed] [Google Scholar]
- 37. Bhat TA, Nambiar D, Tailor D, Pal A, Agarwal R, Singh RP. Acacetin inhibits in vivo and in vitro angiogenesis and down-regulates Stat signaling and VEGF expression. Cancer Prev Res (Phila). 2013;6:1128-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chien ST, Lin SS, Wang CK, et al. Acacetin inhibits the invasion and migration of human non-small cell lung cancer A549 cells by suppressing the p38α MAPK signaling pathway. Mol Cell Biol. 2011;350:135-148. [DOI] [PubMed] [Google Scholar]
- 39. Shen KH, Hung SH, Yin LT, et al. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol Cell Biol. 2010;333:279-291. [DOI] [PubMed] [Google Scholar]
- 40. Keely PJ, Fong AM, Zutter MM, Santoro SA. Alteration of collagen-dependent adhesion, motility, and morphogenesis by the expression of antisense alpha 2 integrin mRNA in mammary cells. J Cell Sci. 1995;108(pt 2):595-607. [DOI] [PubMed] [Google Scholar]
- 41. Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3:a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537-540. [DOI] [PubMed] [Google Scholar]
- 43. Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361-1374. [DOI] [PubMed] [Google Scholar]
- 44. Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O’Connor K, Chen M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases. 2013;4:141-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsai YM, Yang CJ, Hsu YL, et al. Glabridin inhibits migration, invasion, and angiogenesis of human non-small cell lung cancer A549 cells by inhibiting the FAK/rho signaling pathway. Integr Cancer Ther. 2011;10:341-349. [DOI] [PubMed] [Google Scholar]
- 47. Wang L, Ling Y, Chen Y, et al. Flavonoid baicalein adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010;297:42-48. [DOI] [PubMed] [Google Scholar]
- 48. Chen P, Lu N, Ling Y, et al. Inhibitory effects of wogonin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Toxicology. 2011;282:122-128. [DOI] [PubMed] [Google Scholar]
- 49. Nguyen C, Baskaran K, Pupulin A, et al. Hibiscus flower extract selectively induces apoptosis in breast cancer cells and positively interacts with common chemotherapeutics. BMC Complement Altern Med. 2019;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bartmanska A, Tronina T, Poplonski J, Milczarek M, Filip-Psurska B, Wietrzyk J. Highly cancer selective antiproliferative activity of natural prenylated flavonoids. Molecules. 2018;23:E2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bhaumik I, Pal K, Debnath U, Karmakar P, Jana K, Misra AK. Natural product inspired allicin analogs as novel anti-cancer agents. Bioorg Chem. 2019;86:259-272. [DOI] [PubMed] [Google Scholar]