Abstract
The discovery of epidermal growth factor receptor (EGFR) somatic mutations and the availability of tyrosine kinase inhibitors (TKIs) as targeted therapies have altered the therapeutic prospects of advanced non-small-cell lung cancer (NSCLC). G719X and S768I are uncommon mutations, and they often exist as compound mutations. A few reports have described the efficacy of first- and second-generation EGFR-TKIs. However, the efficacy of osimertinib in patients with these uncommon compound mutations is unknown. In this study, we reported the postoperative outcome of a patient with NSCLC and uncommon compound EGFR G719X and S768I mutations. After postoperative recurrence, the patient was treated with osimertinib, and an excellent and long-lasting clinical response was achieved. The patient has taken osimertinib for 31.0 months and exhibited a partial response, and her follow-up is ongoing.
Keywords: Epidermal growth factor receptor, non-small-cell lung cancer, osimertinib, compound mutation, tyrosine kinase inhibitor, uncommon mutation
Introduction
The discovery of epidermal growth factor receptor (EGFR) activating mutations in non-small-cell lung cancer (NSCLC) and the success of tyrosine kinase inhibitors (TKIs) have changed the paradigm of cancer therapy from empirical cytotoxic chemotherapy to molecular targeted therapy. Excluding the L858R mutation and exon 19 deletion, other EGFR mutations are considered uncommon, accounting for approximately 4% to 13% of all mutations of this gene. These uncommon mutations include a heterogeneous group of molecular alterations within exons 18 to 21 (G719X, 5%; S768I, 1%; L861Q, 3%; T790M, 3%, and exon 20 insertion, 3%).1,2 G719X and L861Q are considered moderately sensitive to first-generation EGFR-TKIs.3,4 Previous retrospective studies with small sample sizes and case reports indicated that patients with these mutations are more sensitive to the second-generation EGFR-TKI afatinib.5,6 Patients with exon 20 insertion have a poor response to EGFR-TKIs.7,8 However, there are little data on the third-generation EGFR-TKI osimertinib in patients with uncommon EGFR mutations. Uncommon mutations often exist as compound mutations. Different uncommon EGFR mutations, especially compound uncommon EGFR mutations, have different reactivities to TKIs, but most studies did not report survival data for specific compound EGFR mutation types.
In this study, we have reported a patient with G719X and S768I compound mutations who has taken osimertinib for 31.0 months and exhibited a partial response (PR), and her follow-up is ongoing.
Case presentation
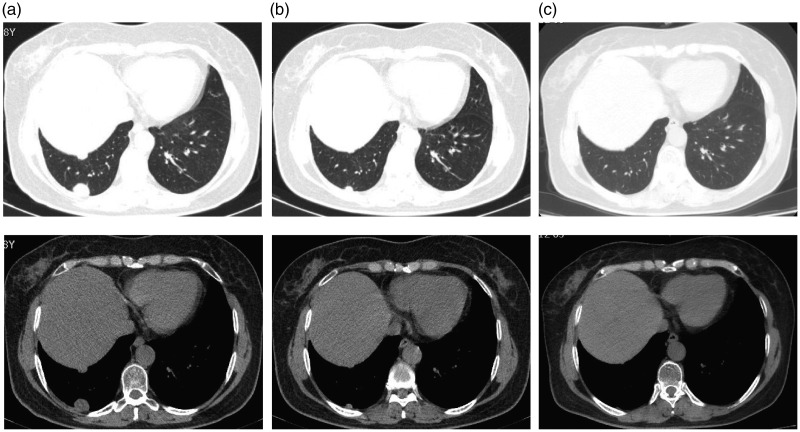
A 56-year-old woman (never-smoker) was referred to the hospital in December 2015 after a lung nodule was detected on chest computed tomography (CT) during a physical examination, and no particular personal or family medical history was reported for this patient. CT identified a ground-glass nodule in the right upper lobe (2.4 cm × 1.4 cm). No enlarged lymph nodes or distant metastasis was detected via brain magnetic resonance imaging and positron emission tomography-CT. The right upper lobe was resected. Postoperative pathological examination led to a diagnosis of acinar predominant adenocarcinoma (pathologic tumor-node-metastasis stage: T1N0M0). Gene sequencing of the tumor confirmed the G719X mutation in exon 18 and the S768I mutation in exon 20 of EGFR. Regular check-up illustrated that the disease was stable until April 2017. At this visit, CT disclosed two pleural nodules in the lower lobe of the right lung (Figure 1a). Systemic examination did not reveal lymph node metastasis or distant metastasis. Percutaneous lung biopsy was performed on the larger nodule, and pathology confirmed adenocarcinoma, suggesting the progression of lung cancer. The size of the specimen was insufficient for genetic testing, and the patient refused our advice to undergo another biopsy or blood testing to determine the mutation status of the recurrent nodule. Combined with the result of the first genetic test of surgically resected tumor tissue, we considered that compound G719X and S768I EGFR gene mutations still existed in the pleural nodules of the patient. The patient refused chemotherapy. We selected the third-generation EGFR-TKI osimertinib. Since the end of April 2017, the patient has been administered 80 mg of osimertinib per day. After 2 months of treatment, lung CT illustrated that the two tumor nodules had significantly shrank, and the efficacy evaluation was PR (Figure 1b). To date, the patient has taken osimertinib for 31.0 months with no adverse effects, and the two tumor nodules have further decreased in size (Figure 1c). No significant clinical or biological adverse effects of osimertinib have been observed during the treatment course.
Figure 1.
Imaging results for the patient during treatment with osimertinib. (a) After postoperative recurrence, two pleural dissemination nodules with diameters of 0.5 and 2.4 cm, respectively, were detected in the right lower lobe. (b) After 2.0 months of osimertinib treatment, the two nodules were significantly smaller, and the efficacy was judged as a partial response. (c) After 31.0 months of osimertinib treatment, the two nodules have further decreased in size.
This report was approved by the Ethics Committee of The First Hospital of Jilin University (18K052-001), and the patient provided informed consent for publication of this case report.
Discussion
This case report described a patient with NSCLC carrying the uncommon compound G719X and S768I mutations in EGFR. After tumor recurrence, she was treated with osimertinib, and her progression-free survival (PFS) has exceeded 31.0 months.
Previous studies illustrated that uncommon mutations often coexist with other uncommon or common mutations. A possible explanation is that these uncommon mutations harbor inadequate tumor-driving abilities, and therefore, they must coexist with another mutation to initiate tumorigenesis.5,9 The Taiwan Lung Cancer Clinical Trial Consortium study was a large retrospective cohort of patients harboring uncommon EGFR mutations.10 In this study, patients harboring compound uncommon mutations (G719X plus L861Q and G719X plus S878I) had a significantly higher overall response rate (ORR) (68.4% vs. 37.8%, P = 0.011) and significantly better PFS (11.9 vs. 6.5 months, P = 0.010) than those with a single uncommon mutation. Passaro et al. reported that the median PFS times of patients with EGFR exon 18 mutation and double mutations of EGFR who received first- and second-generation TKI treatment were 8.3 and 12.3 months (hazard ratio = 0.65, P = 0.06), respectively, which were similar to the survival times of patients with common EGFR mutations. Comparative analysis illustrated that patients with compound mutations exhibited longer survival than those carrying the exon 18 mutation, but there was no correlation between survival and the presence of exon 20 mutations.11 It is currently accepted that the efficacy of TKIs in patients with compound uncommon mutations is roughly equivalent to that in patients with TKI-sensitive mutations. Most studies did not distinguish between single and compound uncommon mutations when evaluating therapeutic efficacy. This may explain why these studies reported high sensitivity to EGFR-TKIs among patients with uncommon mutations.
Patients carrying the EGFR G719X mutation are considered moderately sensitive to first-generation EGFR-TKIs, exhibiting an average response rate of 35.1% to 53.3%.3,9,12 The median PFS ranges 6.3 to 8.1 months in such patients,3,9,10,13 which is shorter than that of patients with common mutations. Patients carrying the S768I mutation are generally considered resistant to first-generation EGFR-TKIs based on a median PFS of 2.7 to 5.0 months.14,15 Compared with the findings for first-generation EGFR-TKIs, patients with G719X and S768I mutations in EGFR are more sensitive to the second-generation EGFR-TKI afatinib. The combined post hoc analysis of the Lux-Lung 2, Lux-Lung 3, and Lux-Lung 6 studies included 18 patients with G719X and 8 patients with S768I. The PFS times of patients with G719X and S768I were 13.8 and 14.7 months, respectively. Notably, most of these patients harbored compound mutations.6 Yang et al.16 reported the efficacy of afatinib in 35 EGFR-TKI–naïve patients with compound uncommon EGFR mutations. The median time to treatment failure was 14.7 months, and the ORR was 77.1%. The clinical data for osimertinib in patients with compound uncommon mutations are limited. A post-analysis of phase I and II of the AURA trial (ClinicalTrials.gov, NCT01802632) included only five patients with uncommon mutations (G719X [n = 2], G719X/S768I [n = 2], and L861Q [n = 1]), and the median PFS was 8.3 months (95% confidence interval = 2.8–19.0 months) in patients treated with once-daily osimertinib at a dose of 80 or 160 mg.17 A phase II clinical study in Korea included 36 patients with uncommon mutations who were treated with osimertinib. The PFS times of patients carrying the L861Q, G719X, and S768I mutations were 15.2, 8.2, and 12.3 months, respectively. This study included four patients who harbored compound uncommon EGFR mutations, including two patients each carrying G719X plus L861Q and G719X plus S768I.18 However, the study did not report the effects of these compound uncommon mutations. An in vitro study found that the IC50 of afatinib in G719X-mutant cells was 0.9 nM, and that in S768I-mutant cells was 0.7 nM. Meanwhile, the IC50 of osimertinib was 53 nm in G719X-mutant cells and 49 nM in S768I-mutant cells. However, clinically achievable concentrations should be considered in the interpretation of in vitro sensitivities.5
The FLAURA and LUX-LUNG 3 clinical trials reported median PFS times for osimertinib and afatinib in the first-line treatment of patients with NSCLC and a common EGFR mutation (exon 19 deletion or L858R) of 18.9 and 13.6 months, respectively.19,20 In our study, a Chinese patient with uncommon compound G719X and S768I mutations in EGFR has been treated with osimertinib for 31.0 months. Efficacy has been judged as PR, and her follow-up is ongoing. The efficacy in this patient has exceeded that in patients with common mutations.
Conclusion
The results of this case report indicate that the third-generation TKI osimertinib is more effective than first-generation TKIs and the second-generation TKI afatinib for patients with NSCLC who carry uncommon and compound G719X and S768I mutations in EGFR. However, further clinical data for patients with NSCLC harboring compound uncommon mutations are required to provide more powerful evidence.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
This report was approved by the Ethics Committee of The First Hospital of Jilin University (18K052-001), and the patient provided informed consent.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
References
- 1.Massarelli E, Johnson FM, Erickson HS, et al. Uncommon epidermal growth factor receptor mutations in non-small cell lung cancer and their mechanisms of EGFR tyrosine kinase inhibitors sensitivity and resistance. Lung Cancer 2013; 80: 235–241. [DOI] [PubMed] [Google Scholar]
- 2.O’Kane GM, Bradbury PA, Feld R, et al. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer 2017; 109: 137–144. [DOI] [PubMed] [Google Scholar]
- 3.Li K, Yang M, Liang N, et al. Determining EGFR-TKI sensitivity of G719X and other uncommon EGFR mutations in non-small cell lung cancer: perplexity and solution. Oncol Rep 2017; 37: 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JY, Yu CJ, Chang YC, et al. Effectiveness of Tyrosine Kinase Inhibitors on ‘Uncommon’ Epidermal Growth Factor Receptor Mutations of Unknown Clinical Significance in Non-Small Cell Lung Cancer. Clin Cancer Res 2011; 17: 3812–3821. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci 2016; 107: 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang JCH, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015; 16: 830–838. [DOI] [PubMed] [Google Scholar]
- 7.Beau-Faller M, Prim N, Ruppert A-M, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 2014; 25: 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidoo J, Sima CS, Rodriguez K, et al. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: clinical outcomes and response to erlotinib: EGFR Exon 20 Insertions. Cancer 2015; 121: 3212–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu TH, Hsiue EHC, Lee JH, et al. New data on clinical decisions in NSCLC patients with uncommon EGFR mutations. Expert Rev Respir Med 2017; 11: 51–55. [DOI] [PubMed] [Google Scholar]
- 10.Chiu CH, Yang CT, Shih JY, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas with G719X/L861Q/S768I Mutations. J Thorac Oncol 2015; 10: 793–799. [DOI] [PubMed] [Google Scholar]
- 11.Passaro A, Prelaj A, Bonanno L, et al. Activity of EGFR TKIs in Caucasian Patients With NSCLC Harboring Potentially Sensitive Uncommon EGFR Mutations. Clin Lung Cancer 2019; 20: e186–e194. [DOI] [PubMed] [Google Scholar]
- 12.Klughammer B, Brugger W, Cappuzzo F, et al. Examining Treatment Outcomes with Erlotinib in Patients with Advanced Non–Small Cell Lung Cancer Whose Tumors Harbor Uncommon EGFR Mutations. J Thorac Oncol 2016; 11: 545–555. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe S, Minegishi Y, Yoshizawa H, et al. Effectiveness of Gefitinib against Non–Small-Cell Lung Cancer with the Uncommon EGFR Mutations G719X and L861Q. J Thorac Oncol 2014; 9: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu X, Bai Q, Lu Y, et al. Response to Tyrosine Kinase Inhibitors in Lung Adenocarcinoma with the Rare Epidermal Growth Factor Receptor Mutation S768I: a Retrospective Analysis and Literature Review. Target Oncol 2017; 12: 81–88. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Song Z, Cheng G. Clinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutations. Onco Targets Ther 2016; 9: 4181–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JCH, Schuler M, Popat S, et al. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: a Database of 693 Cases. J Thorac Oncol. Epub ahead of print 10 January 2020. DOI: 10.1016/j.jtho.2019.12.126. [DOI] [PubMed] [Google Scholar]
- 17.Ramalingam SS, Yang JCH, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation–Positive Advanced Non–Small-Cell Lung Cancer. J Clin Oncol 2018; 36: 841–849. [DOI] [PubMed] [Google Scholar]
- 18.Cho JH, Lim SH, An HJ, et al. Osimertinib for Patients With Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: a Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J Clin Oncol 2020; 38: 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018; 378: 113–125. [DOI] [PubMed] [Google Scholar]
- 20.Sequist LV, Yang JCH, Yamamoto N, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. J Clin Oncol 2013; 31: 3327–3334. [DOI] [PubMed] [Google Scholar]