Abstract
The p75 neurotrophin receptor (p75NTR) can regulate multiple cellular functions including proliferation, survival, and apoptotic cell death. The p75NTR is widely expressed in the developing brain and is downregulated as the nervous system matures, with only a few neuronal subpopulations retaining expression into adulthood. However, p75NTR expression is induced following damage to the adult brain, including after traumatic brain injury, which is a leading cause of mortality and disability worldwide. A major consequence of traumatic brain injury is the progressive neuronal loss that continues secondary to the initial trauma, which ultimately contributes to cognitive decline. Understanding mechanisms governing this progressive neuronal death is key to developing targeted therapeutic strategies to provide neuroprotection and salvage cognitive function. In this study, we demonstrate that a cortical impact injury to the sensorimotor cortex elicits p75NTR expression in apoptotic neurons in the injury penumbra, confirming previous studies. To establish whether preventing p75NTR induction or blocking the ligands would reduce the extent of secondary neuronal cell death, we used a noninvasive intranasal strategy to deliver either siRNA to block the induction of p75NTR, or function-blocking antibodies to the ligands pro-nerve growth factor and pro-brain-derived neurotrophic factor. We demonstrate that either preventing the induction of p75NTR or blocking the proneurotrophin ligands provides neuroprotection and preserves sensorimotor function.
Keywords: neuroprotection; pro-brain-derived neurotrophic factor; pro-nerve growth factor; p75 neurotrophin receptor; traumatic brain injury, intranasal delivery
Growth factors that are produced after brain injury critically affect whether injured neurons survive or die, which in turn influence the neurological outcome for the affected individual. The neurotrophin family of trophic factors can support neuronal survival or promote neuronal death depending upon which receptor complex and which signaling pathways are activated. Nerve growth factor (NGF) and the related neurotrophins are known to support the survival of many neuronal populations acting through the Trk family of receptor tyrosine kinases (Huang and Reichardt, 2003; Reichardt, 2006). In contrast, activation of the p75 neurotrophin receptor (p75NTR) can lead to cell death (Frade and Barde, 1998; Friedman, 2000). Neurotrophins are initially synthesized as precursor proneurotrophins and are cleaved to generate their mature forms, which signal through their cognate Trk receptors. However, the precursor proneurotrophins, which are selective, high-affinity ligands for p75NTR with its coreceptor sortilin, can also be secreted, inducing p75NTR-mediated apoptosis (Lee et al., 2001; Nykjaer et al., 2004). Although p75NTR is transiently expressed in many central nervous system (CNS) neuronal populations during development, this receptor is not widely expressed in the normal adult brain. However, after injury, expression of p75NTR is induced in numerous CNS neurons and has been shown to regulate cell death following several types of brain injury, including seizures (Troy et al., 2002), corticospinal transection (Harrington et al., 2004), and spinal cord injury (Beattie et al., 2002). This contrasts with the role of mature neurotrophins that stimulate Trk receptors to prevent inappropriate developmental death (Oppenheim, 1989) and to promote neuronal survival after injury. Thus, neurotrophins have opposing actions on neuronal viability depending upon whether the precursor proneurotrophin or the mature neurotrophin protein is secreted and with which receptor complex it engages. Our previous work has shown that CNS injury increases p75NTR expression and proneurotrophin secretion (Volosin et al., 2008; Le and Friedman, 2012), shifting the balance of neurotrophin signaling toward cell death.
Traumatic brain injury (TBI) is a leading cause of death and disability, resulting from relatively common occurrences—car accidents, falls, sport- and work-related injuries, among others. The effects are far-reaching and detrimental, often disrupting cognitive function and normal routines, and causing long-term debilitating effects on memory, reasoning, sensation, language abilities, and emotional understanding (Robertson, 2008). Primary damage occurs in the tissue directly underneath the area of impact, involving mechanical damage to neurons, glia, and blood vessels. However, the secondary damage may evolve over hours and days following the initial insult, resulting from metabolic and biochemical changes that induce delayed neuronal apoptosis, leading to functional impairment (Raghupathi et al., 2000; Nathoo et al., 2004; Loane and Faden, 2010). Although it will be challenging to preserve those neural elements that have sustained the initial mechanical injury, therapeutic strategies are targeted at minimizing neuronal loss due to secondary damage to preserve neural circuitry and brain function. Such strategies require comprehensive understanding of the mechanisms governing post-TBI neuronal death. Recent studies have implicated p75NTR as an important player in mediating neuronal cell death in several different models of TBI (Sebastiani et al., 2015; Alder et al., 2016; Delbary-Gossart et al., 2016). Here we provide data to corroborate the role of p75NTR and the participation of the proneurotrophin ligands in secondary neuronal cell death after TBI. We show that delivering therapeutics that neutralize either the proneurotrophins or their receptor, using a noninvasive intranasal strategy, reduces secondary neuronal cell death and improves sensorimotor function.
Materials and Methods
Animals
All animal studies were conducted using the National Institutes of Health guidelines for the ethical treatment of animals with approval of the Rutgers University Animal Care and Facilities Comittee. Adult mice (C57BI/6) between the ages of 2 and 3 months were maintained on a 12-hr light/dark cycle with free access to food and water.
Controlled Cortical Impact
Male mice at 10 to 12 weeks old were subjected to controlled cortical impact (CCI) injury. The animals were anesthetized with a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg ip). Once fully anesthetized, the scalp was cleansed, and an incision along the midline was created to expose the skull. The animals were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). A 4-mm craniectomy was produced using a trephine (Meisinger, Centennial, CO, USA, Cat# 229 030) midway between bregma and lambda, 2.5 mm lateral to the sagittal suture (somatosensory cortex). The brain injury was generated using the electronically-driven CCI device (Custom Design and Fabrication at Virginia Commonwealth University, Ritchmond, VA, USA) fitted with a 3-mm diameter impactor tip.The velocity of the impactor was set at 4.0 m/s, depth of penetration was 1.5 mm, and the duration of deformation was 150 ms. Animals were randomly assigned to receive either sham injury or brain injury. The animals were placed on heating pads at 37° and monitored continuously for 2 hr after surgery. Buprenorphine (0.05 mg/kg) was administered subcutaneously postoperatively. In addition, all animals received 3% body weight of 0.9% saline subcutaneously to prevent dehydration.
Intranasal Infusion Treatment
siRNA directed against the p75NTR sequence (sense, SSUGGAACAGCUGCAAACAAAUU) or luciferase sequence (sense, SSCGUACGCGGAAUACUUCGAUU) was synthesized (Horizon Discovery, Dharmacon, Lafayette, CO, USA) and linked to Penetratin-1 (Davidson et al., 2004). Two µl drops of 80 nM p75NTR siRNA or luciferase siRNA (control siRNA) were administered under anesthesia to each nostril every 2 min for a total of 20 µl. Alternatively, 3 µg of antiserum against proNGF (provided by Dr. Barbara Hempstead and validated by us previously; Volosin et al., 2008) or pro-brain-derived neurotrophic factor (proBDNF; Alomone Labs, Jerusalem, Israel, Cat# ANT-006, RRID:AB_2039758) were infused intranasally immediately after the surgery, with 2 µl drops to each nostril alternating every 2 min for a total of 10 µl. Animals were randomly assigned to each treatment. Control animals received purified rabbit immunoglobulin G (IgG) antibody (BD Biosciences, San Jose, CA, USA, Cat# 550875, RRID:AB_393942).
Immunohistochemistry
Animals were deeply anesthetized with ketamine/xylazine and perfused transcardially with saline followed by 4% paraformaldehyde. The brains were removed and postfixed in 4% paraformaldehyde for 2 hr and cryoprotected in 30% sucrose. Sections (20 µm) were cut on a cryostat (Leica Biosystems, Buffalo Grove, IL, USA) and mounted onto charged slides. Sections were blocked in 1%bovine serum albumin (BSA)/5% donkey serum and permeabilized with PBS/0.3% Triton X-100 and then exposed to primary antibodies overnight at 4°C in PBS/1% BSA. Slides were then washed three times in PBS, exposed to secondary antibodies coupled to different fluorophores at room temperature (RT) for 1 hr. Sections were washed again three times, with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO, USA; 1: 10,000) present in the final wash. Sections were coverslipped with antifading medium (ProLong Gold; Thermo Fisher Scientific, Waltham, MA, USA, RRID:SCR_015961) and analyzed by fluorescence (Nikon Eclipse TE200) and confocal microscopy (Zeiss LSM 510 META). The following were the primary antibodies used: anti-NeuN (1:500; Cell Signaling Technology, Danvers, MA, USA, Cat# 12943, RRID:AB_2630395), anti-proBDNF (1:500; Alomone Labs, Jerusalem, Israel, Cat# ANT-006, RRID:AB_2039758), anti-glial fibrillary acidic protein (GFAP; 1:500; R&D Systems, Minneapolis, MN, USA, Cat# AF2594, RRID:AB_2109656), and anti-p75NTR (1:500; R&D Systems, Cat# AF1157, RRID:AB_2298561).
Western Blot
Tissue from olfactory bulb (OB) and cortex were dissected and homogenized using 1% nonylphenyl polyethylene glycol (NP-40), 1% triton, and 10% glycerol in Tris-buffered saline (TBS) buffer (50 mM Tris, pH 7.6, 150 mM NaCl) with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The protein lysates were sonicated and centrifuged for 15 min at 4°C. Proteins were quantified using the Bradford assay (Bio-Rad, Hercules, CA, USA, Cat# 500-006) and equal amounts of protein were loaded onto Sodium Dodecyl Sulfate (SDS) gels and transferred to nitrocellulose membranes. Membranes were blocked in 5% nonfat dried skim milk in TBS-T for 2 hr at RT. Primary antibody (anti-p75NTR, Millipore, Burlington, MA, USA, Cat# 07-476, RRID:AB_310649) diluted 1:1000 in 1% BSA was applied overnight at 4°C. Membranes were washed with TBS-T 3 × 10 min each and incubated with secondary anti-rabbit horseradish peroxidase-conjugated IgG antibody for 1 hr at RT (Jackson ImmunoResearch, West Grove, PA, USA). To confirm equal protein levels, blots were reprobed for actin. Bands were visualized by enhanced chemical luminescence (Pierce, Rockford, IL, USA) and quantified using ImageJ Version 1.52e (National Institutes of Health, USA).
Terminal Deoxynucleotidyl Transferase Deoxyuridine Triphosphate Nick End Labeling Staining
The number of apoptotic cells following TBI was assessed by labeling with terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end according to the manufacture’s protocol (Click-iT TUNEL assay, Thermo Fisher Scientific, Cat# C10617). Sections were then immunostained for p75NTR and counterstained with DAPI. TUNEL-positive cells were analyzed on a Zeiss spinning disk confocal microscope using the tiling function to measure 10 fields of view of the lesion site and surrounding tissue. Quantification of TUNEL-positive cells was made using ImageJ Version 1.51 (National Institutes of Health, USA).
Determination of the Area of Damage
A total of 12 sections (20 µm thickness, spaced every 200 µm) through the injured cortex (bregma -0.5 mm to –1.80 mm) were stained with cresyl violet and coverslipped with Permount mounting media or stained for NeuN and coverslipped with antifading medium with DAPI (ProLong Gold with DAPI, Thermo Fisher Scientific, Cat# P36931, RRID:SCR_015961). The area of tissue loss in the injured hemisphere was traced using the contralateral (CL) hemisphere superimposed on top of the lesioned hemisphere. The area that had been damaged in the ipsilateral (IL) hemisphere, which included the area of tissue loss and the penumbra, was quantified and divided by the total area of the contralateral hemisphere. Area measurements were obtained from at least 3 animals per group using ImageJ Version 1.51.
Behavioral Analysis
Mice were subjected to a battery of behavioral test by an investigator who was blinded to the experimental groups. Animals were handled 1 day before and on the day after the CCI to reduce the effects that handling stress might have on the behavioral tests.
Modified Neurological Severity Score
Animals were analyzed using a battery of tests to assess motor, balance, sensory, exploratory, and reflex behaviors that make up the modified neurological severity score (mNSS; Chen et al., 2001; Flierl et al., 2009; Wu et al., 2010). Successful completion of each task results in a “0” score, while failure results in a “1” score. Scores for each task are added to create a total composite score out of 12. High final mNSS scores were indicative of task failures and interpreted as neurological impairment.
Hang Test
Mice were allowed to grab onto a thin, elevated, horizontal metal rod by their forelimbs. The length of time that the mouse spent on the metal rod without falling was measured. A maximum time of 3 min on the rod was allotted per trial. Mice were tested 3 times consecutively.
Horizontal Ladder Test
This test has been used to evaluate injury to the sensorimotor cortex (Soblosky et al., 1997; Zhao et al., 2012; Madathil et al., 2013). We modified the apparatus so that the 4-mm diameter rungs were irregularly spaced, with a minimum spacing of 12 mm and a maximum spacing of 24 mm. The ladder was suspended horizontally 18 inches above the ground. One end contained a hollow black goal box where a sugar-rich cereal treat was placed. Rungs were suspended along an 8-cm wide beam. A video camera was placed directly in front of the apparatus, and a mirror was situated below the apparatus so that foot slips were readily visible. Training consisted of a 5-min acclimation period in the goal box, followed by at least three trials where the animal was directed to run across the ladder beam toward the goal box. On the testing day, each animal completed three runs where they completely traversed the ladder at a constant rate without turning around. Between each test run, the animal was left in the goal box for 1 min. A foot slip was scored when either of the limbs dropped below the plane of the rungs due to misplacement on either the rung ahead or behind. The number of IL and CL forelimb and hindlimb foot slips was counted.
Statistical Analyses
Data are expressed as mean values ± SEM, and experimental groups were compared using GraphPad Prism, Version 8. One-way analysis of variance followed by Tukey’s post hoc analysis was used for parametric values, and Kruskal–Wallis test followed by Dunn’s multiple comparison test was used for nonparametric values. Unpaired one-tailed Student’s t test was used for any two-group comparisons. As appropriate, p < .05 was considered significant. Statistical results are presented in the figure legends.
Results
Cortical Impact Elicits p75NTR Expression in Apoptotic Cells in the Injury Penumbra
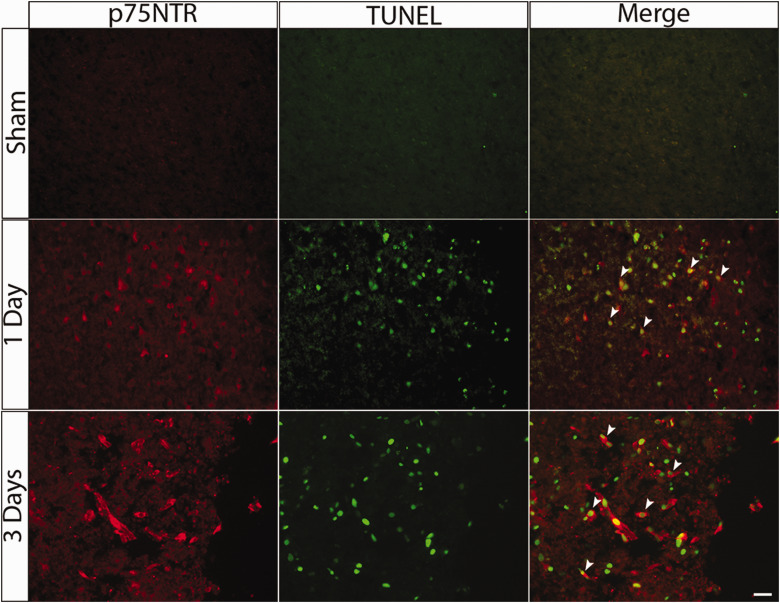
The p75NTR is induced after multiple different types of injury to the CNS, including seizures (Roux et al., 1999; Troy et al., 2002), spinal cord injury (Beattie et al., 2002), and corticospinal transection (Harrington et al., 2004). The neurons that show induction of p75NTR in those injury paradigms are apoptotic, and p75NTR was shown to mediate neuronal death in response to proneurotrophin ligands in several of these injury conditions (Troy et al., 2002). To assess whether p75NTR and its ligands might also play a role in mediating neuronal death following TBI, we used the CCI model to induce a focal injury in mice and examined p75NTR expression during the subacute period of recovery following the injury. Adult male C57Bl/6 mice were subjected to CCI and perfused 1 and 3 days after injury. Sham animals that had been anesthetized and subjected to the craniotomy were used as controls. Following CCI injury, p75NTR expression was induced in the penumbral area adjacent to the injury, confirming results from previous studies (Sebastiani et al., 2015; Alder et al., 2016; Delbary-Gossart et al., 2016). At 1 and 3 days after the injury, cells with high levels of p75NTR are also labeled with TUNEL (Figure 1), supporting the conclusion that cells expressing p75NTR were undergoing cell death.
Figure 1.
p75NTR-Positive Cells After CCI Are Apoptotic. Adult mice were subjected to CCI and perfused 1 or 3 days after injury. Representative images showing double labeling with TUNEL staining and anti-p75NTR adjacent to the area of tissue damage 1 and 3 days after the injury. Arrowheads show colocalization of p75NTR and TUNEL-positive cells. Scale bar = 50 µm.
p75NTR = p75 neurotrophin receptor; TUNEL = terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling.
p75NTR Knockdown Decreases the Extent of Injury and Improves Sensorimotor Function
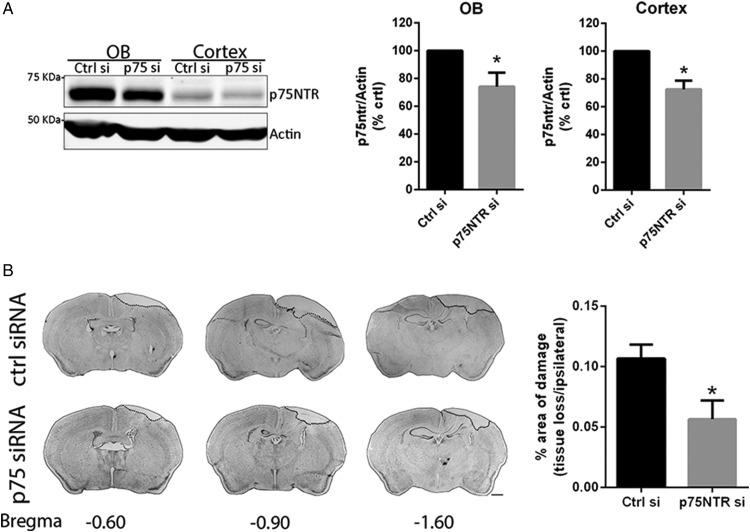
Previous studies have shown that mice lacking p75NTR showed less neuronal cell death following different types of TBI (Sebastiani et al., 2015; Alder et al., 2016). To assess whether acutely blocking the induction of p75NTR that occurs following injury would provide the same neuroprotection seen in the p75NTR null mice, we administered a siRNA directed against p75NTR immediately following the injury. The siRNA was linked to Penetratin (Pen-siRNA) to facilitate entry of the siRNA into cells (Davidson et al., 2004). To avoid an invasive injection protocol, the siRNA was infused intranasally. Intranasal delivery has been used to deliver a wide variety of therapeutic compounds (Jin et al., 2003; Thorne et al., 2004; Cantarella et al., 2008; Tian et al., 2012; Scafidi et al., 2014). To assess the efficiency of the intranasal p75NTR siRNA, control mice were infused with the p75NTR siRNA and compared with a siRNA to luciferase (control siRNA). One day after infusion, the OB and cortex were analyzed for p75NTR levels. Both brain regions analyzed showed reduced p75NTR levels in the animals that received the p75NTR Pen-siRNA infusion compared with the control infusion (Figure 2A; p < .05). p75NTR Pen-siRNA or luciferase Pen-siRNA was applied intranasally to mice immediately following the CCI injury, and the mice were allowed to recover for 2 to 3 days. Morphological analyses of sections stained with cresyl violet revealed that the mice that received the p75NTR Pen-siRNA showed a significant reduction in neocortical damage compared with the mice that received siRNA control (Figure 2B; p < .05).
Figure 2.
Intranasal Infusion of p75NTR Pen-siRNA Reduces Damage Following CCI. (A) Infusion of p75NTR Pen-siRNA reduced p75NTR expression in the OB and cortex as measured by Western blot. Controls received luciferase Pen-siRNA. Values represent the means ± SEM. Asterisks indicate significance by two-tailed, unpaired Student’s t test with p = .04 for OB and p = .01 for cortex. n = 3 mice/treatment. (B) Adult mice were subjected to CCI and immediately infused intranasally with either p75NTR Pen-siRNA or luciferase Pen-siRNA control. Representative images of cresyl violet-stained coronal sections from luciferase or p75NTR Pen-siRNA-treated mice marked with their coordinates to bregma. Scale bar = 1 mm. The percentage of the area of damage (region of tissue loss and penumbra, indicated by the dotted line) shows less damage in the mice that received the p75NTR Pen-siRNA. Values represent the means ± SEM. Asterisks indicate significance by two-tailed, unpaired Student’s t test with p = .03. n = 3 mice/treatment.
p75NTR = p75 neurotrophin receptor; OB = olfactory bulb.
Prior to perfusion, the mice were analyzed using a series of tests to determine whether the Pen-siRNA provided behavioral as well as morphological sparing (Table 1).
Table 1.
Modified Neurological Severity Scoring (mNSS).
| Task | Description | Success | Failure |
|---|---|---|---|
| Circle exit | Ability and initiative to exit a circle of 30 cm diameter (time limit: 3 min) | 0 | 1 |
| Mono/Hemiparesis | Paresis of upper and/or lower limb | 0 | 1 |
| Straight walk | Alertness, initiative, and motor ability to walk straight | 0 | 1 |
| Tail position | Tail position is either up (normal) or down (impaired) while walking | 0 | 1 |
| Startle reflex | Innate reflex: The mouse will bounce in response to a loud handclap | 0 | 1 |
| Seeking behavior | Physiological behavior as a sign of interest in the environment | 0 | 1 |
| Grip test | Ability to grip forceps with all four limbs | 0 | 1 |
| Beam balancing | Ability to balance on a beam of 7 mm width for at least 10 s | 0 | 1 |
| Round stick balancing | Ability to balance on a round stick 5 mm diameter for at least 10 s | 0 | 1 |
| Beam walk: 1.5 cm | More than twice the average sham animal slips | 0 | 1 |
| Beam walk: 1 cm | More than twice the average sham animal slips | 0 | 1 |
| Beam walk: 0.7 cm | More than twice the average sham animal slips | 0 | 1 |
| Maximal score | 12 |
Note. Summary of the motor, balance, sensory, exploratory, and reflex tests that go into the overall composite mNSS score. Successful completion of each task results in a “0” score, while failure results in a “1” score. Scores for each task are added to create a total composite score out of 12. Mice were evaluated by an experimenter blinded to the identity of the subjects.
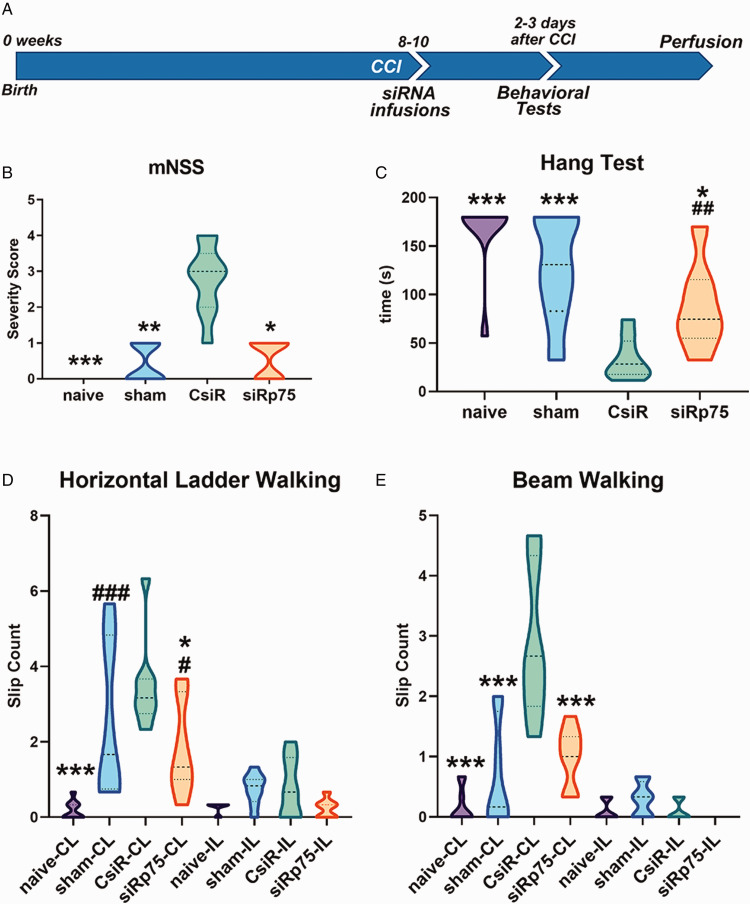
Mice that sustained a CCI and had received p75NTR Pen-siRNA showed significantly preserved sensorimotor function 2 days after surgery compared with the CCI group that was given control Pen-siRNA (Figure 3A; p < .05). On the mNSS test, p75NTR Pen-siRNA-treated mice consistently scored better than control Pen-siRNA-treated mice and were comparable with sham-operated mice (Figure 3B; p < .05). When their ability to hang onto a horizontal metal rod was measured, the p75NTR Pen-siRNA-treated mice showed some muscle weakness (as reflected by short durations hanging onto the rod) when compared with the naïve animals, but their performance was significantly better than the control Pen-siRNA group (Figure 3C; p < .05). Similar results were obtained for the horizontal ladder test. As expected, the CCI-injured mice treated with the control Pen-siRNA made foot slips when using their limbs CL to the CCI, whereas they made few foot slips using their limbs IL to the lesion (Figure 3D). The p75NTR Pen-siRNA-treated mice had fewer foot slips than control Pen-siRNA-treated mice (p < .05). There was no significant difference in IL foot slips among groups indicating the specificity of both injury and recovery of sensorimotor function after treatment (Figure 3D). Foot slips were also measured on horizontal beam walk test as part of the mNSS battery. p75NTR Pen-siRNA-treated mice had significantly fewer CL foot slips (p < .001) than control Pen-siRNA-treated mice on a 1.0-cm wide horizontal beam (Figure 3E). We also assessed the groups on 0.7-cm and 1.5-cm wide horizontal beams. p75NTR Pen-siRNA-treated mice exhibited improvements over control Pen-siRNA mice on both beams; however, these differences did not reach statistical significance (data not shown).
Figure 3.
Behavioral Analyses of Mice Receiving Intranasal p75NTR Pen-siRNA or Control Pen-siRNA. (A) Outline of the experimental paradigm of CCI injury and behavioral testing. Control and p75NTR Pen-siRNA were infused intranasally to each nostril every 2 min for a total of 20 µl immediately after CCI. (B) Composite mNSS scores for naive, sham-treated, control Pen-siRNA-treated, or p75NTR Pen-siRNA-treated mice evaluated 2 days following the injury. (C) Hang test measured in time (seconds) 2 days following the injury. (D) Average foot slips per run on horizontal ladder with irregularly placed rugs evaluated 3 days following the injury. (E) Average foot slips per run on 1.0-cm wide balance beam evaluated 3 days following the injury. Data were collected across 7 to 9 animals per group; *p < .05, **p < .001, ***p < .0001 for groups compared with control Pen-siRNA-treated mice; #p < .05, ##p < .001, ###p < .0001 for groups compared with naive mice using analysis of variance followed by Tukey’s multiple comparisons test for parametric values and Kruskal–Wallis test followed by Dunn’s multiple comparison test for nonparametric values.
CCI = controlled cortical impact; CsiR = control siRNA; siRp75 = p75NTR siRNA; CL= contralateral; IP = ipsilateral to the injury.
Blocking proNGF or proBDNF Ligands Provides Neuroprotection and Improves Neurological Function
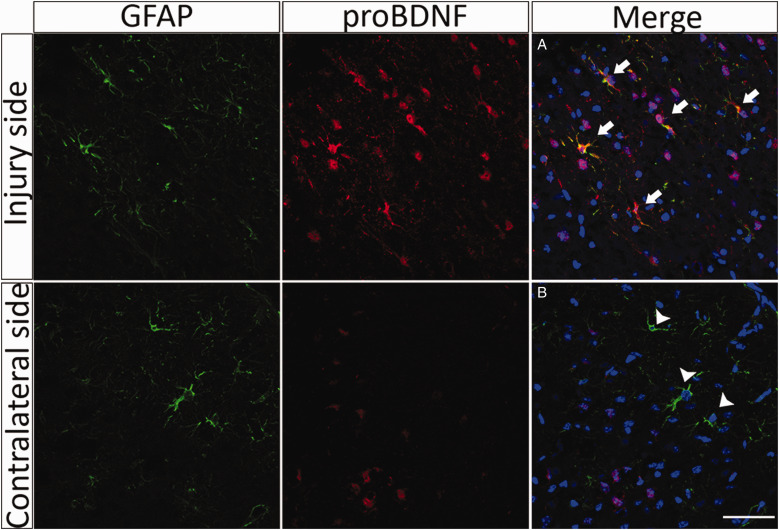
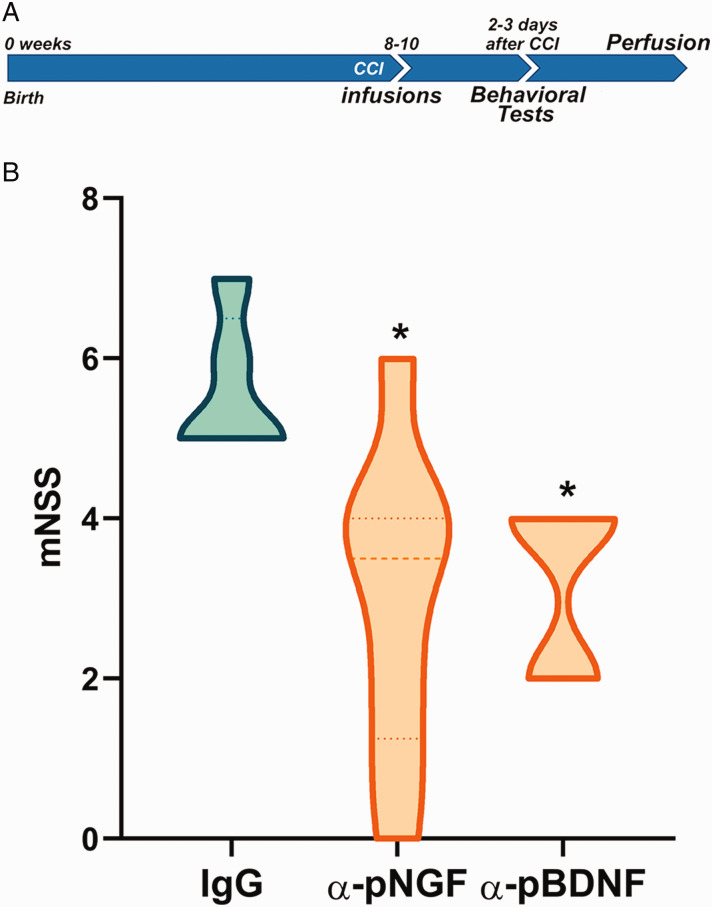
A recent study has shown that the levels of proNGF increased after TBI in astrocytes and microglia (Delbary-Gossart et al., 2016). To assess whether proBDNF was also upregulated after TBI, mice were subjected to CCI and perfused 3 days after injury. Morphological analysis of sections stained for proBDNF (red) demonstrated an increase in the expression of this protein surrounding the injury site (Figure 4). proBDNF was induced in GFAP-expressing astrocytes as well as in other cell types following TBI (Figure 4). Considering these results and taking into account that the levels of proNGF are also upregulated after injury (Alder et al., 2016; Delbary-Gossart et al., 2016), we investigated whether inhibition of these proneurotrophin ligands that activate p75NTR would prevent neuronal death and functional loss after TBI. Neutralizing antibodies to either proNGF or proBDNF were provided to the mice intranasally immediately following the CCI injury. Controls received an equal amount of pre-immune IgG. Two days following the injury, sensorimotor function was analyzed using the mNSS test battery. The neutralizing antibodies to either proNGF or proBDNF provided significant functional sparing compared with control IgG as assessed by the mNSS (Figure 5; p < .05).
Figure 4.
proBDNF Is Induced in Astrocytes After TBI. Adult mice were subjected to CCI and perfused 3 days after injury. Representative sections through the injury site 3 days after CCI show increased proBDNF labeling (red), some of which colocalizes with GFAP (green) adjacent to the area of tissue damage. In contrast, sections through the contralateral side 3 days after the injury show little expression of proBDNF. (A) Arrows indicate colocalization of proBDNF and GFAP-positive cells. (B) Arrowheads indicate GFAP-positive cells that do not express proBDNF. Scale bar = 50 µm.
GFAP = glial fibrillary acidic protein; proBDNF = pro-brain-derived neurotrophic factor.
Figure 5.
Behavioral Analysis of Mice Receiving Intranasal Neutralizing Antibodies to proNGF or proBDNF Following CCI. (A) Outline of the experimental paradigm of CCI injury and behavioral testing. Control IgG, anti-proNGF, or anti-proBDNF was infused intranasally to each nostril every 2 min for a total of 20 µl immediately after CCI. (B) The mNSS score showed behavioral sparing of mice that received either anti-proNGF or anti-proBDNF compared with mice that received control IgG following CCI. Data were collected across 5 to 8 animals per group. Asterisks indicate significant difference from IgG control by Kruskal–Wallis test followed by Dunn’s multiple comparison test for nonparametric values, with p = .0204 for IgG control versus proNGF-treated mice; p = .045 for IgG control versus proBDNF-treated mice.
CCI = controlled cortical impact; mNSS = modified neurological severity scoring; IgG = immunoglobulin G; BDNF = brain-derived neurotrophic factor; NGF = nerve growth factor.
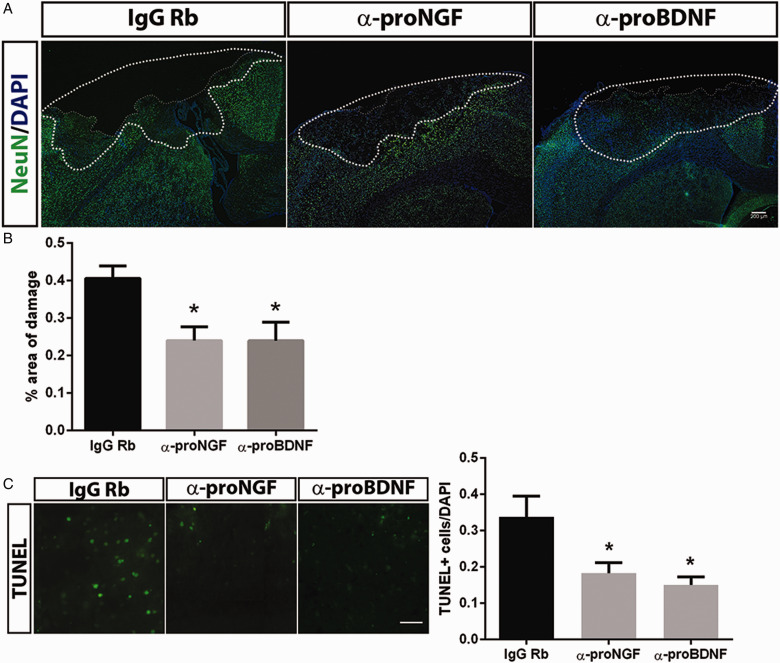
Morphological analysis of the brains from the animals that had received the blocking antibodies to proNGF or proBDNF showed that the area of total damage (the area of tissue loss and the penumbra) was reduced by the application of the antibodies to either ligand (Figure 6A, B; p < .05). Moreover, the number of TUNEL-positive cells in the penumbra was reduced by 50% following administration of the proneurotrophin antibodies (Figure 6C; p < .05). These data demonstrate that the proneurotrophin-p75NTR pathway contributes to delayed cell death following TBI and that either preventing induction of the receptor or blocking the ligands can provide neuroprotection and rescue sensorimotor function.
Figure 6.
Neutralizing Antibodies to proNGF and proBDNF Provide Neuroprotection. (A) Mice were infused intranasally with anti-proNGF, anti-proBDNF, or control IgG immediately after the CCI. At 3 days of recovery, sections were stained for NeuN and counterstained with DAPI to reveal the area of damage. (B) The area of total damage comprised of the area of tissue loss and the penumbra (dotted line), where the density of DAPI and NeuN staining was reduced. The percentage of the total area of damage (relative to the contralateral hemisphere) was significantly reduced by the antiproneurotrophin antibodies. Scale bar = 200 µm. (C) Representative images of TUNEL staining in the penumbra showed fewer apoptotic cells in the mice that received anti-proNGF or anti-proBDNF. Scale bar = 50 µm. Data were collected from 3 to 4 animals per group. Graphs depict the means ± SEM. Asterisks indicate significance by one-way analysis of variance followed by Tukey’s post hoc analysis with p < .05.
IgG = immunoglobulin G; proBDNF = pro-brain-derived neurotrophic factor; proNGF = pro-nerve growth factor; DAPI = 4′,6′-diamidino-2-phenylindole; TUNEL = terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling.
Discussion
TBI can occur from many different causes and elicits neuronal loss leading to numerous detrimental effects. Understanding mechanisms by which neurons die is critical for developing therapeutic approaches to mitigate the devastating consequences of TBI.
The p75NTR has been shown to mediate neuronal death following various types of brain injury, including seizures, spinal cord injury, and TBI (Troy et al., 2002; Harrington et al., 2004; Volosin et al., 2008; Sebastiani et al., 2015; Alder et al., 2016). Here we confirm that the CCI model of severe brain injury elicits induction of p75NTR in dying cells. Previous studies using p75NTR knockout mice reported reduced neuronal loss, indicating that this receptor plays an important role in initiating neuronal death, confirming predictions based on previous studies (Sebastiani et al., 2015; Alder et al., 2016). One goal of the studies we report here was to assess the therapeutic efficacy of acutely blocking the proneurotrophin-p75NTR signaling cascade. We made use of a Penetratin-linked siRNA (Davidson et al., 2004) directed against p75NTR and infused the siRNA intranasally (Akpan et al., 2011) to acutely prevent upregulation of the receptor following the injury. Neuroprotection was then assessed using histopathological measures as well as sensorimotor tests. Preventing p75NTR induction following injury reduced the extent of damage and also provided sparing of behavioral function, a critical aspect of amelioration of the injury response.
Previous studies have used pharmacological tools to inhibit p75NTR signaling after injury. The efficacy of different p75NTR blockers may depend on the injury context and the duration of time for which they are administered. The p75NTR antagonist LM11A-31, which blocks the binding of proNGF to p75NTR, prevented neuronal death and promoted functional sparing following spinal cord injury and in a model of Alzheimer’s disease (Knowles et al., 2013; Tep et al., 2013). While LM11A-31 failed to prevent neuronal death following pilocarpine-induced seizures (Grabenstatter et al., 2014), other methods of blocking p75NTR were neuroprotective following pilocarpine-induced seizures, thus supporting the hypothesis that reagents that will block this receptor might be effective therapeutics (Troy et al., 2002; Volosin et al., 2008). After CCI, the intranasal delivery of LM11A-31 prevented neuronal death and improved outcomes when administered each day for 7 days after the injury (Shi et al., 2013). Similarly, a different p75NTR antagonist, EVT901, which prevents oligomerization of the receptor, also had a neuroprotective effect and improved neurological function when it was delivered intravenously for 1 week (Delbary-Gossart et al., 2016). In the current study, we show that a single intranasal application of an siRNA to p75NTR that prevents the upregulation of the receptor immediately after the injury provided neuroprotection for at least 3 days following the injury. Taken together, these studies highlight the potential benefit of inhibiting p75NTR signaling as a therapeutic approach to prevent secondary progressive brain damage after TBI.
Blocking Proneurotrophins Is Neuroprotective Following TBI
The p75NTR is a multifunctional receptor and can mediate numerous cellular activities, depending on the cell context (Gentry et al., 2004). Importantly, the p75NTR often functions as a coreceptor. It interacts with Trk receptors to increase their affinity and selectivity for their neurotrophin ligands and under this circumstance promotes cell survival (Hempstead et al., 1991). Alternatively, the p75NTR can partner with a member of the sortilin family to bind proneurotrophins, which then stimulates apoptotic death signaling (Nykjaer et al., 2004). We have previously shown that a p75NTR/sortilin complex mediates neuronal death following seizures in response to elevated levels of proNGF (Le and Friedman, 2012). Although p75NTR has been shown to mediate apoptosis in injured neurons, this receptor can mediate other cellular functions (Bronfman and Fainzilber, 2004). In particular, it has been demonstrated that p75NTR can mediate astrocyte migration (Cragnolini et al., 2018) as well as inhibit proliferation during scar formation after injury (Cragnolini et al., 2009); therefore, it may not always be advantageous to inhibit this receptor in all circumstances. The proneurotrophin ligands proNGF and proBDNF are also increased in several areas of the brain following different types of injury (Volosin et al., 2006, 2008), including TBI (Alder et al., 2016; Delbary-Gossart et al., 2016). We and others found that proNGF is induced in astrocytes after seizures (Volosin et al., 2006, 2008) and TBI (Delbary-Gossart et al., 2016). In this study, we showed that proBDNF is also induced in astrocytes and increased in other cell types after CCI. With increased proBDNF in different cell types, it is unclear which of these cells may secrete the precursor form that promotes cell death or the cleaved form of BDNF to promote cell survival. Moreover, whether the proBDNF detected in astrocytes after injury is an attempt to take up and clear these proneurotrophins from the extracellular space to provide neuroprotection, or is being produced and secreted by the astrocytes to promote apoptosis of neurons following the injury, is a question that still needs to be addressed. Because these proneurotrophins are induced following injury and activate p75NTR-mediated apoptosis, we evaluated whether we could achieve neuroprotection by blocking these ligands using function-blocking antibodies, also by intranasal application. Intranasal delivery of peptides and proteins is an effective noninvasive method to provide therapeutic molecules, including antibodies, to the brain (Malerba et al., 2011). Although antibodies are large molecules, intranasal delivery of antibodies has been successfully used to block amyloid pathology in a mouse model of Alzheimer’s disease (Cattepoel et al., 2011). We found that blocking either proNGF or proBDNF with intranasal infusion of the appropriate antibody ameliorated both morphological and behavioral deterioration, comparable with the protection observed when blocking the upregulation of the receptor. These results are consistent with another study showing that intranasal delivery of recombinant human tissue plasminogen activator (tPA) decreases proBDNF levels by promoting its cleavage and improves functional recovery after TBI (Meng et al., 2014). Because the tPA-plasmin system can convert proneurotrophins to mature neurotrophins (Lee et al., 2001), it is likely that the levels of proNGF were also affected by tPA administration.
The induction of proneurotrophins and upregulation of p75NTR to promote apoptosis is a common feature of many different types of brain injury. Inhibiting this ligand/receptor axis therefore represents a reasonable target for therapeutic approaches to prevent or attenuate neuronal loss following TBI. The intranasal infusion of either siRNA to prevent receptor induction or antibodies to the proneurotrophin ligands is an effective, noninvasive way of gaining access for these reagents into the brain, and we have determined that these approaches are efficacious for promoting both morphological and functional rescue.
Summary
Mice with severe head trauma exhibit an increase in p75NTR expression in apoptotic neurons and deficit in cognition. The use of a noninvasive intranasal strategy to block the induction of p75NTR or its ligands provides neuroprotection and preserves sensorimotor function.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the New Jersey Commission for Brain Injury Research CBIR14IRG006 awarded to W. J. F. and CBIR17IRG019 awarded to S. W. L.
ORCID iDs
Hur D. Kanal https://orcid.org/0000-0002-6863-3651
Wilma J. Friedman https://orcid.org/0000-0002-3638-3504
References
- Akpan N., Serrano-Saiz E., Zacharia B. E., Otten M. L., Ducruet A. F., Snipas S. J., Liu W., Velloza J., Cohen G., Sosunov S. A., Frey W. H., 2nd, Salvesen , G. S., Connolly E. S., Jr., Troy C. M. (2011). Intranasal delivery of caspase-9 inhibitor reduces caspase-6-dependent axon/neuron loss and improves neurological function after stroke. J Neurosci, 31, 8894–8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder J., Fujioka W., Giarratana A., Wissocki J., Thakkar K., Vuong P., Patel B., Chakraborty T., Elsabeh R., Parikh A., Girn H. S., Crockett D., Thakker-Varia S. (2016). Genetic and pharmacological intervention of the p75NTR pathway alters morphological and behavioural recovery following traumatic brain injury in mice. Brain Inj, 30, 48–65. [DOI] [PubMed] [Google Scholar]
- Beattie M. S., Harrington A. W., Lee R., Kim J. Y., Boyce S. L., Longo F. M., Bresnahan J. C., Hempstead B. L., Yoon S. O. (2002). ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron, 36, 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfman F. C., Fainzilber M. (2004). Multi-tasking by the p75 neurotrophin receptor: Sortilin things out? EMBO Rep, 5, 867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarella C., Cayre M., Magalon K., Durlec P. (2008). Intranasal HB-EGF administration favors adult SVZ cell mobilization to demyelinated lesions in mouse corpus callosum. Dev Neurobiol, 68, 223–236. [DOI] [PubMed] [Google Scholar]
- Cattepoel S., Hanenberg M., Kulic L., Nitsch R. M. (2011). Chronic intranasal treatment with an anti-Aβ30-42 scFv antibody ameliorates amyloid pathology in a transgenic mouse model of Alzheimer’s disease. PLoS One, 6(4): e18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Sanberg P. R., Li Y., Wang L., Lu M., Willing A. E., Sanchez-Ramos J., Chopp M. (2001). Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke, 32, 2682–2688. [DOI] [PubMed] [Google Scholar]
- Cragnolini A. B., Huang Y., Gokina P., Friedman W. J. (2009). Nerve growth factor attenuates proliferation of astrocytes via the p75 neurotrophin receptor. Glia, 57, 1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragnolini A. B., Montenegro G., Friedman W. J., Mascó D. H. (2018). Brain-region specific responses of astrocytes to an in vitro injury and neurotrophins. Mol Cell Neurosci, 88, 240–248. [DOI] [PubMed] [Google Scholar]
- Davidson T. J., Harel S., Arboleda V. A., Prunell G. F., Shelanski M. L., Greene L. A., Troy C. M. (2004). Highly efficient small interfering RNA delivery to primary mammalian neurons induces microRNA-like effects before mRNA degradation. J Neurosci, 24, 10040–10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbary-Gossart S., et al. (2016). A novel inhibitor of p75-neurotrophin receptor improves functional outcomes in two models of traumatic brain injury. Brain, 139, 1762–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl M. A., Stahel P. F., Beauchamp K. M., Morgan S. J., Smith W. R., Shohami E. (2009). Mouse closed head injury model induced by a weight-drop device. Nat Protoc, 4, 1328–1337. [DOI] [PubMed] [Google Scholar]
- Frade J. M., Barde Y. A. (1998). Nerve growth factor: Two receptors, multiple functions. BioEssays, 20, 137–145. [DOI] [PubMed] [Google Scholar]
- Friedman W. J. (2000). Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci, 20, 6340–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry J. J., Barker P. A., Carter B. D. (2004). The p75 neurotrophin receptor: Multiple interactors and numerous functions. Prog Brain Res, 146, 25–39. [DOI] [PubMed] [Google Scholar]
- Grabenstatter H. L., Carlsen J., Raol Y. H., Yang T., Hund D., Cruz Del Angel Y., White A. M., Gonzalez M. I., Longo F. M., Russek S. J., Brooks-Kayal A. R. (2014). Acute administration of the small-molecule p75NTR ligand does not prevent hippocampal neuron loss or development of spontaneous seizures after pilocarpine-induced status epilepticus. J Neurosci Res, 92, 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington A. W., Leiner B., Blechschmitt C., Arevalo J. C., Lee R., Mörl K., Meyer , M., Hempstead B. L., Yoon S. O., Giehl K. M. (2004). Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A, 101, 6226–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. (1991). High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature, 350, 678–683. [DOI] [PubMed] [Google Scholar]
- Huang E. J., Reichardt L. F. (2003). Trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem, 72, 609–642. [DOI] [PubMed] [Google Scholar]
- Jin K., Xie L., Childs J., Sun Y., Mao X. O., Logvinova A., Greenberg D. A. (2003). Cerebral neurogenesis is induced by intranasal administration of growth factors. Ann Neurol, 53, 405–409. [DOI] [PubMed] [Google Scholar]
- Knowles J. K., Simmons D. A., Nguyen T. V., Vander Griend L., Xie Y., Zhang H., Yang T., Pollak J., Chang T., Arancio O., Buckwalter M. S., Wyss-Coray T., Massa S. M., Longo F. M. (2013). A small molecule p75NTR ligand prevents cognitive deficits and neurite degeneration in an Alzheimer’s mouse model. Neurobiol Aging, 34, 2052–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A. P., Friedman W. J. (2012). Matrix metalloproteinase-7 regulates cleavage of pro-nerve growth factor and is neuroprotective following kainic acid-induced seizures. J Neurosci, 32, 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R., Kermani P., Teng K. K., Hempsted B. L. (2001). Regulation of cell survival by secreted proneurotrophins. Science, 294, 1945–1948. [DOI] [PubMed] [Google Scholar]
- Loane D. J., Faden A. I. (2010). Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci, 31, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madathil S. K., Carlson S. W., Brelsfoard J. M., Ye P., D’Ercole A. J., Saatman K. E. (2013). Astrocyte-specific overexpression of insulin-like growth factor-1 protects hippocampal neurons and reduces behavioral deficits following traumatic brain injury in mice. PLoS One, 8(6), e67204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerba F., Paoletti F., Capsoni S., Cattaneo A. (2011). Intranasal delivery of therapeutic proteins for neurological diseases. Expert Opin Drug Deliv, 8, 1277–1296. [DOI] [PubMed] [Google Scholar]
- Meng Y., Chopp M., Zhang Y., Liu Z., An A., Mahmood A., Xiong Y. (2014). Subacute intranasal administration of tissue plasminogen activator promotes neuroplasticity and improves functional recovery following traumatic brain injury in rats. PLoS One, 9(9): e106238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathoo N., Narotam P. K., Agrawal D. K., Connolly C. A., van Dellen J. R., Barnett G. H., Chetty R. (2004). Influence of apoptosis on neurological outcome following traumatic cerebral contusion. J Neurosurg, 101, 233–240. [DOI] [PubMed] [Google Scholar]
- Nykjaer A., Lee R., Teng K. K., Jansen P. (2004). Sortilin is essential for proNGF-induced neuronal cell death. Nature, 427, 15–20. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W. (1989). The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci, 12, 252–255. [DOI] [PubMed] [Google Scholar]
- Raghupathi R., Graham D. I., McIntosh T. K. (2000). Apoptosis after traumatic brain injury. J Neurotraum, 17, 927–938. [DOI] [PubMed] [Google Scholar]
- Reichardt L. F. (2006). Neurotrophin-regulated signalling pathways. Philos Trans R Soc B Biol Sci, 361, 1545–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I. H. (2008). Traumatic brain injury: Recovery, prediction, and the clinician. Arch Phys Med Rehabil, 89, S1–S2. [DOI] [PubMed] [Google Scholar]
- Roux P. P., Colicos M. A., Barker P. A., Kennedy T. E. (1999). P75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci, 19, 6887–6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafidi J., Hammond T. R., Scafidi S., Ritter J., Jablonska B., Roncal M., Szigeti-Buck K., Coman D., Huang Y., McCarter R. J., Jr., Hyder F., Horvath T. L., Gallo V. (2014). Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature, 506, 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani A., Gölz C., Werner C., Schäfer M., Engelhard K., Thal S. C. (2015). Pro-neurotrophin binding to p75 neurotrophin receptor (p75NTR) is essential for brain lesion formation and functional impairment after experimental traumatic brain injury. J Neurotrauma, 32, 1599–1607. [DOI] [PubMed] [Google Scholar]
- Shi J., Longo F. M., Massa S. M. (2013). A small molecule p75NTR ligand protects neurogenesis after traumatic brain injury. Stem Cells, 31, 2561–2574. [DOI] [PubMed] [Google Scholar]
- Soblosky J. S., Colgin L. L., Chorney-Lane D., Davidson M. E., Carey J. F. (1997). Some functional recovery and behavioral sparing occurs independent of task-specific practice after injury to the rat’s sensorimotor cortex. Behav Brain Res, 89(1–2), 51–59. [DOI] [PubMed] [Google Scholar]
- Tep C., Lim T. H., Ko P. O., Getahun S., Ryu J. C., Goettl V. M., Massa S. M., Basso M., Longo F. M., Yoon S. O. (2013). Oral administration of a small molecule targeted to block proNGF binding to p75 promotes myelin sparing and functional recovery after spinal cord injury. J Neurosci, 33, 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne R. G., Pronk G. J., Padmanabhan V., Frey W. H. (2004). Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience, 127, 481–496. [DOI] [PubMed] [Google Scholar]
- Tian L., Guo R., Yue X., Lv Q., Ye X., Wang Z., Chen Z., Wu B., Xu G., Liu X. (2012). Intranasal administration of nerve growth factor ameliorate β-amyloid deposition after traumatic brain injury in rats. Brain Res, 1440, 47–55. [DOI] [PubMed] [Google Scholar]
- Troy C. M., Friedman J. E., Friedman W. J. (2002). Mechanisms of p75-mediated death of hippocampal neurons: Role of caspases. J Biol Chem, 277, 34295–34302. [DOI] [PubMed] [Google Scholar]
- Volosin M., Song W., Almeida R. D., Kaplan D. R., Hempstead B. L., & Friedman W. J. (2006). Interaction of Survival and Death Signaling in Basal Forebrain Neurons: Roles of Neurotrophins and Proneurotrophins. J Neurosci, 26(29), 7756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M., Trotter C., Cragnolini A., Kenchappa R. S., Light M., Hempstead B. L., Carter B. D., Friedman W. J. (2008). Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J Neurosci, 28, 9870–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Chen X., Hu C., Li J., Yu Z., Cai W. (2010). Transplantation of neural stem cells expressing hypoxia-inducible factor-1α (HIF-1α) improves behavioral recovery in a rat stroke model. J Clin Neurosci, 17, 92–95. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Loane D. J., Murray M. G., Stoica B. A., Faden , A. I. (2012). Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J Neurotrauma, 29(15), 2475–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]