Abstract
Background:
Several treatment options are available for stable massive rotator cuff tears, including partial repair with or without tissue augmentation, tendon transfer, superior capsular reconstruction (SCR), and reverse shoulder arthroplasty.
Purpose/Hypothesis:
The purpose of this study was to compare the outcomes and effectiveness of partial rotator cuff repair with SCR using the long head of the biceps tendon (PRCR-SCRB) and SCR with a tensor fasciae latae autograft (SCRTF) for the treatment of rotator cuff tears with severe fatty degeneration. The hypothesis of this study was that SCRTF would be superior to PRCR-SCRB in functional and anatomic outcomes.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A total of 26 consecutive patients with massive and fatty degenerative rotator cuff tears were treated surgically. Patients were divided into either the PRCR-SCRB group (n = 14) or SCRTF group (n = 12). Functional outcomes were assessed at final follow-up, and the acromiohumeral distance (AHD) was measured.
Results:
All functional scores significantly improved in both groups at final follow-up. The PRCR-SCRB group showed better overall outcomes in terms of the visual analog scale for pain; American Shoulder and Elbow Surgeons score; and Quick Disabilities of the Arm, Shoulder and Hand, but these differences were not statistically significant. Better outcomes were found for only the AHD for the PRCR-SCRB group without statistical significance (P = .4). No statistical difference was found in terms of retear rate.
Conclusion:
PRCR-SCRB had comparable outcomes and improvement in AHD compared with SCRTF without the need for additional graft harvesting.
Keywords: massive rotator cuff tear, fatty degeneration, superior capsular reconstruction, tensor fasciae latae, long head of the biceps tendon, rotator cuff repair
Massive rotator cuff tears are classified as reparable or irreparable. In reparable tears, the surgeon is able to cover all the exposed footprint to rebuild the normal anatomy of the rotator cuff.2,6 Unrepaired rotator cuffs could cause permanent pain and loss of function and lead to rotator cuff arthropathy if not treated.
Massive rotator cuff tears are often difficult to repair because of muscle atrophy and fatty degeneration. Thus, patients are not satisfied with the results, and the retear rate can be as high as 94.4%.13,19,23 In long-standing cases of massive rotator cuff tears, bringing together the torn edges without causing inappropriate tension on the repaired tissue is almost impossible.14,23 Thus, massive rotator cuff tears are secondarily classified as stable (nonpseudoparalytic) or unstable (pseudoparalytic), which is important for the type of surgery performed.21,28
Several treatment options including rotator cuff medialization, partial repair with or without tissue augmentation, tendon transfer, superior capsular reconstruction (SCR), and reverse arthroplasty can be performed when stable massive rotator cuff tears occur, depending on patient age, type of tendon involved, and accompanying arthritis.1,7,13,14,16,19
SCR has been recently presented as a possible alternative in treating irreparable rotator cuff tears.17 Patients with irreparable rotator cuff tears have a defect of the superior shoulder capsule, which plays a role in superior stability of the shoulder joint. SCR aims to restore superior glenohumeral stability and function in the shoulder joint in case of irreparable rotator cuff tears.8 The graft used in the original procedure is a tensor fasciae latae (TFL) autograft that is attached medially to the superior glenoid and laterally to the greater tuberosity.17 However, 2 major problems exist with this treatment option. The first is the viability of the graft after 2 years.9 Although early results of SCR are successful, the long-term results are not as satisfactory, as revealed in recent studies.14,28 The second is the need for either another operative procedure for TFL harvesting or the additional cost to use a dermal graft as an alternative. To overcome these problems, the long head of the biceps tendon (LHBT) has been proposed for use in SCR with partial rotator cuff repair.
The purpose of this study was to compare the outcomes and effectiveness of partial rotator cuff repair with SCR using the LHBT (PRCR-SCRB) and SCR with an autogenous TFL graft (SCRTF) for rotator cuff tears with severe fatty degeneration. The hypothesis of this study was that SCRTF would be superior to PRCR-SCRB in functional and anatomic outcomes.
Methods
Patient Enrollment
In this retrospective comparative study, 26 consecutive patients (mean age, 63.7 years; range, 56-73 years) with stable massive rotator cuff tears were treated surgically between 2016 and 2017. Ethical approval was given for this retrospective cohort study by an institutional review board, and informed consent was obtained. All 26 patients had nonpseudoparalytic massive rotator cuff tears, as evidenced on magnetic resonance imaging (MRI). All patients had fatty degeneration of the supraspinatus tendon above grade 2. None of the patients had chronic pseudoparalysis for more than 4 weeks. Among the cohort, 14 patients underwent arthroscopic PRCR-SCRB (PRCR-SCRB group). The remaining 12 patients underwent arthroscopic SCRTF (SCRTF group). The mean follow-up was 30.9 months (range, 18-40 months).
To assess functional outcomes, visual analog scale (VAS) for pain; American Shoulder and Elbow Surgeons (ASES); and Quick Disabilities of the Arm, Shoulder and Hand (QuickDASH) scores were collected preoperatively and at final follow-up. Forward flexion, external rotation at the side, external rotation at 90°, and internal rotation behind the back were also measured and evaluated with a goniometer by an author (G.F.), who was blinded to group allocation (Table 1).
TABLE 1.
Preoperative Demographics and Characteristicsa
| PRCR-SCRB (n = 14) | SCRTF (n = 12) | |
|---|---|---|
| Age, y | 64.6 ± 8.4 | 62.5 ± 6.5 |
| Mean follow-up, mo | 28 | 32 |
| Intraoperative supraspinatus tear size, mm | ||
| Anterior-posterior | 36.8 ± 6.5 | 35.5 ± 5.5 |
| Retraction | 40.2 ± 5.6 | 39.6 ± 8.5 |
| Preoperative clinical score | ||
| VAS for pain | 8.5 ± 3.5 | 8.0 ± 2.5 |
| ASES | 46.2 ± 16.2 | 48.5 ± 15.5 |
| QuickDASH | 52.5 ± 12.8 | 53.6 ± 15.2 |
| Preoperative shoulder ROM | ||
| Forward flexion, deg | 135.0 ± 15.5 | 136.2 ± 24.4 |
| External rotation at side, deg | 35.0 ± 1.0 | 38.0 ± 15.0 |
| External rotation at 90°, deg | 60.5 ± 22.0 | 62.5 ± 15.0 |
| Internal rotation behind back | T11 ± 2.5 | T11 ± 3.0 |
aValues are presented as mean ± SD unless otherwise specified. All P values are not significant. ASES, American Shoulder and Elbow Surgeons; NS, not significant; QuickDASH, Quick Disabilities of the Arm, Shoulder and Hand; ROM, range of motion; VAS, visual analog scale.
To evaluate fatty degeneration, the Goutallier classification was used preoperatively and performed by a blinded musculoskeletal radiologist who was not involved in the current study.5 All 4 rotator cuff muscles, including the teres minor, were evaluated. Furthermore, the mean global fatty degeneration index of the supraspinatus, infraspinatus, subscapularis, and teres minor was calculated10 (Table 2).
TABLE 2.
Fatty Degenerationa
| PRCR-SCRB | SCRTF | P | |
|---|---|---|---|
| Preoperative global fatty degeneration index | 2.6 ± 0.5 | 2.5 ± 0.8 | .62 |
| Supraspinatus | 3.8 ± 0.6 | 3.6 ± 0.8 | .22 |
| Infraspinatus | 2.6 ± 0.5 | 2.5 ± 0.6 | .33 |
| Subscapularis | 0.8 ± 0.4 | 1.0 ± 0.2 | .28 |
| Teres minor | 1.2 ± 0.6 | 1.4 ± 0.8 | .24 |
aValues are presented as mean ± SD.
The acromiohumeral distance (AHD) was also measured on preoperative and postoperative anterior-posterior radiographs, which were taken while standing with normal deltoid activation.
The anterior-posterior dimension and medial retraction of the torn rotator cuff were measured using a probe with 5-mm markings (AR-10010; Arthrex) after debridement of the torn end. Healing of the rotator cuff tear was monitored throughout the study by ultrasound. At final follow-up, all patients underwent MRI to assess for failure of the repair site.
Surgical Procedure
The senior author (B.K.) performed all surgical procedures. The patient was placed in the beach-chair position under general anesthesia with a regular arm-holding system (Trimano; Arthrex). Using the posterior portal as the viewing portal, intra-articular abnormalities, including biceps lesions, were evaluated. After glenohumeral inspection, subacromial decompression was performed, and acromioplasty was carried out if the patient had a Neer type 3 acromion and osteophytes compressing the rotator cuff during the Hawkins test while undergoing arthroscopic surgery.
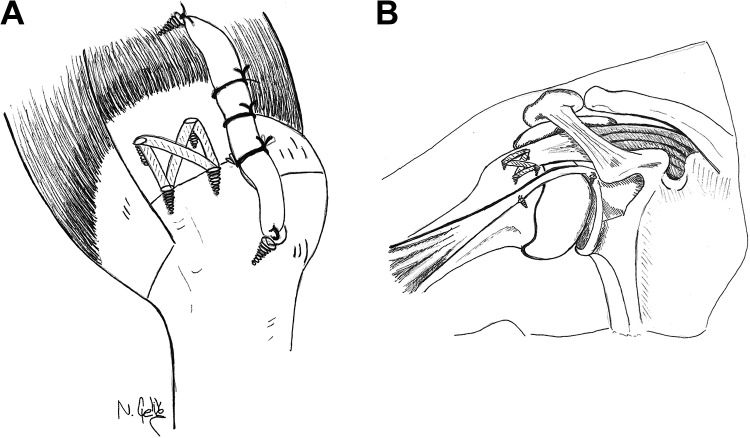
In the PRCR-SCRB group, degenerative supraspinatus tendons were debrided and released from both the glenoid and subacromial area. Footprints were medialized up to 1 cm by resecting the articular cartilage. In cases of subscapularis tears, repair was performed using one 5.5-mm SwiveLock anchor (Arthrex). For bridging with an autogenous LHBT in partial superior reconstruction, the LHBT was attached to its original insertion on the superior glenoid by a 3.5-mm PushLock anchor (Arthrex) using the same technique as that used in superior labrum anterior-posterior (SLAP) repair. At 45° of shoulder abduction, the distal parts of the LHBT were attached to the humeral head at the anterior footprint of the supraspinatus tendon with 5.5-mm SwiveLock anchors to obtain adequate tension (Figure 1A).
Figure 1.
(A) Bridging of the long head of the biceps tendon between the glenoid and humeral head as well as the medialized footprint. (B) Partial rotator cuff repair with superior capsular reconstruction using the long head of the biceps tendon.
After preparing the bleeding surface on the medialized footprint, supraspinatus repair was performed using 2 medial sutures and 2 lateral 5.5-mm SwiveLock knotless anchors. The first 5.5-mm double-loaded SwiveLock suture anchor was inserted at the anterior site of the prepared bone bed and the second anchor at the posterior site. The limbs of the suture anchors were passed through the rotator cuff tendon, and matching sutures were passed through the LHBT. Finally, sutures were crossed and fixed laterally using two 5.5-mm SwiveLock anchors (Figure 1B).
In the SCRTF group, a vertical skin incision was performed over the lateral thigh around the greater trochanter of the femur, and a section of the fascia lata 3 times the size of the superior capsular defect was harvested. Graft optimization consisted of rolling the fascia lata 3 times (mean graft size after folding was 5.5 cm mediolaterally and 3.5 cm anteroposteriorly) to ensure a minimal thickness of 8 mm and stitching to keep it from unfurling.
The graft was inserted into the subacromial space through the lateral portal and then the medial side of the fascia lata attached to the superior glenoid using 2 knotless 3.5-mm PushLock anchors with 2 No. 2 FiberWire nonabsorbable sutures (Arthrex), which were inserted into the superior glenoid at the 10- to 11-o’clock and 11- to 12-o’clock positions and the 1- to 2-o’clock and 12- to 1-o’clock positions for the right and left shoulders, respectively.
The fascia lata graft was attached to the rotator cuff footprint on the greater tuberosity by using a double-row technique and a suture bridge at 45° of shoulder abduction. To achieve this, 2 double-loaded 5.5-mm SwiveLock anchors were placed medially at the edge of the articular cartilage and laterally 5 to 10 mm inferior to the highest point of the greater tuberosity. The sutures were placed through the fascia lata by using either a suture shuttle (SutureLasso; Arthrex) or a suture-passing device (Scorpion suture passer; Arthrex). Finally, side sutures were passed and tied through the infraspinatus tendon and within the graft (Figure 2).
Figure 2.
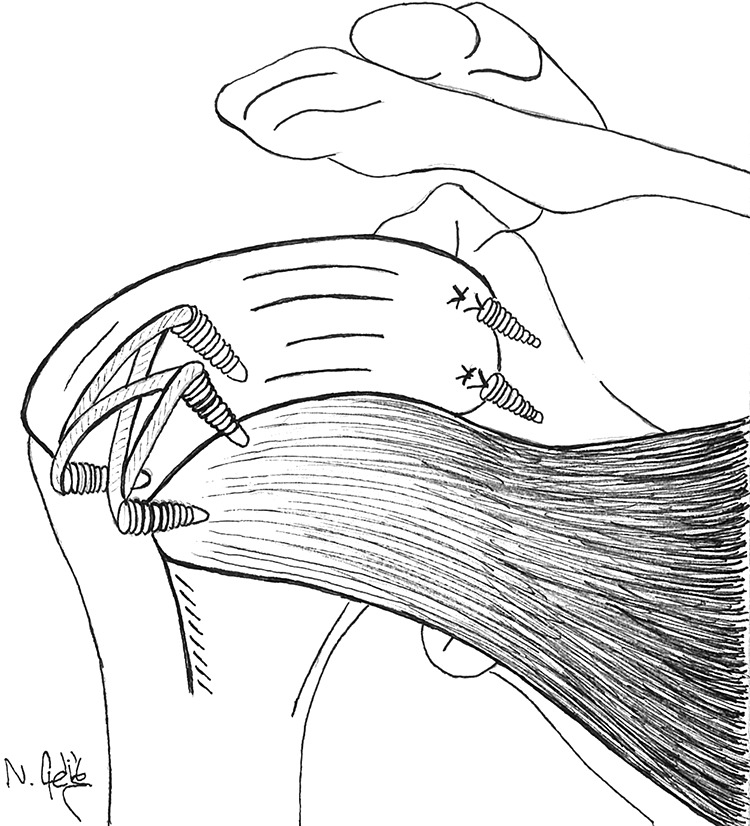
Superior capsular reconstruction with a tensor fasciae latae graft.
In both groups, if there was a partial tear of the subscapularis tendon, it was repaired after tendon debridement and preparation of the lesser tubercle footprint. After preparing the bleeding surface, repair was performed using one 5.5-mm SwiveLock knotless anchor.
During the postoperative period for both groups, an abduction pillow (Ottobock) was used for immobilization for 6 weeks after reconstruction. After the immobilization period, passive- and active-assisted exercises were initiated. Then, 8 weeks postsurgically, patients began to perform exercises to strengthen the rotator cuff and scapular stabilizers.
Statistical Analysis
All statistical analyses were performed using SPSS Version 21.0 (IBM). The level of significance was set at P < .05. The Mann-Whitney U test was used to compare the 2 groups. The Wilcoxon signed rank test was conducted to evaluate differences between the preoperative and postoperative variables.
Results
Functional Outcomes
In the PRCR-SCRB group, all functional scores significantly improved at final follow-up (Table 3). The preoperative VAS for pain score was 8.5 ± 3.5, which decreased postoperatively to 1.4 ± 0.8 (P = .001). The ASES score increased from 46.2 ± 16.2 to 85.2 ± 12.4 (P = .005), and the QuickDASH score improved from 52.5 ± 12.8 to 12.6 ± 18.0 (P = .012). In the SCRTF group, the preoperative VAS for pain score was 8.0 ± 2.5, which decreased postoperatively to 1.6 ± 2.4 (P = .001). The ASES score increased from 48.5 ± 15.5 to 82.6 ± 15.0 (P = .02), and the QuickDASH score improved from 53.6 ± 15.2 to 12.5 ± 5.0 (P = .001). At final follow-up, there was no significant difference in VAS, ASES, and QuickDASH scores between groups (Table 4).
TABLE 3.
Comparison of Initial and Final Visit Dataa
| Initial Visit | Final Visit | P | |
|---|---|---|---|
| PRCR-SCRB | |||
| VAS for pain | 8.5 ± 3.5 | 1.4 ± 0.8 | .001 |
| ASES | 46.2 ± 16.2 | 85.2 ± 12.4 | .005 |
| QuickDASH | 52.5 ± 12.8 | 12.6 ± 18.0 | .012 |
| Shoulder ROM | |||
| Forward flexion, deg | 135.0 ± 15.5 | 162.5 ± 32.0 | .03 |
| External rotation at side, deg | 35.0 ± 1.0 | 52.8 ± 25.0 | .02 |
| External rotation at 90°, deg | 60.5 ± 22.0 | 76.5 ± 16.0 | NS |
| Internal rotation behind back | T11 ± 2.5 | T10 ± 3.0 | NS |
| AHD, mm | 7.0 ± 1.5 | 10.2 ± 2.5 | .02 |
| SCRTF | |||
| VAS for pain | 8.0 ± 2.5 | 1.6 ± 2.4 | .001 |
| ASES | 48.5 ± 15.5 | 82.6 ± 15.0 | .02 |
| QuickDASH | 53.6 ± 15.2 | 12.5 ± 5.0 | .001 |
| Shoulder ROM | |||
| Forward flexion, deg | 136.2 ± 24.4 | 160.0 ± 14.5 | .02 |
| External rotation at side, deg | 38.0 ± 15.0 | 50.3 ± 23.4 | .03 |
| External rotation at 90°, deg | 62.5 ± 15.0 | 68.0 ± 12.5 | NS |
| Internal rotation behind back | T11 ± 3.0 | T10 ± 2.0 | NS |
| AHD, mm | 7.8 ± 2.8 | 9.3 ± 3.0 | .04 |
aValues are presented as mean ± SD. AHD, acromiohumeral distance; ASES, American Shoulder and Elbow Surgeons; NS, not significant; QuickDASH, Quick Disabilities of the Arm, Shoulder and Hand; ROM, range of motion; VAS, visual analog scale.
TABLE 4.
Final Outcomesa
| PRCR-SCRB | SCRTF | |
|---|---|---|
| Clinical score | ||
| VAS for pain | 1.4 ± 0.8 | 1.6 ± 2.4 |
| ASES | 85.2 ± 12.4 | 82.6 ± 15.0 |
| QuickDASH | 12.6 ± 18.0 | 12.5 ± 5.0 |
| Shoulder ROM | ||
| Forward flexion, deg | 162.5 ± 32.0 | 160.0 ± 14.5 |
| External rotation at side, deg | 52.8 ± 25.0 | 50.3 ± 23.4 |
| External rotation at 90°, deg | 76.5 ± 16.0 | 68.0 ± 12.5 |
| Internal rotation behind back | T10 ± 3.0 | T10 ± 2.0 |
| AHD, mm | 10.2 ± 2.5 | 9.3 ± 3.0 |
| Retear rate, n (%) | 3/14 (21) | 2/12 (17) |
aValues are presented as mean ± SD unless otherwise specified. All P values are nonsignificant. AHD, acromiohumeral distance; ASES, American Shoulder and Elbow Surgeons; NS, not significant; QuickDASH, Quick Disabilities of the Arm, Shoulder and Hand; ROM, range of motion; VAS, visual analog scale.
Range of Motion
In the PRCR-SCRB group, shoulder forward flexion and external rotation at the side improved postoperatively (from 135.0° ± 15.5° to 162.5° ± 32.0° [P = .03] and from 35.0° ± 1.0° to 52.8° ± 25.0° [P = .02], respectively), but external rotation at 90° and internal rotation behind the back were not significantly different (from 60.5° ± 22.0° to 76.5° ± 16.0° and from T11 ± 2.5 to T10 ± 3.0, respectively).
In the SCRTF group, shoulder forward flexion and external rotation at the side improved postoperatively (from 136.2° ± 24.4° to 160.0° ± 14.5° [P = .02] and from 38.0° ± 15.0° to 50.3° ± 23.4° [P = .03], respectively), but external rotation at 90° and internal rotation behind the back were not significantly different (from 62.5° ± 15.0° to 68.0° ± 12.5° and from T11 ± 3.0 to T10 ± 2.0, respectively) (Table 3). At final follow-up, there was no significant difference in range of motion (ROM) between the groups (Table 4).
Anatomic Findings
The AHD significantly increased after surgery in both groups. In the PRCR-SCRB group, the AHD increased from 7.0 ± 1.5 mm before surgery to 10.2 ± 2.5 mm at final follow-up (P = .02). In the SCRTF group, the AHD increased from 7.8 ± 2.8 mm before surgery to 9.3 ± 3.0 mm at final follow-up (P = .04) (Table 3). At final follow-up, the PRCR-SCRB group showed better outcomes in terms of the AHD without statistical significance (P = .4). No statistical difference was found in terms of the retear rate detected by MRI at final follow-up (Table 4).
Discussion
Postoperative pain and functional scores were significantly improved in both groups compared with preoperative values. However, the 2 groups did not show any difference in terms of postoperative ROM, functional scores, or retear rates. We also found that PRCR-SCRB did not significantly improve the AHD more than SCRTF, which rejected the hypothesis of the study.
Migration of the humeral head superiorly is a result of rotator cuff tear arthropathy secondary to rotator cuff failure.15 Young and active patients who sustain irreparable rotator cuff tears are not appropriate candidates for reverse total shoulder replacement. The superior capsule is known as the key component of static stabilization.15,28 This can be the mechanism of action of SCR on relieving severe pain and improving disability from irreparable massive rotator cuff tears. In a cadaveric study, Ishihara et al10 revealed that a defect in the superior capsule, as observed in massive rotator cuff tears, can advance glenohumeral translation in all planes. This means that any change in static stabilization of the shoulder joint caused by the lack of a superior capsule can lead to the progression of rotator cuff tear arthropathy.10
Recent studies14,15,17,27 have reported clinical improvement in patients with irreparable massive rotator cuff tears after SCR by using a TFL autograft and dermal allograft. In a prospective observational study of 24 patients who underwent SCR with a TFL graft, Mihata et al15 found that all patients experienced significant pain relief, improvement in ROM, and enhancement of strength with improved ASES scores at 3-year follow-up. Considering the earlier literature, arthroscopic SCR with a TFL graft is an accepted surgical option for patients with massive rotator cuff tears, with short-term improvements in pain, ROM, and function. Although early results are satisfactory, late results of the graft’s viability because of its avascularity remain a problem.
Across all studies, the combined clinical and radiographic failure and retear rates of SCR procedures ranged from 3.4% to 36.1% in 44 of 350 shoulders.2 A total of 41 patients across all studies underwent revision, ranging from 0% to 10.4%. Of those patients for whom the location of the graft tear was reported, 4 had failure of the posterior aspect of the graft with separation from and subsequent tearing of the infraspinatus, 27 had graft failure in the lateral anchor area, 6 showed midsubstance graft failure, and 4 had graft failure at the medial anchors.2,27
A vascular or local graft could be used to resolve the abovementioned problem. An additional problem is the need for another incision for harvesting the TFL graft.12,15,27 The LHBT could be an option for SCR, which may overcome morbidity from a second operative procedure. The use of the LHBT instead of the autologous TFL or dermal allograft has many positive effects.3,11,22 The LHBT can be used easily during arthroscopic shoulder surgery because of the proximity of this tendon, and donor site morbidity can be avoided.18,20,25 The most important complaint in those patients was postoperative pain, which could be seen 6 to 8 weeks after surgery. The study by El-Shaar et al4 revealed that both the LHBT and TFL are equal when it comes to the biomechanical aspect of the SCR procedure. To increase the survival of degenerative rotator cuff repair, the LHBT could be used.4 Several studies11,18,20,24–26 investigated the use of the LHBT for augmentation of massive rotator cuff tear repair. The LHBT is not as wide as the TFL or dermal allograft and may not be suitable for all massive tears if the gap is too large. In that case, the LHBT could be used with partial rotator cuff repair.
Most patients with massive irreparable rotator cuff tears have problems with the LHBT in which its attachment to the glenoid is not sufficiently tight. A large proportion of patients have a loose glenoid anchor because of degeneration and aging. To achieve better outcomes of capsular reconstruction, both the glenoid and humeral head portions should be fixed and tensioned at 45° of shoulder abduction. This was the weakest part of previous studies14–16 that emphasized the use of the LHBT either for SCR or rotator cuff repair augmentation. All prior studies fixed the humeral portion without fixing the glenoid and expected the glenoid attachment to be healthy. After fixation of only the humeral head, the glenoid anchor of the LHBT could be loose. Yet, we have to keep in mind that many patients with massive rotator cuff tears have a ruptured or severely degenerated LHBT that would preclude its use for SCR.
We demonstrated an arthroscopic SCR technique with the LHBT and medialized repair of the fatty degenerative supraspinatus tendon. In the PRCR-SCRB group, we preferred the LHBT instead of a fascia lata autograft, which Mihata et al15 originally reported. Our technique of bridging the LHBT between the glenoid and humeral head and also partially repaired tendon created a downward force to the humeral head; thus, AHD calculations showed better down-migration of the humeral head when we compared it with preoperative values. When we compared PRCR-SCRB and SCRTF, the AHD was significantly improved in both groups compared with preoperatively. PRCR-SCRB maintained AHD better than SCRTF, and both techniques provided pain relief and prevented further degenerative wear.
This study has several limitations. There was no randomization between groups. Additionally, the number of patients was small, and a larger number of patients may show significant differences between groups. There were no strength measurements, and the minimal clinically important difference for the AHD is unknown. LHBT's were tenodized at both glenoid and humeral head which put stress on tendons. This stress might cause pain along the biceps muscle at an early stage after surgery. Further evaluation is needed to distinguish the origin of postoperative pain. Another limitation is that, in the case of a completely torn LHBT, this procedure cannot be indicated. To perform this technique, a preoperative evaluation of the LHBT by MRI or an intraoperative evaluation of the glenohumeral joint should be performed. Finally, the follow-up period was short, and a longer follow-up will be needed to see whether the good clinical results of both groups are maintained.
Conclusion
Postoperative pain and functional scores were significantly improved in both groups compared with preoperative values. The 2 groups did not differ in terms of postoperative ROM, functional scores, or retear rates. PRCR-SCRB maintained AHD better than SCRTF. SCRTF offered no superior outcomes over PRCR-SCRB without the need for additional operative procedures for graft harvesting.
Footnotes
Final revision submitted January 14, 2020; accepted February 12, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Acibadem University (No. 2018-6/1).
References
- 1. Burkhart S, Denard J, Adams R, Brady C, Hartzler U. Arthroscopic superior capsular reconstruction for massive irreparable rotator cuff repair. Arthrosc Tech. 2016;5:e1407–e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Catapano M, de Sa D, Ekhtiari S, Lin A, Bedi A, Lesniak BP. Arthroscopic superior capsular reconstruction for massive, irreparable rotator cuff tears: a systematic review of modern literature. Arthroscopy. 2019;35(4):1243–1253. [DOI] [PubMed] [Google Scholar]
- 3. Cho S, Yi W, Rhee G. Arthroscopic biceps augmentation for avoiding undue tension in repair of massive rotator cuff tears. Arthroscopy. 2009;25:183–191. [DOI] [PubMed] [Google Scholar]
- 4. El-Shaar R, Soin S, Nicandri G, Maloney M, Voloshin I. Superior capsular reconstruction with a long head of the biceps tendon autograft: a cadaveric study. Orthop J Sports Med. 2018;6(7):2325967118785365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT scans. Clin Orthop Relat Res. 1994;(304):78–83. [PubMed] [Google Scholar]
- 6. Han F, Kong H, Hasan Y, Ramruttun K, Kumar P. Superior capsular reconstruction for irreparable supraspinatus tendon tears using the long head of biceps: a biomechanical study on cadavers. Orthop Traumatol Surg Res. 2019;105(2):257–263. [DOI] [PubMed] [Google Scholar]
- 7. Hermanowicz K, Góralczyk A, Malinowski K, Jancewicz P, Domżalski ME. Long head biceps tendon: natural patch for massive irreparable rotator cuff tears. Arthrosc Tech. 2018;7(5):e473–e478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4:e637–e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirahara M, Andersen J, Panero J. Superior capsular reconstruction: clinical outcomes after minimum 2-year follow-up. Am J Orthop. 2017;46(6):266–278. [PubMed] [Google Scholar]
- 10. Ishihara Y, Mihata T, Tamboli M, et al. Role of the superior shoulder capsule in passive stability of the glenohumeral joint. J Shoulder Elbow Surg. 2014;23:642–648. [DOI] [PubMed] [Google Scholar]
- 11. Ji JH, Shafi M, Jeong JJ, Park SE. Arthroscopic repair of large and massive rotator cuff tears using the biceps incorporating technique: mid-term clinical and anatomical results. Eur J Orthop Surg Traumatol. 2014;24(8):1367–1374.24085654 [Google Scholar]
- 12. Kim S, Lee J, Park I, et al. Arthroscopic in situ superior capsular reconstruction using the long head of the biceps tendon. Arthrosc Tech. 2018;7(2):e97–e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45(13):3149–3157. [DOI] [PubMed] [Google Scholar]
- 14. Mihata T. Editorial commentary. Superior capsule reconstruction: grafts for superior capsular reconstruction must be thick and stiff. Arthroscopy. 2019;35(8):2535–2536. [DOI] [PubMed] [Google Scholar]
- 15. Mihata T, Lee TQ, Hasegawa A, et al. Superior capsule reconstruction for reinforcement of arthroscopic rotator cuff repair improves cuff integrity. Am J Sports Med. 2019;47(2):379–388. [DOI] [PubMed] [Google Scholar]
- 16. Mihata T, Lee TQ, Hasegawa A, et al. Arthroscopic superior capsule reconstruction can eliminate pseudoparalysis in patients with irreparable rotator cuff tears. Am J Sports Med. 2018;46(11):2707–2716. [DOI] [PubMed] [Google Scholar]
- 17. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459–470. [DOI] [PubMed] [Google Scholar]
- 18. Obma PR. Free biceps tendon auto graft to augment arthroscopic rotator cuff repair. Arthrosc Tech. 2013;2:e441–e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pandey R, Tafazal S, Shyamsundar S, Modi A, Singh HP. Outcome of partial repair of massive rotator cuff tears with and without human tissue allograft bridging repair. Shoulder Elbow. 2017;9:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park SR, Sun DH, Kim J, Lee HJ, Kim JB, Kim YS. Is augmentation with the long head of the biceps tendon helpful in arthroscopic treatment of irreparable rotator cuff tears? J Shoulder Elbow Surg. 2018;27(11):1969–1977. [DOI] [PubMed] [Google Scholar]
- 21. Patte D. Classification of rotator cuff lesions. Clin Orthop Relat Res. 1990;(254):81–86. [PubMed] [Google Scholar]
- 22. Pauzenberger L, Hurley ET. Editorial commentary. Finally, something positive about the long head of the biceps tendon? Shoulder superior capsular reconstruction. Arthroscopy. 2018;34(9):2601–2603. [DOI] [PubMed] [Google Scholar]
- 23. Petri M, Warth RJ, Horan MP, Greenspoon JA, Millett PJ. Outcomes after open revision repair of massive rotator cuff tears with biologic patch augmentation. Arthroscopy. 2016;32:1752–1760. [DOI] [PubMed] [Google Scholar]
- 24. Rhee SM, Oh JH. Bridging graft in irreparable massive rotator cuff tears: autogenic biceps graft versus allogenic dermal patch graft. Clin Orthop Surg. 2017;9(4):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511–1518. [DOI] [PubMed] [Google Scholar]
- 26. Sano H, Mineta M, Kita A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310–316. [DOI] [PubMed] [Google Scholar]
- 27. Sochacki KR, McCulloch PC, Lintner DM, Harris JD. Superior capsular reconstruction for massive rotator cuff tear leads to significant improvement in range of motion and clinical outcomes: a systematic review. Arthroscopy. 2019;35(4):1269–1277. [DOI] [PubMed] [Google Scholar]
- 28. Tokish JM, Alexander TC, Kissenberth MJ, Hawkins RJ. Pseudoparalysis: a systematic review of term definitions, treatment approaches, and outcomes of management techniques. J Shoulder Elbow Surg. 2017;26:e177–e187. [DOI] [PubMed] [Google Scholar]