Abstract
Wound healing in the Sunda porcupine is believed to occur quickly, although the wound is large and severe. Wound enclosure involves many processes to restore the lost or damaged skin structure where conjugated polysaccharide-protein and collagen, as the main components deposited in wound tissue to restore it. The aim of this study was to evaluate alteration of polysaccharide contents and collagen in untreated full-thickness wound healing in the thoracodorsal and lumbosacral regions in the Sunda porcupines. Histological analysis was performed by periodic acid Schiff, alcian blue pH 2.5, picrosirius red staining method and Low Vacuum Scanning Electron Microscope (LV-SEM) imaging to obtain the fundamental data of healing process. Wound healing began with re-epithelization followed by progressive wound contraction with 4 overlapping stages in about 30–50 days until the wound closed (21–30 days in thoracodorsal and 30–50 days in lumbosacral). Neutral polysaccharide was more widely distributed compared to the acid polysaccharide in almost all stages of wound healing. The ratio of collagen I to III appeared to be higher in the thoracodorsal region than the lumbosacral region during healing process. LV-SEM imaging showed changes in connective tissue structure in the wound border and granulation tissue which appeared abundant and mixed of thin and thick fiber. In conclusion, cutaneous full thickness wound healing in the Sunda porcupine occurred faster in the thoracodorsal region, which might be correlated to the role of neutral polysaccharide and a high ratio of collagen I to III.
Keywords: collagen, granulation tissue, matrix extracellular, neutral polysaccharide, re-epithelization
The acute skin wound healing is a process to restore the lost or damaged tissue through several stages in predicted time. The wound healing process involves several mediators, an extracellular matrix component, cutaneous cells and blood cells in 4 overlapping stages: hemostatic, inflammation, tissue formation (migration and proliferation) and remodeling [57, 59]. The phase of new tissue formation is characterized by the formation of granulation tissue. Intense fibroblast proliferation and migration occur in order to synthesize the new extracellular matrix in the damaged tissue area [28, 61, 65]. Once in the damaged tissue area, fibroblasts synthesize new matrix elements such as proteoglycans, glycosaminoglycans (GAGs) and collagens, the main components of the extracellular matrix, which are deposited in the damaged area to replace the initial provisional matrix, comprised initially of fibrin [9, 28, 65]. Proteoglycans and GAGs are the conjugated polysaccharide to protein or amino acid chain presented in skin to fill mostly interstitial space between tissue and protein fibers [35]. The most abundant proteoglycans and GAGs in skin are hyaluronic acid, decorin, and versican [11, 49]. Hyaluronic acid is known to play a crucial role in wound healing, such as facilitated inflammation, cell migration, proliferation, and collagen deposition [13].
The existence or ration between certain collagen types affect the function of fibroblast. The study conducted by Volk et al. [56] exhibited that collagen III deficient of mice shows increasing myofibroblast differentiation, resulting in stronger wound contraction. In addition, it has been reported that the mice show abnormality of fibrillogenesis of collagen type I in the skin and other organs, and that collagen type III may play a role in regulating collagen type I synthesis [38]. Scarless wound healing in some fetus of small and large mammals show a high ratio of collagen type III to collagen type I [6, 10, 16, 43].
A simple observation in the captivity of the Sunda porcupine (Hystrix javanica) exhibit a quick enclosure of large and severe wounds with only small scar formation. This observation was performed spontaneously with no controlled environment, resulting in asepsis and non-steril conditions, thus exposure of infectious agents around the animals potentially disturbs the healing. Although in those condition, the wound healing in the Sunda porcupine occurs well.
Fundamental and comprehensive data of morphological wound healing in the Sunda porcupine is important to get a better understanding of the wound healing process. There were no reports about the study of polysaccharide content and collagen composition in the cutaneous wound healing process in the Sunda porcupine. The aim of this study is to evaluate and analyze the changes of polysaccharide contents, as well as structure and composition of collagen (types I and III) in the full-thickness skin wound healing process in the Sunda porcupine.
MATERIALS AND METHODS
Animals
In this study, we used skin samples from 6 adult Sunda porcupines from Small Mammals Captivity of Indonesian Institute of Science. The animals were aged 2–4 years and weighing 6–8 kg. The age was determined by the record of each animal. The skin samples were collected by biopsy procedure from thoracodorsal (TD) and lumbosacral (LS) regions. The regions were selected due to their specific quill type which grow in the region. The biopsy procedures were conducted in 4 animals, while the other 2 animals were used to observe the normal appearances of wound healing. The animals were sampled for skin biopsy and the procedures were conducted under anesthesia with 10% HCl ketamine (2.5 mg/kg body weight (BW) Ketamil, Illium, Troy Laboratories, NSW, Australia) and 2% xylazine HCl (1 mg/kg BW, Xylazil, Ilium; Troy Laboratories). All procedures were performed in accordance with the ethical approval of The Ethical Clearance Subcommittee of Life Science, Indonesian Institute of Sciences No. B-12695/K/KS.02.04/XII/2017.
Wounding and biopsies
Full thickness wounding until subcutaneous muscle, approximately 11 cm2 was conducted by incision method in all animals in TD and LS regions. The wounds were untreated with any medication until the end of observation for normal healing evaluation. The skin collected from wounding (day 0) was then fixed in buffered neutral formalin (BNF) 10%. The gross observation of the wounds was conducted in all animals, however the photographs of the clinical appearances of the normal healing were only from 2 animals (non biopsy animal). In addition, wound areas (wound size in TD and LS) were measured in days 0, 3, 7, 10, 14, 21, 30, and 40 post wounding. Repeated biopsies in 4 animals were conducted in days 3, 7, 14, 21, 30, 40 and 50 post wounding by incision on wound edge until deep dermis, approximately 2 cm2 to be evaluated histologically. The skin samples were then fixed in BNF 10%.
Histological staining and analysis
Fixed skin samples proceeded to the standard paraffin histological procedure. The samples were then sectioned at 5–10 µm serially. Subsequently, the hematoxylin and eosin staining method were performed to observe the general structure. Image photographs were conducted on normal dermis (superficial dermis + deep dermis), wound border and granulation tissue in 5 serial sections of the skin. Epidermal thickness measurement was performed in 3–4 objective fields on all sections using the ImageJ 2.0 software (National Institute of Health, Bethesda, MD, USA).
Periodic acid Schiff (PAS) and Alcian Blue (AB) pH 2.5 staining methods were used to identify the distribution of neutral and acid polysaccharide in the tissue. Moreover, the picrosirius red staining method was used to identify collagen types I and III composition in the tissue. The analysis of the collagen composition was performed under light microscope (IX71, Olympus, Tokyo, Japan) with a modified polarized filter. Polarized observation was performed using 2 linier polarized filters. One filter was placed above the source of light and another one was placed between the section and objective lens. Polarized light effect was performed by rotating the filter above the light source to 90°. Observation was carried out at 4× and 10× objective magnification, each of 2 fields of view on normal dermis (superficial dermis+deep dermis), wound border and granulation tissue on all sections.
The percentage of red and green fibers was measured using the ImageJ 2.0 software (National Institute of Health) to determine the ratio of thick fibers (Collagen I) and thin fibers (Collagen III). The polarized PSR images were converted to the greyscale RGB (Red Green Blue) format then proceeded to the area percentage in each red and green channel images in the grey value ranged 60–255.
LV-SEM imaging
Dewaxed and dried sections were immersed in 2.5% glutaraldehyde in 0.1 M PBS for 15 min in room temperature, washed and dehydrated in graded ethanol 90–100%. In the next stages the section in the LV-SEM unit (Hitachi TM 3030, Hitachi, High-Tech Corp., Tokyo, Japan) were observed. The photographs were taken on 1,000× magnification of each section on normal dermis (superficial dermis+deep dermis), wound border and granulation tissue.
Data analysis
Microscopic images were analyzed descriptively, while the measurement of epidermal thickness and a percentage of collagen (types I and III) were analyzed using ANOVA single way and Kruskall-Wallis method at P<0.05. The significant value in ANOVA and Kruskall-Wallis’ analysis was tested by the Duncan and Dunn method to acknowledge the significance in each region and time.
RESULTS
General morphology and polysaccharide distribution in wound healing
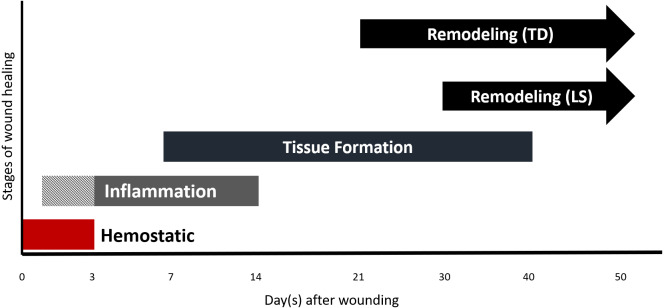
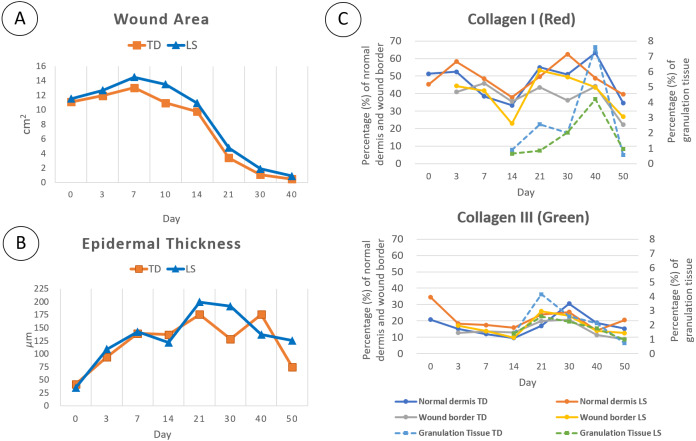
Generally, the wound healing in the Sunda porcupine began with re-epithelization followed by progressive wound contraction. The wound healing process consists of 4 overlapping stages according to its morphological characteristic (Fig. 1). Hemostatic (red bar) appeared as soon as wounding performed and began to formed a clot during day 0 to day 3 post wounding. Gross observation during day 0 to day 3 post wounding showed reddish color on the skin around the wound which indicated the inflammation stage started during hemostatic stage (Fig. 1: pattern-inflammation bar) and then followed by overlapping stage where inflammation and tissue formation began (Fig. 1: grey-inflammation bar). Wound enclosures in TD region occurred faster than LS region, the pattern of closure between ratios was similar. Wound area initially increased until day 7 post wounding, then enclosed significantly from day 10 to 40 post wounding (Fig. 2A and Clinical in Figs. 4 and 5). In gross observation, the pattern of wound closure in biopsy animals was similar to non biopsy where the main wound still closed in almost similar rate and pattern both in TD and LS, leaving only small wound of post biopsy procedure. At the end of observation (day 50 post wounding), the scar in TD appeared smaller than LS. In addition, the quills in TD region grow faster than LS region, so that the scar in TD region was covered by surrounding quills.
Fig. 1.
Schematic of wound healing events in the Sunda porcupine. The wound healing occurred in 4 overlapping stages, namely hemostatic, inflammation, tissue formation, and remodeling.
Fig. 2.
The alteration of wound area (A), epidermal thickness (B) and collagen (types I and III) percentages (C) in wound healing of the Sunda porcupine. The wound closure in TD occurred a slightly faster than LS, where the alteration of epidermal thickness and collagen composition were fluctuated both in TD and LS in each part of skin.
Fig. 4.
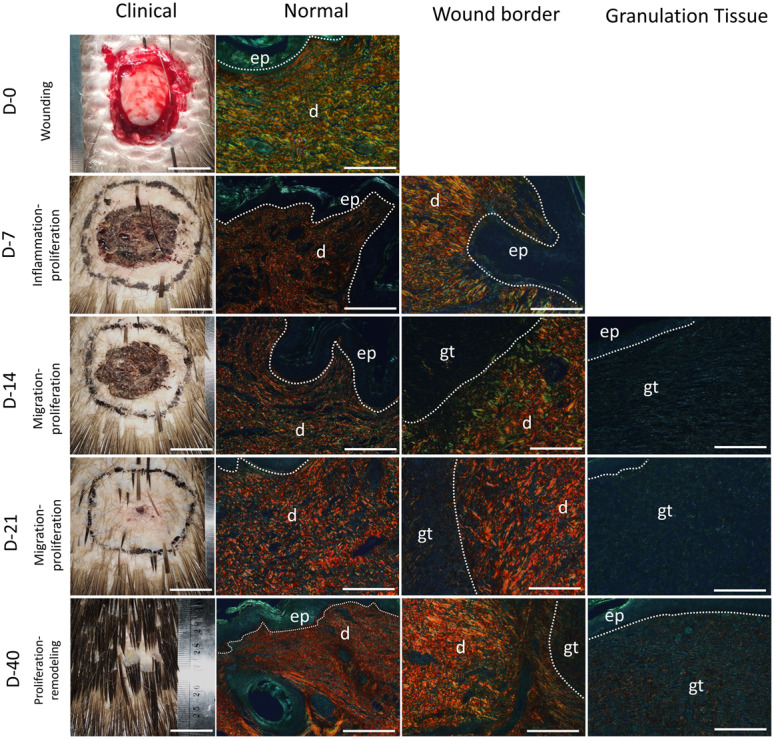
Picrosirius red images under polarized light of wound healing in the Sunda porcupine were shown in gross appearances images (Clinical) and histological images (normal, wound border, and granulation tissue). d, dermis; ep, epidermis; gt, granulation tissue. Bar Clinical: 2 cm, Bar normal, wound border, and granulation tissue: 200 µm.
Fig. 5.
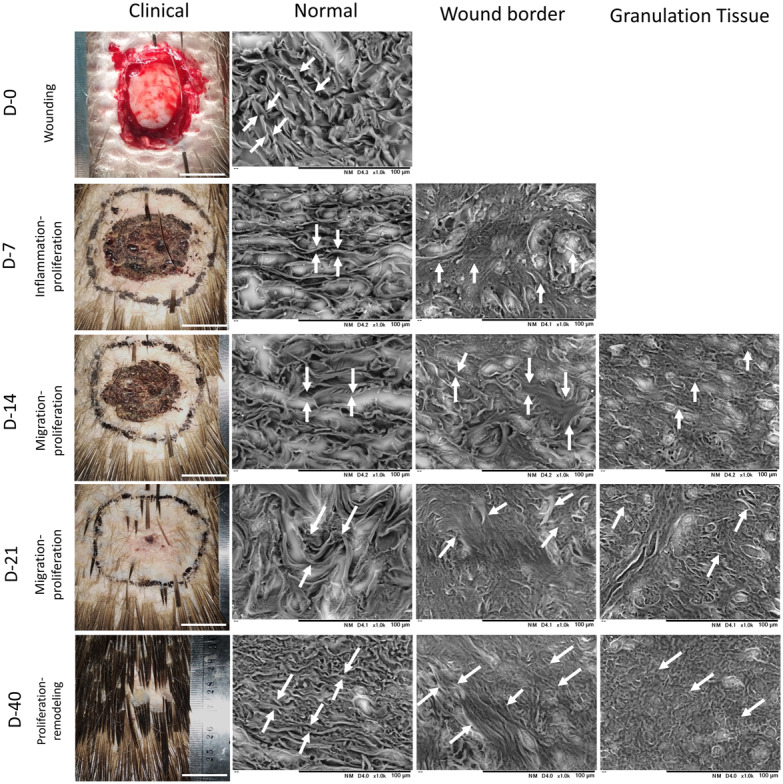
Low Vacuum Scanning Electron Microscope (LV-SEM) images of skin wound healing in the Sunda porcupine were shown in gross appearances images (Clinical) and histological images (normal, wound border, and granulation tissue). White arrow indicates the thickness and morphology of fiber. Fiber in normal part of the skin showed rigid, thick, and clear edge; fiber in wound border showed mixed thick and small fibers with no clear edge; fibers in granulation tissue showed abundant small and compact fibers. Bar Clinical: 2 cm, Bar normal, wound border, and granulation tissue: 100 µm.
Day 0 exhibited normal skin structure with thin epidermis, reticular formation of connective tissue, less blood vessels and some fibroblasts. PAS and AB staining revealed that superficial dermis consisted of a lot of fibroblasts, which reacted strongly to staining, while the fibers in connective tissue showed a weak reaction to staining (Fig. 3A). Wounding caused alteration of skin structure.
Fig. 3.
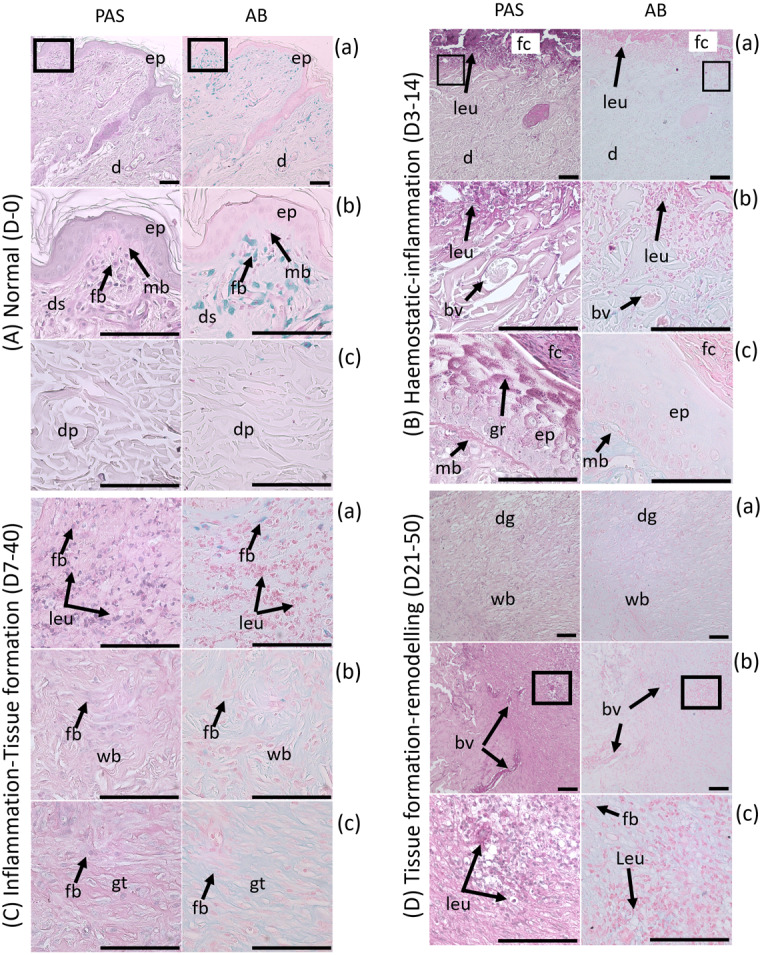
Polysaccharide content detected in Periodic acid Schiff (PAS)-Alcian Blue (AB) staining in stages of wound healing in the Sunda porcupine. The alteration of polysaccharide was shown from Normal skin (A) to Hemostatic-Inflammation stage (B), Inflammation-Tissue Formation (C), and Tissue formation-Remodeling (D). Several parts of the skin were shown in (a), (b), and (c) in each stage.Magenta color represents neutral polysaccharide, blue represent acid polysaccharide. bv, blood vessels; d, dermis; dg, degraded matrix; ds, dermis superficialis; dp, dermis profundus; ep, epidermis; fb, fibroblast; fc, fibrin clot; gr, glycogen granules; gt, granulation tissue; leu, leucocyte; mb, membrana basalis; wb, wound border. Bar: 100 µm.
During day 0–3 post wounding, the epidermis around the wound started to thicken and become proliferated (hypertrophy and hyperplasia), with a wound border attached to the temporary clot (Fig. 3Ba and 3Bb). Histologically, the wound border contained an abundance of migrating cells, probably leucocyte and assembled on the edge of skin connective tissue and clot. During this period, the wound begins with the hemostatic and inflammation stages. During 3–7 days post wounding, proliferating epidermis expanded to the wound edge, called re-epithelization (Fig. 2B). In this period, epidermal cells multiply increasingly until day 21 post wounding (Supplementary Table 1) and expand to cover wound border between connective tissue and clot (Fig. 3Bc), resulting in detachment of scab and clot from skin tissue.
Hemostatic and inflammation stages in days 3–14 post wounding showed a different polysaccharide distribution pattern. Neutral polysaccharide was identified in almost all parts of the skin, such as clot, membrana basalis, secretion in wound edge and leucocyte in skin tissue. In contrast, acid polysaccharide was identified only in collagen fibers in the skin dermis (Fig. 3B).
Granulation tissue (tissue formation stage: migration and proliferation stage) formed primarily in day 7–21 post wounding, started below the epidermis layer (Fig. 3Ca). This event caused the epidermis layer to move upward. In this stage, granulation tissue contained a lot of small blood vessels, blood cells, and fibroblast (Fig. 3Cb and 3Cc). In addition, a clear border was observed between granulation tissue and normal skin connective tissue, which was marked by different arrangement and composition in both of them. Granulation tissue grew larger and complex in day 21–30 post wounding, especially in the LS region. Wound in TD region showed remodeling stages in day 21 post wounding, marked with the degradation of several spots in granulation tissue (Fig. 3Da). Wound contraction began since the granulation tissue was formed, around day 7–14 post wounding.
The inflammation and tissue formation (migration and proliferation) stage showed that fibroblast around superficial dermis contained acid polysaccharide, but was absent for neutral polysaccharide. However, neutral polysaccharide was found abundantly in leucocyte (Fig. 3Ca). This circumstance differed from the normal condition, marked by a reactive fibroblast to PAS dan AB staining. Migrating and proliferating fibroblast to wound border and granulation tissue showed no reaction to PAS and AB staining, but was reactive to fiber and extracellular matrix (Fig. 3Cb and 3Cc).
The wound healing process in days 30–50 post wounding showed remodeling (maturation) stages, especially in the LS region which characterized with decreased granulation tissue and degraded extracellular matrix in several parts of wounded and normal skin . Tissue formation (migration and proliferation) and remodeling (maturation) stage in around days 21–50 showed degraded extracellular matrix, especially in granulation tissue, which was marked by decreased integrity, loose and a lot of space of connective tissue structure (Fig. 3Da). Leucocyte contained neutral polysaccharide was identified in degraded extracellular matrix (Fig. 3Db and 3Dc). The activity of epidermis thickening started to decrease in days 40–50 (Fig. 2B).
Collagen composition changes in wound healing
Generally, the percentage of collagen type I (red) was higher than collagen type III (green) (Fig. 2C). Collagen type I was found abundantly in normal dermis and wound border both in the TD and LS region (Fig. 2C and Fig. 4: Normal, Wound Border). Normal dermis consisted of large fiber collagen, predominantly collagen type I. Meanwhile, wound border was highly variated in connective tissue and morphology, which mostly composed of collagen type I, but several parts composed of collagen type III and other types in all stages of wound healing. Granulation tissue was composed by other types of collagens (non polarized components−black color), predominantly beside collagen types I and III according to polarized color. However, in certain days of observation of granulation tissue (day 21–30), the percentage of collagen type I showed higher than collagen type III, but distributed locally in certain spots. In contrast, collagen type III distributed greater in granulation tissue (Fig. 2C and Fig. 4). The alteration of these collagens showed significant differences during observation.
Generally, the alteration of collagen types I and III was similar in normal dermis and wound border both in TD and LS region, where the decreased percentage was observed in days 3–14 post wounding, and started to increase in days 14–30 post wounding, reaching an equivalent percentage to normal skin percentage (day 0) (Supplementary Table 2). Subsequently, the decreased percentage was observed in days 40–50, both in collagen types I and III in TD and LS region. In contrast to normal dermis and wound border, the percentage of collagen types I and III in granulation tissue showed a different pattern. In granulation tissue the collagen type I started to increase rapidly in days 30 to 40, reaching to peak, while collagen type III increased from day 14 to 21, reaching to peak, then both collagen type percentages decreased until day 50 (Fig. 2C and Fig. 4, Supplementary Table 2).
The ratio of collagen type I to collagen type III in the TD region tends to be higher than LS region both in normal dermis, wound border, and granulation tissue. This circumstance indicated that a percentage of collagen type III in the TD region tends to be lower compared to the LS region and contributed to its pattern of collagen alteration during wound healing (Table 1).
Table 1. Ratio of collagen type I to collagen type III in each part of skin and days.
| Day | Ratio collagen I: III |
|||||
|---|---|---|---|---|---|---|
| Normal dermis |
Wound border |
Granulation tissue |
||||
| TD | LS | TD | LS | TD | LS | |
| 0 | 2.47 | 1.31 | ||||
| 3 | 3.48 | 3.18 | 3.22 | 2.59 | ||
| 7 | 3.22 | 2.79 | 3.37 | 3.02 | ||
| 14 | 3.49 | 2.39 | 2.77 | 2.36 | 0.65 | 0.46 |
| 21 | 3.26 | 2.10 | 2.19 | 2.06 | 0.62 | 0.32 |
| 30 | 1.67 | 2.46 | 1.75 | 2.15 | 0.78 | 0.91 |
| 40 | 3.36 | 3.45 | 3.91 | 3.14 | 3.60 | 2.39 |
| 50 | 2.27 | 1.93 | 2.55 | 2.14 | 0.79 | 0.97 |
TD, thoracodorsal; LS, lumbosacral.
Through LV-SEM imaging, fiber in normal dermis was thick band, rigid and clear edge, formed with reticular arrangement to other fiber. During wound healing, several parts in dermis superficial altered morphologically to be abundant, smaller and shorter fibers. Fiber in the wound border composed of thick and thin fiber randomly, where no clear edge between fibers. In addition, the thick fiber originated from normal dermis, while thin fibers were new fibers produced in granulation tissue to facilitate wound healing. Granulation tissue composed of abundant small, short and thin fibers without clear edge mixed with many cells (Fig. 5).
DISCUSSION
The process of wound healing in the Sunda porcupine skin is generally the same as other mammals, which includes the process of hemostatic, inflammation, tissue formation, and remodeling. The process of wound healing in rodents is contraction type, which is believed to be the same in the Sunda porcupine, which involves the function of myofibroblast and panniculus carnosus to reduce the size of the wound [36, 42]. This process helps wound closure to be faster than the type of re-epithelization in human [29].
The healing of open wounds in the Sunda porcupine was similar to rats, cats and humans [6, 29, 36, 67] compared to spiny mice, Acomys sp. [58]. Generally, wound healing in human is characterized by a re-epithelialization and granulation formation process while almost rodent’s wound healing is characterized by rapid contraction and re-epithelization. The healing process is characterized by re-epithelialization and granulation tissue formation at the beginning of healing (similar in humans and rats), which is then followed by progressive wound contractions similar in cats’ wound healing. On the other hand, wound healing in spiny mice is characterized by a process of quick re-epithelialization and wound contraction at the beginning of the healing process. One similar factor between wound healing in the Sunda porcupines and humans is related to their hair density. Characteristics of rat skin that have high hair density (1,548 hairs/cm2) are very different from humans (29 hairs/cm2) [40] and the Sunda porcupine (0.38–1 quill clusters/cm2 with approximately of 5–20 hairs/quill clusters) [53], which contribute to the rapid re-epithelialization process in early wound healing in rats [33].
Picrosirius red staining is a simple and sensitive method developed by Junqueira et al. [34] to identify collagen structure in tissue section. Picrosirius red is a strong anionic dye associated with cationic collagen fiber that enhanced its natural birefringence under polarized light [34, 45]. Recently, many studies use the picrosirius red staining to identify the type of collagen based on its color under polarized light [3, 4, 12, 14, 52]. Generally, yellow-red birefringence can be associated with collagen type I, while green birefringence can be associated with collagen type III [34, 45]. Caetano et al. [8] revealed that the picrosirius red staining method is equivalent to the quantified method by measuring hydroxyproline of sample to obtain the total collagen percentage in wound healing.
The role of connective tissue, especially collagen types I and III is important in forming the structure of the skin. The increased wound size in the early days of healing corresponds to the decreased percentage of collagen types I and III, thus indicating the integrity of skin is affected by collagen composition. The alteration of collagen types I and III during wound healing in the Sunda porcupine, indicates that there might be an interaction between collagen types I and III in the process of wound healing in porcupine skin. Collagen type III in the skin is distributed as a sheet on the outside of collagen type I fibers. These fibers are the main components in the reticular tissue of the skin [23]. Collagen type III is thought to play a role in the process of fibrillogenesis of collagen type I [23, 38]. In addition, the presence or ratio between certain types of collagen fibers can affect the function of fibroblasts [66]. In full thickness wound healing of the Sunda porcupine, the ratio of collagen type I to collagen type III in normal dermis and wound border tends to be stable during wound healing, thus indicating no abnormalities in the process of fibrillogenesis.
This study shows that the stage of tissue formation (migration and proliferation) is a stage that produces a lot of collagen types I and III fibers both on normal skin, wound border and granulation tissues. In addition, the components of collagen types I and III are not the main components in granulation tissue, although these tissues exhibited the red and green color, but the polarization of light indicates that there are other different components besides collagen types I and III. The pattern of connective tissue composition alteration in the wound healing process in the Sunda porcupine skin has a similar pattern to some other studies, such as in rats [8], sheep fetus [16] and pigs [39]. The pattern of change is preceded by a decrease in the composition of collagen until a certain day, followed by an increase and a decline again. However, the percentage of both collagen types I and III varies and differs with the Sunda porcupine.
The alteration in the percentage of collagen types I and III fibers on day 7–40 post wounding has similarities with the alteration in epidermal thickness on day 7–40 post wounding. There might be an interaction between the epidermal proliferation process and collagen synthesis at this stage. Some researchers found that the process of migrating cells of the epidermis or keratinocytes requires interaction between keratinocytes and the extracellular matrix. On day 7–40 post wounding is the stage of synthesis of both extracellular matrix fibers such as collagen and proteoglycans. The interaction between keratinocytes and extracellular matrix increases the migration from the basal layer to the wound area [48, 59]. Platelet-derived growth factor (PDGF) secreted by keratinocytes can modulate the organization of the extracellular matrix by attracting fibroblasts, mononuclear cells and blood vessels [20]. Interleukin I produced by leukocytes can increase keratinocyte migration and collagen synthesis in the early wound healing process [55, 63].
Granulation tissue in the wound healing process in the Sunda porcupine, which was observed on day 14 post wounding, might have begun around days 7–14 post wounding. Granulation tissue is composed of extracellular matrix, fibroblasts and new blood vessels [21, 44, 56]. Two main processes, the deposition of extracellular matrix and angiogenesis work in parallel for tissue repair during the proliferation stage. Angiogenesis is slightly slower than the formation of new extracellular matrix. Oxygen is an important component for collagen-producing fibroblasts that aim to perfect granulation tissue [62], thus angiogenesis is an important stage in the wound healing process.
The decreased collagen types I and III percentage at the end of the wound healing process of the Sunda porcupines is related to the degradation activity of extracellular matrix along with the smaller wound size. Generally, fibroblasts are directed to differentiate to become myofibroblasts by macrophages via TGF-β1 signals approaching the final stages of proliferation [2, 17, 18, 31]. Myofibroblast is a contractile cell that capable to forms a new matrix. Contractions are important because they provide mechanical strength to the granulation tissue and reduce the size of the wound [17, 18, 31]. Collagen type I is produced in the form of fiber, which is parallel to the direction of the myofibroblast, thus strengthening the network and providing mechanical tension. At this stage, myofibroblast begins to degrade primary collagen type III matrix through the mechanism of action of matrix metalloproteinase enzyme and marks a transition phase from proliferation to remodeling characterized by maturation of the granulation tissue into scar [17, 41, 68].
The process of wound contraction plays a major role in reducing wound size in the wound healing process and has different characteristics in some animals, such as rats, rabbits, guinea pigs, cats and dogs [5, 36]. Wound contraction in the Sunda porcupine was observed, starting from day-7 with high progressivity until day-21, whereas in other animals, the process of wound contraction occurred in early wound healing, but with constant progressivity until day 21. Some theories regarding wound contraction reveal that the myofibroblast population at the edge of the wound is responsible for the pull that results in inward contraction of the wound, whereas the granulation tissue that has formed inside of the wound, results in the full contraction of the myofibroblast [1, 25]. The delay in wound contraction in the Sunda porcupine might be related to the initial process of granulation tissue formation and re-epithelialization in the wound, as well as the type of contraction that occurs. According to the wound closure graphic, the wound area increased until day 7, which occurred re-epithelialization and early process of granulation tissue formation at the wound edge until the base of the wound is sufficient to produce tissue containing myofibroblast population to contract. In addition, this might be also play a role in activating the contraction of wounds from the subcutaneous muscle (panniculus carnosus) so that when these components were fulfilled, contractions of the myofibroblast and subcutaneous muscles produce a high progression of wound closure from days 7 to 21.
Cells, such as fibroblast, that form connective tissue in hair follicles are believed to play a role in repairing the damaged dermis [15, 32, 50, 54]. Quill follicles in the Sunda porcupine have a large and complex structure compared to hair follicles [53], so that the activity of fibroblasts in forming quill follicles is believed to be higher. The existence of this condition is believed to support the ability of fibroblasts in repairing the dermis of damaged skin much better in the Sunda porcupine skin.
PAS staining can be used to stain neutral polysaccharide and oligosaccharide, such as glycogen and glycoprotein in tissue [37]. PAS staining showed that clot reacted strongly in early stages of wound healing. Glycogen in skin normally detected in epidermis and hair follicle [51]. Re-epithelization during wound healing in the Sunda porcupine showed presence of glycogen granules which indicated that migration and proliferation of keratinocytes needed and deposited glycogen. One of the glycoprotein is fibronectin, which plays important roles in wound healing, such as haemostatic and tissue formation stage. Fibronectin along with fibrin and clotting factors form blood clot which play a role in haemostatic [26, 27, 46, 47, 64]. Moreover, fibronectin also capable to associate with collagen type III [22]. This ability contributed to the fibrin clot reacted to the PAS staining and displayed a green color, collagen type III, in picrosirius red staining.
AB staining can be used to stain the proteoglycans and GAGs in connective tissue. Several proteoglycans and GAGs, such as versican, perlecan, syndecan, and decorin are reported play several roles in wound healing, such as support fibroblast differentiation to myofibroblast, angiogenesis induction, stimulate keratinocytes and endotel migration, and regulate collagen fibers association [7, 19, 24, 30, 60, 69]. However, the intensity of AB staining was lower than PAS staining during wound healing in the Sunda porcupine, which might be indicate that the proteoglycans and GAGs contribute less than glycoprotein.
In conclusion, full thickness of skin wound healing in the Sunda porcupine began with re-epithelization followed by progressive wound contraction with 4 overlapping stages in about 30–50 days until the wound closed (21–30 days in thoracodorsal and 30–50 days in lumbosacral). The wound healing in the Sunda porcupine mostly involves neutral polysaccharide components and a similar pattern of collagen types I and III alteration in thoracodorsal and lumbosacral region. However, higher ratio of collagen I to III in thoracodorsal region compared to lumbosacral region might be contributed to the wound enclosure time in each region.
Supplementary
Acknowledgments
We would like to thank to Muhammad Risman Wahid, Yuliastuti, Desrayni Hanadhita, Anisa Rahma and Katsuhiko Warita for the assistance in this work of publication. Also special thanks to the Directorate of Human Resource Qualification, the Directorate General of Resources for Science and Technology and Higher Education (DG-RSTHE), Ministry of Research, Technology and Higher Education of the Republic of Indonesia, Enhanced International Publication (EIP) program No. 1406.2/D3/PG/2018 and Program Magister Doktor untuk Sarjana Unggul (PMDSU) No. 29/SP2H/PTNBH/DRPM/2018 and 1468/IT3.11/PN/2018 for the funding of the research.
REFERENCES
- 1.Abercrombie M., Flint M. H., James D. W.1956. Wound contraction in relation to collagen formation in scorbutic Guinea-pigs. J. Embryol. Exp. Morphol. 4: 167–175. [Google Scholar]
- 2.Ariel A., Timor O.2013. Hanging in the balance: endogenous anti-inflammatory mechanisms in tissue repair and fibrosis. J. Pathol. 229: 250–263. doi: 10.1002/path.4108 [DOI] [PubMed] [Google Scholar]
- 3.Bayounis A. M., Alzoman H. A., Jansen J. A., Babay N.2011. Healing of peri-implant tissues after flapless and flapped implant installation. J. Clin. Periodontol. 38: 754–761. doi: 10.1111/j.1600-051X.2011.01735.x [DOI] [PubMed] [Google Scholar]
- 4.Binnebösel M., Klink C. D., Otto J., Conze J., Jansen P. L., Anurov M., Schumpelick V., Junge K.2010. Impact of mesh positioning on foreign body reaction and collagenous ingrowth in a rabbit model of open incisional hernia repair. Hernia 14: 71–77. doi: 10.1007/s10029-009-0580-4 [DOI] [PubMed] [Google Scholar]
- 5.Bohling M. W., Henderson R. A., Swaim S. F., Kincaid S. A., Wright J. C.2004. Cutaneous wound healing in the cat: a macroscopic description and comparison with cutaneous wound healing in the dog. Vet. Surg. 33: 579–587. doi: 10.1111/j.1532-950X.2004.04081.x [DOI] [PubMed] [Google Scholar]
- 6.Burd D. A., Longaker M. T., Adzick N. S., Harrison M. R., Ehrlich H. P.1990. Foetal wound healing in a large animal model: the deposition of collagen is confirmed. Br. J. Plast. Surg. 43: 571–577. doi: 10.1016/0007-1226(90)90122-G [DOI] [PubMed] [Google Scholar]
- 7.Cattaruzza S., Perris R.2005. Proteoglycan control of cell movement during wound healing and cancer spreading. Matrix Biol. 24: 400–417. doi: 10.1016/j.matbio.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 8.Caetano G. F., Fronza M., Leite M. N., Gomes A., Frade M. A. C.2016. Comparison of collagen content in skin wounds evaluated by biochemical assay and by computer-aided histomorphometric analysis. Pharm. Biol. 54: 2555–2559. doi: 10.3109/13880209.2016.1170861 [DOI] [PubMed] [Google Scholar]
- 9.Campos A. C., Groth A. K., Branco A. B.2008. Assessment and nutritional aspects of wound healing. Curr. Opin. Clin. Nutr. Metab. Care 11: 281–288. doi: 10.1097/MCO.0b013e3282fbd35a [DOI] [PubMed] [Google Scholar]
- 10.Carter R., Jain K., Sykes V., Lanning D.2009. Differential expression of procollagen genes between mid- and late-gestational fetal fibroblasts. J. Surg. Res. 156: 90–94. doi: 10.1016/j.jss.2009.03.056 [DOI] [PubMed] [Google Scholar]
- 11.Carrino D. A., Sorrell J. M., Caplan A. I.2000. Age-related changes in the proteoglycans of human skin. Arch. Biochem. Biophys. 373: 91–101. doi: 10.1006/abbi.1999.1545 [DOI] [PubMed] [Google Scholar]
- 12.Cavallo J. A., Roma A. A., Jasielec M. S., Ousley J., Creamer J., Pichert M. D., Baalman S., Frisella M. M., Matthews B. D., Deeken C. R.2014. Remodeling characteristics and collagen distribution in synthetic mesh materials explanted from human subjects after abdominal wall reconstruction: an analysis of remodeling characteristics by patient risk factors and surgical site classifications. Surg. Endosc. 28: 1852–1865. doi: 10.1007/s00464-013-3405-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W. Y. J., Abatangelo G.1999. Functions of hyaluronan in wound repair. Wound Repair Regen. 7: 79–89. doi: 10.1046/j.1524-475X.1999.00079.x [DOI] [PubMed] [Google Scholar]
- 14.Coen M., Menegatti E., Salvi F., Mascoli F., Zamboni P., Gabbiani G., Bochaton-Piallat M. L.2013. Altered collagen expression in jugular veins in multiple sclerosis. Cardiovasc. Pathol. 22: 33–38. doi: 10.1016/j.carpath.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 15.Cotsarelis G., Kaur P., Dhouailly D., Hengge U., Bickenbach J.1999. Epithelial stem cells in the skin: definition, markers, localization and functions. Exp. Dermatol. 8: 80–88. doi: 10.1111/j.1600-0625.1999.tb00351.x [DOI] [PubMed] [Google Scholar]
- 16.Cuttle L., Nataatmadja M., Fraser J. F., Kempf M., Kimble R. M., Hayes M. T.2005. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen. 13: 198–204. doi: 10.1111/j.1067-1927.2005.130211.x [DOI] [PubMed] [Google Scholar]
- 17.Darby I. A., Laverdet B., Bonté F., Desmoulière A.2014. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 7: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmoulière A., Chaponnier C., Gabbiani G.2005. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 13: 7–12. doi: 10.1111/j.1067-1927.2005.130102.x [DOI] [PubMed] [Google Scholar]
- 19.Elenius K., Vainio S., Laato M., Salmivirta M., Thesleff I., Jalkanen M.1991. Induced expression of syndecan in healing wounds. J. Cell Biol. 114: 585–595. doi: 10.1083/jcb.114.3.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eming S. A., Yarmush M. L., Krueger G. G., Morgan J. R.1999. Regulation of the spatial organization of mesenchymal connective tissue: effects of cell-associated versus released isoforms of platelet-derived growth factor. Am. J. Pathol. 154: 281–289. doi: 10.1016/S0002-9440(10)65274-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enoch S., Leaper D. J.2008. Basic science of wound healing. Surgery 26: 31–37. [Google Scholar]
- 22.Engvall E., Ruoslahti E., Miller E. J.1978. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J. Exp. Med. 147: 1584–1595. doi: 10.1084/jem.147.6.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleischmajer R., MacDonald E. D., Perlish J. S., Burgeson R. E., Fisher L. W.1990. Dermal collagen fibrils are hybrids of type I and type III collagen molecules. J. Struct. Biol. 105: 162–169. doi: 10.1016/1047-8477(90)90110-X [DOI] [PubMed] [Google Scholar]
- 24.Fukushima K., Badlani N., Usas A., Riano F., Fu F., Huard J.2001. The use of an antifibrosis agent to improve muscle recovery after laceration. Am. J. Sports Med. 29: 394–402. doi: 10.1177/03635465010290040201 [DOI] [PubMed] [Google Scholar]
- 25.Grillo H. C., Watts G. T., Gross J.1958. Studies in wound healing: I. Contraction and the wound contents. Ann. Surg. 148: 145–160. doi: 10.1097/00000658-195808000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grinnell F., Billingham R. E., Burgess L.1981. Distribution of fibronectin during wound healing in vivo. J. Invest. Dermatol. 76: 181–189. doi: 10.1111/1523-1747.ep12525694 [DOI] [PubMed] [Google Scholar]
- 27.Grinnell F.1984. Fibronectin and wound healing. J. Cell. Biochem. 26: 107–116. doi: 10.1002/jcb.240260206 [DOI] [PubMed] [Google Scholar]
- 28.Guo S., Dipietro L. A.2010. Factors affecting wound healing. J. Dent. Res. 89: 219–229. doi: 10.1177/0022034509359125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurtner G. C., Werner S., Barrandon Y., Longaker M. T.2008. Wound repair and regeneration. Nature 453: 314–321. doi: 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- 30.Hattori N., Carrino D. A., Lauer M. E., Vasanji A., Wylie J. D., Nelson C. M., Apte S. S.2011. Pericellular versican regulates the fibroblast-myofibroblast transition: a role for ADAMTS5 protease-mediated proteolysis. J. Biol. Chem. 286: 34298–34310. doi: 10.1074/jbc.M111.254938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinz B.2007. Formation and function of the myofibroblast during tissue repair. J. Invest. Dermatol. 127: 526–537. doi: 10.1038/sj.jid.5700613 [DOI] [PubMed] [Google Scholar]
- 32.Inaba M., Anthony J., McKinstry C.1979. Histologic study of the regeneration of axillary hair after removal with subcutaneous tissue shaver. J. Invest. Dermatol. 72: 224–231. doi: 10.1111/1523-1747.ep12530773 [DOI] [PubMed] [Google Scholar]
- 33.Ito M., Cotsarelis G.2008. Is the hair follicle necessary for normal wound healing? J. Invest. Dermatol. 128: 1059–1061. doi: 10.1038/jid.2008.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Junqueira L. C., Bignolas G., Brentani R. R.1979. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 11: 447–455. doi: 10.1007/BF01002772 [DOI] [PubMed] [Google Scholar]
- 35.Järveläinen H., Sainio A., Koulu M., Wight T. N., Penttinen R.2009. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol. Rev. 61: 198–223. doi: 10.1124/pr.109.001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy D. F., Cliff W. J.1979. A systematic study of wound contraction in mammalian skin. Pathology 11: 207–222. doi: 10.3109/00313027909061947 [DOI] [PubMed] [Google Scholar]
- 37.Kumar G. L., Gill G. W.2010. Introduction to special stains. Connection. [Google Scholar]
- 38.Liu X., Wu H., Byrne M., Krane S., Jaenisch R.1997. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA 94: 1852–1856. doi: 10.1073/pnas.94.5.1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu J.2017. Evaluation of the Wound Healing Process by Immunohistochemistry and Pircrosirius Red Staining [tesis]. Universitas Temple, Philadelphia. [Google Scholar]
- 40.Mangelsdorf S., Vergou T., Sterry W., Lademann J., Patzelt A.2014. Comparative study of hair follicle morphology in eight mammalian species and humans. Skin Res. Technol. 20: 147–154. doi: 10.1111/srt.12098 [DOI] [PubMed] [Google Scholar]
- 41.McCawley L. J., Matrisian L. M.2001. Matrix metalloproteinases: they’re not just for matrix anymore! Curr. Opin. Cell Biol. 13: 534–540. doi: 10.1016/S0955-0674(00)00248-9 [DOI] [PubMed] [Google Scholar]
- 42.McGrath M. H., Simon R. H.1983. Wound geometry and the kinetics of wound contraction. Plast. Reconstr. Surg. 72: 66–73. doi: 10.1097/00006534-198307000-00015 [DOI] [PubMed] [Google Scholar]
- 43.Merkel J. R., DiPaolo B. R., Hallock G. G., Rice D. C.1988. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc. Soc. Exp. Biol. Med. 187: 493–497. doi: 10.3181/00379727-187-42694 [DOI] [PubMed] [Google Scholar]
- 44.Monaco J. L., Lawrence W. T.2003. Acute wound healing an overview. Clin. Plast. Surg. 30: 1–12. doi: 10.1016/S0094-1298(02)00070-6 [DOI] [PubMed] [Google Scholar]
- 45.Montes G. S., Junqueira L. C.1991. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem. Inst. Oswaldo Cruz 86 Suppl 3: 1–11. doi: 10.1590/S0074-02761991000700002 [DOI] [PubMed] [Google Scholar]
- 46.Mosher D. F.1975. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J. Biol. Chem. 250: 6614–6621. [PubMed] [Google Scholar]
- 47.Mosher D. F.1976. Action of fibrin-stabilizing factor on cold-insoluble globulin and alpha2-macroglobulin in clotting plasma. J. Biol. Chem. 251: 1639–1645. [PubMed] [Google Scholar]
- 48.Munshi H. G., Wu Y. I., Ariztia E. V., Stack M. S.2002. Calcium regulation of matrix metalloproteinase-mediated migration in oral squamous cell carcinoma cells. J. Biol. Chem. 277: 41480–41488. doi: 10.1074/jbc.M207695200 [DOI] [PubMed] [Google Scholar]
- 49.Okamoto O., Fujiwara S.2006. Dermatopontin, a novel player in the biology of the extracellular matrix. Connect. Tissue Res. 47: 177–189. doi: 10.1080/03008200600846564 [DOI] [PubMed] [Google Scholar]
- 50.Oliver R. F.1966. Whisker growth after removal of the dermal papilla and lengths of follicle in the hooded rat. J. Embryol. Exp. Morphol. 15: 331–347. [PubMed] [Google Scholar]
- 51.Parakkal P. F.1969. The fine structure of anagen hair follicle of the mouse. Adv. Skin. Biol. 9: 441–469. [Google Scholar]
- 52.Peeters E., De Hertogh G., Junge K., Klinge U., Miserez M.2014. Skin as marker for collagen type I/III ratio in abdominal wall fascia. Hernia 18: 519–525. doi: 10.1007/s10029-013-1128-1 [DOI] [PubMed] [Google Scholar]
- 53.Prawira A. Y., Novelina S., Darusman H. S., Farida W. R., Agungpriyono S.2018b. The dorsal skin structure contributes to the surface bacteria populations of Sunda Porcupine (Hystrix javanica). Anat. Histol. Embryol. 47: 591–598. doi: 10.1111/ahe.12401 [DOI] [PubMed] [Google Scholar]
- 54.Prouty S. M., Lawrence L., Stenn K. S.1996. Fibroblast-dependent induction of a murine skin lesion with similarity to human common blue nevus. Am. J. Pathol. 148: 1871–1885. [PMC free article] [PubMed] [Google Scholar]
- 55.Sauder D. N., Kilian P. L., McLane J. A., Quick T. W., Jakubovic H., Davis S. C., Eaglstein W. H., Mertz P. M.1990. Interleukin-1 enhances epidermal wound healing. Lymphokine Res. 9: 465–473. [PubMed] [Google Scholar]
- 56.Schaffer C. J., Nanney L. B.1996. Cell biology of wound healing. Int. Rev. Cytol. 169: 151–181. doi: 10.1016/S0074-7696(08)61986-5 [DOI] [PubMed] [Google Scholar]
- 57.Schultz G. S.1999. Molecular regulation of wound healing. In: Acute and Chronic Wounds: Nursing Management 2nd ed. (Bryant, R. A. ed.), Mosby, St. Louis. [Google Scholar]
- 58.Seifert A. W., Kiama S. G., Seifert M. G., Goheen J. R., Palmer T. M., Maden M.2012. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489: 561–565. doi: 10.1038/nature11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singer A. J., Clark R. A.1999. Cutaneous wound healing. N. Engl. J. Med. 341: 738–746. doi: 10.1056/NEJM199909023411006 [DOI] [PubMed] [Google Scholar]
- 60.Stepp M. A., Gibson H. E., Gala P. H., Iglesia D. D., Pajoohesh-Ganji A., Pal-Ghosh S., Brown M., Aquino C., Schwartz A. M., Goldberger O., Hinkes M. T., Bernfield M.2002. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J. Cell Sci. 115: 4517–4531. doi: 10.1242/jcs.00128 [DOI] [PubMed] [Google Scholar]
- 61.Stroncek J. D., Bell N., Reichert W. M.2009. Instructional PowerPoint presentations for cutaneous wound healing and tissue response to sutures. J. Biomed. Mater. Res. A 90: 1230–1238. doi: 10.1002/jbm.a.32158 [DOI] [PubMed] [Google Scholar]
- 62.Thackham J. A., McElwain D. L., Long R. J.2008. The use of hyperbaric oxygen therapy to treat chronic wounds: A review. Wound Repair Regen. 16: 321–330. doi: 10.1111/j.1524-475X.2008.00372.x [DOI] [PubMed] [Google Scholar]
- 63.Trengove N. J., Bielefeldt-Ohmann H., Stacey M. C.2000. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 8: 13–25. doi: 10.1046/j.1524-475x.2000.00013.x [DOI] [PubMed] [Google Scholar]
- 64.Valenick L. V., Hsia H. C., Schwarzbauer J. E.2005. Fibronectin fragmentation promotes alpha4beta1 integrin-mediated contraction of a fibrin-fibronectin provisional matrix. Exp. Cell Res. 309: 48–55. doi: 10.1016/j.yexcr.2005.05.024 [DOI] [PubMed] [Google Scholar]
- 65.Velnar T., Bailey T., Smrkolj V.2009. The wound healing process: an overview of the cellular and molecular mechanisms. J. Int. Med. Res. 37: 1528–1542. doi: 10.1177/147323000903700531 [DOI] [PubMed] [Google Scholar]
- 66.Volk S. W., Wang Y., Mauldin E. A., Liechty K. W., Adams S. L.2011. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs (Print) 194: 25–37. doi: 10.1159/000322399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong V. W., Sorkin M., Glotzbach J. P., Longaker M. T., Gurtner G. C.2011. Surgical approaches to create murine models of human wound healing. J. Biomed. Biotechnol. 2011: 969618. doi: 10.1155/2011/969618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xue M., Le N. T., Jackson C. J.2006. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin. Ther. Targets 10: 143–155. doi: 10.1517/14728222.10.1.143 [DOI] [PubMed] [Google Scholar]
- 69.Zhou Z., Wang J., Cao R., Morita H., Soininen R., Chan K. M., Liu B., Cao Y., Tryggvason K.2004. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 64: 4699–4702. doi: 10.1158/0008-5472.CAN-04-0810 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.