Abstract
Cerebral ischemia is a neurological disorder that causes permanent disability and is sometimes fatal. Epigallocatechin gallate (EGCG) is a natural polyphenol that exerts beneficial antioxidant and anti-inflammatory effects. The aim of this study was to investigate the neuroprotective effects of EGCG against cerebral ischemia. Middle cerebral artery occlusion was surgically initiated to induce focal cerebral ischemia in adult male rats. EGCG (50 mg/kg) or vehicle was intraperitoneally injected just prior to middle cerebral artery occlusion (MCAO) induction. Neuronal behavior tests were performed 24 hr after MCAO. Brain tissues were isolated to evaluate infarct volume, histological changes, apoptotic cell death, and caspase-3 and poly ADP-ribose polymerase (PARP) levels. MCAO injury led to serious functional neurological deficits and increased infarct volume. Moreover, it induced histopathological lesions and increased the numbers of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells in the cerebral cortex. However, EGCG improved MCAO-induced neurological deficits and reduced infarct volume, alleviated histopathological changes, and decreased TUNEL-positive cells in the cerebral cortex of MCAO rats. Western blot analysis showed increases of caspase-3 and PARP expression levels in MCAO rats with vehicle, whereas EGCG administration alleviated these increases after MCAO injury. These results demonstrate that EGCG exerts a neuroprotective effect by regulating caspase-3 and PARP proteins during cerebral ischemia. In conclusion, we suggest that EGCG acts as a potent neuroprotective agent by modulating the apoptotic signaling pathway.
Keywords: caspase-3, epigallocatechin gallate, neuroprotection, poly ADP-ribose polymerase
Stroke is a neurodegerative disease characterized by high mortality and morbidity [34]. Ischemia is a major cause of neuronal disability and impairment. Ischemic injuries induce reactive oxygen species generation and neuronal cell damage [30]. Cerebral ischemia leads to serious permanent neurological disorders, and damage from ischemic injury is difficult to repair [8]. Moreover, ischemia causes pathological events, including glutamate excitotoxicity, membrane depolarization, calcium accumulation, and toxic free radical generation [7]. These changes induce unrecoverable neuronal cell death, leading to possible functional degeneration [11].
Epigallocatechin gallate (EGCG) is a polyphenolic compound that is abundant in green tea. EGCG is nontoxic and acts as a natural iron chelator, and it is a useful reagent as it penetrates the blood-brain barrier [21]. EGCG has diverse beneficial effects, such as anti-oxidant, anti-inflammation, anti-cancer, and anti-obesity actions [10, 13, 18, 40]. EGCG also exerts a neuroprotective effect against neurological disorders including multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, and stroke [1, 4, 5, 25]. It ameliorates learning and memory deficits in ischemic injury through modulation of oxidative stress and neuroinflammation [36]. EGCG acts as a potent anti-oxidant and protects neuronal cells against oxidative stress-induced damage [24]. EGCG induces neuronal proliferation and differentiation in neural progenitor cells and promotes neurogenesis after ischemic stroke [38]. The neuroprotective mechanisms of EGCG are very complex. Although many previous studies have demonstrated some neuroprotective effects of EGCG, the protective mechanism has not been fully elucidated. Therefore, we investigated the neuroprotective effects of EGCG against focal cerebral ischemia and elucidated its anti-apoptotic signal regulation.
MATERIALS AND METHODS
Experimental animals
Adult male Sprague-Dawley rats were purchased from Samtako Co. (Animal Breeding Center, Osan, Korea). Rats were maintained under light condition (12 hr day/12 hr night) and temperature (22 ± 1°C), and given free access to water and food. All experimental protocols were approved and carried out in accordance to the Guide for Institutional Animal Care and Use Committee of Gyeongsang National University (Approval number: GNU-190218-R0008). Rats were randomly grouped into four groups (n=20 each group) as follows: vehicle+sham, EGCG+sham, vehicle+middle cerebral artery occlusion (MCAO), and EGCG+MCAO. EGCG (Sigma Aldrich, St. Louis, MO, USA) was dissolved in phosphate buffered saline (PBS). EGCG (50 mg/kg) or vehicle was intraperitoneally injected just before MCAO operation [4, 17]. Vehicle group was treated only PBS without EGCG.
Middle cerebral artery occlusion
Rats were anesthetized with Zoletil (50 mg/kg, Virbac, Carros, France) before surgery. MCAO was performed with a previous described method [20]. Animals were kept on an operation table with supine position and a midline neck skin was incised. Right common carotid artery (CCA) was exposed and separated from adjacent tissues, continuously external carotid artery (ECA) and internal carotid artery (ICA) was exposed. Right CCA was temporally blocked using a microvascular clamp and proximal end of ECA was ligated and cut. A 4–0 nylon suture with flame-blunted end was inserted into truncated ECA and put into ICA to the origin of middle cerebral artery. The length of inserted nylon suture was approximately 22 to 24 mm. ECA with inserted filament was ligated and skin incision was sutured with silk. Sham-operated animals were performed as an identical surgical operation except a nylon suture insertion. Animals were put on a heating pad for the keeping of body temperature during surgery. MCAO was maintained for 24 hr and functional neurological deficit test was performed. Animals were quickly sacrificed by cervical dislocation to reduce suffering and whole brain was isolated for further experiment.
Neurological deficit scoring test
We conducted neurological deficit scoring test 24 hr after MCAO induction. Neurological behavior disorders were evaluated by neurological deficit scoring test [14, 32]. Scoring system was conducted based on a five-point scale as follows: normal posture without neurological deficit (no neurological deficit, 0), incomplete extension and weakness of contralateral forelimb (mild neurological deficit, 1), circling spontaneous to contralateral side (moderate neurological deficit, 2), falling down to contralateral side, sensitive response to stimulus, seizure (severe neurological deficit, 3), no spontaneous movement and no conscious (very severe neurological deficit, 4).
Brain edema measurement
Cerebral cortex tissues were isolated from whole brain and placed on a slide glass. Tissues were immediately weighed by GB204 electronic balance (Mettler toledo, Columbus, OH, USA) to measure the wet weight. They were dried in a dry oven for 24 hr at 100°C to obtain the dry weight. Water content of brain tissues (%) was calculated by following formula: [(wet weight–dry weight)/dry weight] ×100.
Triphenyltetrazolium chloride staining
Brain tissues were removed from the skull and placed on brain matrix (Ted Pella, Redding, CA, USA). They were coronally sliced into 2 mm slices and incubated with 1% triphenyltetrazolium chloride (TTC, Sigma Aldrich) solution for 20 min at 37°C. Brain slices were fixed in 10% formalin solution for 24 hr and stained images were scanned by Agfar ARCUS 1200™ (Agfar Gevaert, Mortsel, Belgium). Intact areas were stained as red color, whereas infarct areas were un-stained and remained as white color. Infarct areas of slice were quantified by Image-ProPlus 4.0 software (Media Cybernetics, Silver Spring, MD, USA). Infarct volumes (%) were calculated as follows: (infarct area/whole brain area) ×100.
Hematoxylin and eosin staining
Brain tissues were fixed in 4% neutral buffered paraformaldehyde solution. They were washed with tap water, dehydrated with ethanol from low to high concentration (70 to 100%), and cleaned with xylene. Tissues were embedded with paraplast (Leica, Wetzlar, Germany) in Leica EG1160 paraffin embedding center (Leica) and paraffin blocks were sectioned into 4 µm using a rotary microtome (Leica). Tissue sections were placed on a slide glass, dried on slide warmer, deparaffinized with xylene, and hydrated with high concentration ethanol to low concentration ethanol (100 to 70%). Sections were stained with Harris’ hematoxylin solution (Sigma Aldrich) for 5 min, washed with tap water for 5 min, and then differentiated in 1% HCl solution and 1% ammonia water. They were washed with water and stained with eosin Y solution (Sigma Aldrich) for 2 min. Stained tissues were washed with water, dehydrated with a graded series of ethanol, and cleared with xylene. Finally, stained tissues mounted with Permount mounting medium (Thermo Fisher Scientific, Waltham, MA, USA) for light microscopic observations. Stained tissues were observed and photographed using Olympus microscope (Olympus, Tokyo, Japan).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
TUNEL assay was carried out to detect apoptotic cells using ApopTag® Peroxidase In Situ Apoptosis Detection Kit (Merck, Kenilworth, NJ, USA). Deparaffinized sections were incubated with proteinase K (20 µg/ml) for 5 min and washed with PBS for 5 min. Sections were incubated with 3% hydrogen peroxide in methanol for 5 min to inhibit endogenous peroxidase activity. They were washed with PBS for 5 min and reacted with equilibration buffer for 1 hr at 4°C. And then, they were incubated with reaction buffer and terminal deoxynucleotidyl transferase (TdT) enzyme mixture in humidified chamber for 90 min at 37°C. Sections were incubated with stop buffer for 10 min to terminate TdT enzyme reaction, rinsed with PBS for 5 min, incubated with anti-digoxigenin conjugate for 1 hr, and washed with PBS. They were stained with 3,3′-diaminobenzidine (DAB, Sigma Aldrich) and washed with PBS for 5 min. They were counterstained by hematoxylin solution, and dehydrated in a graded series of ethanol (70 to 100%), and cleaned with xylene. Sections were coverslipped by Permount mounting medium (Thermo Fisher Scientific) and observed using Olympus light microscope (Olympus). Five fields in cerebral cortical images were chosen and TUNEL-positive cells were counted in each field. The result value of TUNEL assay was expressed as a percentage of the number of TUNEL-positive cells to the number of total cells.
Western blot analysis
Right cerebral cortices were carefully separated from brain, quickly frozen in liquid nitrogen, and kept at −70°C. They were homogenized in lysis buffer [1% Triton X-100, 1 mM EDTA in PBS (pH 7.4)] contained phenylmethanesulfonyl fluoride (PMSF, Sigma Aldrich) to extract total protein. Lysates were sonicated and centrifuged at 15,000 g for 1 hr at 4°C. Supernatants were collected and protein concentrations were measured with bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). Total proteins (30 µg) from each sample were loaded into 10% sodium dodecyl sulfate polyacrylamide gel and electrophoresed at 10 mA for 30 min, continuously run at 20 mA for 90 min using Mini-Protean® 3 cell (Bio-Rad, Hercules, CA, USA). They were transferred onto poly-vinylidene fluoride membranes (Sigma Aldrich) at 120 V for 2 hr. Membranes were blocked with 5% skim milk solution in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 hr, washed with TBST, and reacted for overnight at 4°C with specific primary antibodies against anti-caspase 3, anti-poly ADP-ribose polymerase (PARP), and anti-β-actin (diluted 1:1,000, Cell Signaling Technology, Beverly, MA, USA). Membranes were rinsed with TBST and incubated with horseradish peroxide-conjugated anti-rabbit IgG or anti-mouse IgG (diluted 1:5,000, Cell Signaling Technology) for 2 hr at room temperature. They were washed with TBST and incubated with enhanced chemiluminescence reagents (GE Healthcare, Little Chalfont, Buckinghamshire, UK) to detect reacted signals. They were consequently exposed to X-ray film to visualize protein bands. Relative densities for these expressions were normalized using β-actin expression. They were presents as a ratio of these proteins intensity to β-actin intensity.
Statistical analysis
All experiment data were presented as the mean ± standard error of means (S.E.M.). The results of each group were compared by two-way analysis of variance (ANOVA) followed by post-hoc Scheffe’s test. P<0.05 was regarded as statistically significant.
RESULTS
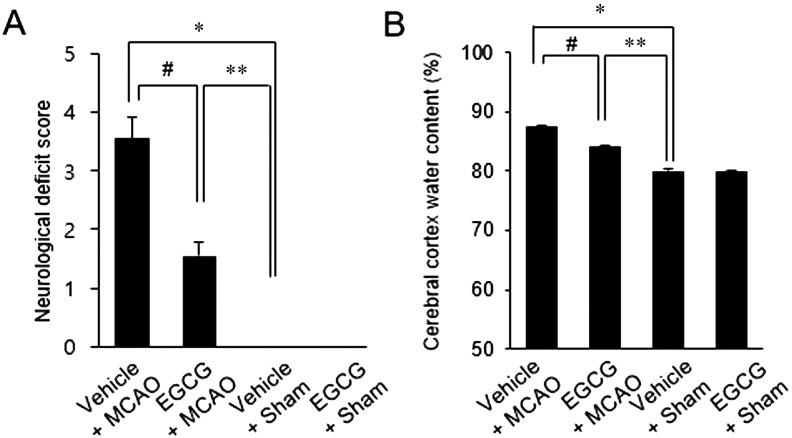
We found that MCAO injury leads to neurological behavioral dysfunction and neuronal damage. Neurological damage was evaluated by neurological deficit scoring and brain edema measurement. Vehicle-treated animals with MCAO showed severe functional neurologic deficits such as spontaneous circling, falling to the left side, lack of spontaneous movement, and seizures. ECGC-treated animals with MCAO showed only mild neurologic deficits, with failure to extend the left forepaw. Impairment on the left side indicates effects on the contralateral side of the ischemic region. However, sham-operated animals did not show any distinct symptoms of neurological deficits regardless of vehicle or EGCG administration. Neurological deficit scores were 3.55 ± 0.37 and 1.54 ± 0.23 in vehicle+MCAO and EGCG+MCAO animals, respectively (Fig. 1A). MCAO injury caused severe cerebral cortex edema, whereas EGCG treatment alleviated this change. The water content of the cerebral cortex was 87.5 ± 0.21% in vehicle+MCAO animals and 84.3 ± 0.15% in EGCG+MCAO animals (Fig. 1B).
Fig. 1.
Neurobehavioral scores (A) and edema measurements (B) in vehicle+sham, epigallocatechin gallate (EGCG)+sham, vehicle+middle cerebral artery occlusion (MCAO), and EGCG+MCAO animals. EGCG attenuated the functional neurological deficits and edema caused by ischemic stroke. Data (n=4) are represented as mean ± S.E.M. *P<0.01, **P<0.05 vs. vehicle+sham animals, #P<0.05 vs. vehicle+MCAO animals.
TTC staining showed that infarct volume markedly increases in MCAO-operated animals (Fig. 2A). However, this increase was alleviated in EGCG-treated animals compared to vehicle-treated animals. Ischemic regions remained white, while intact regions stained red. Infarct volumes were 29.9 ± 1.34% and 14.7 ± 1.13% in vehicle+MCAO and EGCG+MCAO animals, respectively (Fig. 2B). Infarct regions were not observed in sham-operated animals regardless of vehicle or EGCG treatment. Figure 3 shows serious histopathological changes in the cerebral cortex of MCAO animals. Typical pyramidal cells with large and round nuclei were observed in sham-operated animals. However, MCAO-operated animals had distorted pyramidal cells with condensed nuclei and shrunken dendrites, as well as numerous vacuoles in the cytoplasm. These histopathological changes were alleviated by EGCG treatment. Pyknotic nuclei and vacuoles were reduced in EGCG-treated animals with MCAO. Shrunken dendrites were preserved in similar shapes to those of sham-operated animals. We also detected TUNEL-positive signals in the cerebral cortex of MCAO-operated animals. The number of TUNEL-positive cells was significantly increased in vehicle-treated animals with MCAO (Fig. 4A). However, EGCG administration attenuated this MCAO-induced increase (Fig. 4B). The value of TUNEL-positive reactions represents the apoptotic index, which in the cerebral cortex was 78.46 ± 5.27% and 32.17 ± 4.33% in vehicle+MCAO and EGCG+MCAO animals, respectively (Fig. 4E).
Fig. 2.
Representative photograph of Triphenyltetrazolium chloride (TTC) staining (A) and infarct volume (B) in vehicle+sham, epigallocatechin gallate (EGCG)+sham, vehicle+middle cerebral artery occlusion (MCAO), and EGCG+MCAO animals. Intact areas stained red, while ischemic areas remained white in color (A). Infarct volume was calculated as the ratio of infarct area to total brain area (B). EGCG attenuated MCAO-induced infarction. Data (n=4) are represented as mean ± S.E.M. *P<0.01, **P<0.05 vs. vehicle+sham animals, #P<0.05 vs. vehicle+MCAO animals.
Fig. 3.
Representative photograph of hematoxylin and eosin staining (A–D) in vehicle+sham, epigallocatechin gallate (EGCG)+sham, vehicle+middle cerebral artery occlusion (MCAO), and EGCG+MCAO animals. EGCG alleviated histopathological changes caused by MCAO. Filled arrows indicate shrunken and condensed nuclei, and open arrows indicate swelled and vacuolated forms. Scale bar=100 µm.
Fig. 4.
Representative photos of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (A–D) in the cerebral cortex in vehicle+sham, epigallocatechin gallate (EGCG)+sham, vehicle+middle cerebral artery occlusion (MCAO), and EGCG+MCAO animals. The number of TUNEL positive cells was markedly increased in vehicle+MCAO animals, while EGCG decreased the number of these positive cells (A and B). Arrows indicate cells with positive TUNEL staining. Scale bar: 100 µm. Apoptotic index (E) indicates the percentage of TUNEL-positive cells to total cells. Data (n=4) are shown as mean ± S.E.M. *P<0.01, **P<0.05 vs. vehicle+sham animals, #P<0.05 vs. vehicle+MCAO animals.
The results of Western blot analysis showed changes of PARP and caspase-3 expressions in the cerebral cortex of MCAO animals (Fig. 5). PARP and caspase-3 expression levels were significantly increased in MCAO animals with vehicle compared to those of sham animals. However, increases of these proteins were alleviated by EGCG treatment. PARP expression level was 1.49 ± 0.14 in vehicle+MCAO and 0.65 ± 0.04 in EGCG+MCAO animals (Fig. 5B). Moreover, caspase-3 expression level was 1.33 ± 0.05 and 0.69 ± 0.07 in vehicle+MCAO and EGCG+MCAO animals, respectively (Fig. 5D).
Fig. 5.
Western blot analysis of poly ADP-ribose polymerase (PARP) and caspase-3 proteins in vehicle+sham, epigallocatechin gallate (EGCG)+sham, vehicle+middle cerebral artery occlusion (MCAO), and EGCG+MCAO animals. EGCG prevents MCAO-induced increases of PARP and caspase-3 expressions. Densitometric analysis is represented as a ratio of PARP (B) and caspase-3 (D) staining intensity to actin intensity. Data (n=4) are shown as mean ± S.E.M. *P<0.01, **P<0.05 vs. vehicle+sham animals, #P<0.05 vs. vehicle+MCAO animals.
DISCUSSION
Cerebral ischemia is associated with severe neurological deficits, such as cognitive disorders and sensorimotor impairment [6, 33, 39]. It also induces serious neurological impairments, including motor dysfunction and seizure. Previous studies showed that post-treatment of EGCG after MCAO ischemic injury exerts a neuroprotective effect [4, 17]. As a further study, we elucidated that pre-treatment of EGCG protects neuronal cells against focal cerebral ischemia. EGCG is considered as a potent preventative agent. Moreover, EGCG treatment prevents neurological dysfunction and alleviates brain edema caused by MCAO injury. Brain edema is accepted as a representative pathological symptom in ischemic stroke [29]. Moreover, EGCG treatment decreases infarct volume and attenuates histopathological changes. TUNEL-positive reactions indicate cell death, which accompanies the apoptotic process. We confirmed that MCAO injury increases the number of TUNEL-positive cells in the cerebral cortex, and that this increase was alleviated by EGCG treatment. Thus, we clearly demonstrated that EGCG exerts neuroprotective effects and preserves neuronal cells in the cerebral cortex of MCAO-injured animals.
Cerebral ischemic injury generates cytotoxic oxidants [2]. Oxidative stress induces mitochondrial dysfunction and leads to intracellular calcium overload [27]. Excessive calcium concentration induces excitotoxicity and impairs neuronal cells [12]. Excitotoxicity triggers necrosis and apoptosis in brain ischemia [28]. EGCG reduces oxidative damage and exerts neuroprotective effects against glutamate and kainic acid-induced excitotoxicity [15]. Moreover, oxidants destroy the blood-brain barrier and cell structure, which results in neuronal cell damage [35]. EGCG permeates the blood-brain barrier, becomes distributed in the brain parenchyma, and efficiently exerts protective effects during ischemic injury [26]. EGCG alleviates blood-brain barrier damage in cerebral ischemia by regulating tight junctions and protein kinase C signaling [19]. It also contributes to neurogenesis by modulating neuronal differentiation in neuronal precursor cells [38]. Our results clearly show that EGCG mediates neuroprotective functions against brain ischemic injury through various experimental techniques.
Apoptosis is a cell death process caused by oxidative stress and excitotoxicity. We demonstrated that MCAO injury activates the apoptotic signaling pathway and induces apoptotic cell death in the cerebral cortex, whereas EGCG prevents the MCAO-induced apoptotic signaling cascade and attenuates apoptotic neuronal cell death. Caspase-3 and PARP proteins are representative apoptotic indicators. Caspase-3 is a critical participant of apoptosis, and its activation is considered a general marker of apoptotic cell death. Activation of caspase promotes pro-inflammatory responses and cell death [16]. In addition, PARP is an enzyme that is involved in DNA repair to address damage due to stress [31]. Moreover, PARP induces apoptotic cell death by releasing apoptosis inducing factor [37]. Cerebral ischemia increases PARP expression in neurons, astrocytes, and microglial cells. However, brain damage caused by ischemic injury is alleviated through suppression of PARP by genetic modification or PARP inhibitor administration [3, 9, 23]. Thus, up-regulation of PARP during neuronal injury indicates apoptotic neuronal cell death and brain damage [22]. We observed increases of caspase-3 and PARP proteins in the cerebral cortex of MCAO mice. We clearly demonstrated that EGCG prevents MCAO-induced increases of caspase-3 and PARP expressions. Alleviation of such proteins increases indicates suppression of apoptotic cell death. In this study, we showed that EGCG attenuates infarction and apoptotic cell death in the cerebral cortex region during MCAO injury. EGCG also alleviates increases of caspase-3 and PARP expressions caused by MCAO. In conclusion, EGCG prevents MCAO-induced neurological dysfunction, infarction, and histopathological changes. It attenuates apoptotic cell death through inactivation of caspase-3 and PARP in MCAO surgery. Thus, our findings suggest that pre-treatment of EGCG acts as a neuroprotective agent in focal cerebral ischemia by modulating the apoptotic signaling cascade.
CONFLICT OF INTERESTS
The authors declare no competing financial interests.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (NRF-2018R1D1A1B07044074).
REFERENCES
- 1.Aktas O., Prozorovski T., Smorodchenko A., Savaskan N. E., Lauster R., Kloetzel P. M., Infante-Duarte C., Brocke S., Zipp F.2004. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 173: 5794–5800. doi: 10.4049/jimmunol.173.9.5794 [DOI] [PubMed] [Google Scholar]
- 2.Brown G. C.2010. Nitric oxide and neuronal death. Nitric Oxide 23: 153–165. doi: 10.1016/j.niox.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 3.Chiarugi A., Meli E., Calvani M., Picca R., Baronti R., Camaioni E., Costantino G., Marinozzi M., Pellegrini-Giampietro D. E., Pellicciari R., Moroni F.2003. Novel isoquinolinone-derived inhibitors of poly(ADP-ribose) polymerase-1: pharmacological characterization and neuroprotective effects in an in vitro model of cerebral ischemia. J. Pharmacol. Exp. Ther. 305: 943–949. doi: 10.1124/jpet.103.048934 [DOI] [PubMed] [Google Scholar]
- 4.Choi Y. B., Kim Y. I., Lee K. S., Kim B. S., Kim D. J.2004. Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res. 1019: 47–54. doi: 10.1016/j.brainres.2004.05.079 [DOI] [PubMed] [Google Scholar]
- 5.Choi Y. T., Jung C. H., Lee S. R., Bae J. H., Baek W. K., Suh M. H., Park J., Park C. W., Suh S. I.2001. The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 70: 603–614. doi: 10.1016/S0024-3205(01)01438-2 [DOI] [PubMed] [Google Scholar]
- 6.Ding Y., Zhou Y., Lai Q., Li J., Park H., Diaz F. G.2002. Impaired motor activity and motor learning function in rat with middle cerebral artery occlusion. Behav. Brain Res. 132: 29–36. doi: 10.1016/S0166-4328(01)00405-3 [DOI] [PubMed] [Google Scholar]
- 7.Dirnagl U., Iadecola C., Moskowitz M. A.1999. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22: 391–397. doi: 10.1016/S0166-2236(99)01401-0 [DOI] [PubMed] [Google Scholar]
- 8.Donnan G. A., Fisher M., Macleod M., Davis S. M.2008. Stroke. Lancet 371: 1612–1623. doi: 10.1016/S0140-6736(08)60694-7 [DOI] [PubMed] [Google Scholar]
- 9.Endres M., Wang Z. Q., Namura S., Waeber C., Moskowitz M. A.1997. Ischemic brain injury is mediated by the activation of poly(ADP-ribose)polymerase. J. Cereb. Blood Flow Metab. 17: 1143–1151. doi: 10.1097/00004647-199711000-00002 [DOI] [PubMed] [Google Scholar]
- 10.Fujiki H., Sueoka E., Watanabe T., Suganuma M.2015. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. Oncol. 141: 1511–1522. doi: 10.1007/s00432-014-1899-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi T., Abe K.2004. Ischemic neuronal cell death and organellae damage. Neurol. Res. 26: 827–834. doi: 10.1179/016164104X3770 [DOI] [PubMed] [Google Scholar]
- 12.Huang L., Chen N., Ge M., Zhu Y., Guan S., Wang J. H.2010. Ca2+ and acidosis synergistically lead to the dysfunction of cortical GABAergic neurons during ischemia. Biochem. Biophys. Res. Commun. 394: 709–714. doi: 10.1016/j.bbrc.2010.03.056 [DOI] [PubMed] [Google Scholar]
- 13.Hsu S.2015. Antioxidant and antiviral activities of lipophilic epigallocatechin gallate (EGCG) derivatives. Inflamm. Allergy Drug Targets 14: 13–18. doi: 10.2174/1871528114666151022150122 [DOI] [PubMed] [Google Scholar]
- 14.Jin Z., Liang J., Wang J., Kolattukudy P. E.2015. MCP-induced protein 1 mediates the minocycline-induced neuroprotection against cerebral ischemia/reperfusion injury in vitro and in vivo. J. Neuroinflammation 12: 39. doi: 10.1186/s12974-015-0264-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang K. S., Wen Y., Yamabe N., Fukui M., Bishop S. C., Zhu B. T.2010. Dual beneficial effects of (-)-epigallocatechin-3-gallate on levodopa methylation and hippocampal neurodegeneration: in vitro and in vivo studies. PLoS One 5: e11951. doi: 10.1371/journal.pone.0011951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kavanagh E., Rodhe J., Burguillos M. A., Venero J. L., Joseph B.2014. Regulation of caspase-3 processing by cIAP2 controls the switch between pro-inflammatory activation and cell death in microglia. Cell Death Dis. 5: e1565. doi: 10.1038/cddis.2014.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim S. H., Kim H. S., Kim Y. K., Kim T. M., Im S., Chung M. E., Hong B. Y., Ko Y. J., Kim H. W., Lee J. I.2010. The functional effect of epigallocatechin gallate on ischemic stroke in rats. Acta Neurobiol. Exp. (Warsz.) 70: 40–46. [DOI] [PubMed] [Google Scholar]
- 18.Lin J. K., Lin-Shiau S. Y.2006. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol. Nutr. Food Res. 50: 211–217. doi: 10.1002/mnfr.200500138 [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Wang Z., Wang P., Yu B., Liu Y., Xue Y.2013. Green tea polyphenols alleviate early BBB damage during experimental focal cerebral ischemia through regulating tight junctions and PKCalpha signaling. BMC Complement. Altern. Med. 13: 187. doi: 10.1186/1472-6882-13-187 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Longa E. Z., Weinstein P. R., Carlson S., Cummins R.1989. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20: 84–91. doi: 10.1161/01.STR.20.1.84 [DOI] [PubMed] [Google Scholar]
- 21.Mandel S., Amit T., Reznichenko L., Weinreb O., Youdim M. B.2006. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 50: 229–234. doi: 10.1002/mnfr.200500156 [DOI] [PubMed] [Google Scholar]
- 22.Moroni F.2008. Poly(ADP-ribose)polymerase 1 (PARP-1) and postischemic brain damage. Curr. Opin. Pharmacol. 8: 96–103. doi: 10.1016/j.coph.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Moroni F., Meli E., Peruginelli F., Chiarugi A., Cozzi A., Picca R., Romagnoli P., Pellicciari R., Pellegrini-Giampietro D. E.2001. Poly(ADP-ribose) polymerase inhibitors attenuate necrotic but not apoptotic neuronal death in experimental models of cerebral ischemia. Cell Death Differ. 8: 921–932. doi: 10.1038/sj.cdd.4400884 [DOI] [PubMed] [Google Scholar]
- 24.Nagai K., Jiang M. H., Hada J., Nagata T., Yajima Y., Yamamoto S., Nishizaki T.2002. (-)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res. 956: 319–322. doi: 10.1016/S0006-8993(02)03564-3 [DOI] [PubMed] [Google Scholar]
- 25.Nie G., Cao Y., Zhao B.2002. Protective effects of green tea polyphenols and their major component, (-)-epigallocatechin-3-gallate (EGCG), on 6-hydroxydopamine-induced apoptosis in PC12 cells. Redox Rep. 7: 171–177. doi: 10.1179/135100002125000424 [DOI] [PubMed] [Google Scholar]
- 26.Pervin M., Unno K., Nakagawa A., Takahashi Y., Iguchi K., Yamamoto H., Hoshino M., Hara A., Takagaki A., Nanjo F., Minami A., Imai S., Nakamura Y.2017. Blood brain barrier permeability of (-)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem. Biophys. Rep. 9: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Racay P., Tatarkova Z., Chomova M., Hatok J., Kaplan P., Dobrota D.2009. Mitochondrial calcium transport and mitochondrial dysfunction after global brain ischemia in rat hippocampus. Neurochem. Res. 34: 1469–1478. doi: 10.1007/s11064-009-9934-7 [DOI] [PubMed] [Google Scholar]
- 28.Radak D., Katsiki N., Resanovic I., Jovanovic A., Sudar-Milovanovic E., Zafirovic S., Mousad S. A., Isenovic E. R.2017. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr. Vasc. Pharmacol. 15: 115–122. doi: 10.2174/1570161115666161104095522 [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg G. A.1999. Ischemic brain edema. Prog. Cardiovasc. Dis. 42: 209–216. doi: 10.1016/S0033-0620(99)70003-4 [DOI] [PubMed] [Google Scholar]
- 30.Sanderson T. H., Reynolds C. A., Kumar R., Przyklenk K., Hüttemann M.2013. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 47: 9–23. doi: 10.1007/s12035-012-8344-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satoh M. S., Lindahl T.1992. Role of poly(ADP-ribose) formation in DNA repair. Nature 356: 356–358. doi: 10.1038/356356a0 [DOI] [PubMed] [Google Scholar]
- 32.Shamsaei N., Erfani S., Fereidoni M., Shahbazi A.2017. Neuroprotective Effects of Exercise on Brain Edema and Neurological Movement Disorders Following the Cerebral Ischemia and Reperfusion in Rats. Basic Clin. Neurosci. 8: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J. H., Tan L., Yu J. T.2014. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann. Transl. Med. 2: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thrift A. G., Howard G., Cadilhac D. A., Howard V. J., Rothwell P. M., Thayabaranathan T., Feigin V. L., Norrving B., Donnan G. A.2017. Global stroke statistics: An update of mortality data from countries using a broad code of “cerebrovascular diseases”. Int. J. Stroke 12: 796–801. doi: 10.1177/1747493017730782 [DOI] [PubMed] [Google Scholar]
- 35.van der Vliet A., Hoen P. A., Wong P. S., Bast A., Cross C. E.1998. Formation of S-nitrosothiols via direct nucleophilic nitrosation of thiols by peroxynitrite with elimination of hydrogen peroxide. J. Biol. Chem. 273: 30255–30262. doi: 10.1074/jbc.273.46.30255 [DOI] [PubMed] [Google Scholar]
- 36.Wu K. J., Hsieh M. T., Wu C. R., Wood W. G., Chen Y. F.2012. Green tea extract ameliorates learning and memory deficits in ischemic rats via its active component polyphenol epigallocatechin-3-gallate by modulation of oxidative stress and neuroinflammation. Evid. Based Complement. Alternat. Med. 2012: 163106. doi: 10.1155/2012/163106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu S. W., Andrabi S. A., Wang H., Kim N. S., Poirier G. G., Dawson T. M., Dawson V. L.2006. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. USA 103: 18314–18319. doi: 10.1073/pnas.0606528103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J. C., Xu H., Yuan Y., Chen J. Y., Zhang Y. J., Lin Y., Yuan S. Y.2017. Delayed Treatment with Green Tea Polyphenol EGCG promotes neurogenesis after ischemic stroke in adult mice. Mol. Neurobiol. 54: 3652–3664. doi: 10.1007/s12035-016-9924-0 [DOI] [PubMed] [Google Scholar]
- 39.Zhang L., Schallert T., Zhang Z. G., Jiang Q., Arniego P., Li Q., Lu M., Chopp M.2002. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J. Neurosci. Methods 117: 207–214. doi: 10.1016/S0165-0270(02)00114-0 [DOI] [PubMed] [Google Scholar]
- 40.Zhong Y., Chiou Y. S., Pan M. H., Shahidi F.2012. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 134: 742–748. doi: 10.1016/j.foodchem.2012.02.172 [DOI] [PubMed] [Google Scholar]