Abstract
Background
Despite the availability of aggressive lipid-lowering strategies, many patients remain at risk of cardiovascular events. C-reactive protein is a marker of inflammation elevated in patients at high risk of cardiovascular events. C-reactive protein has demonstrated value as a predictor of cardiovascular risk; however, it is unclear whether targeting C-reactive protein levels improves outcomes. This systematic review aimed to characterise the relationship between C-reactive protein and cardiovascular outcomes and to assess whether the magnitude of C-reactive protein reduction correlates to the extent of cardiovascular risk reduction.
Methods
A systematic review was conducted to identify randomised controlled trials that measured C-reactive protein before and after administration of therapies for cardiovascular disease and measured incidence of cardiovascular events. A meta-analysis of placebo-controlled studies assessed the relationship between extent of C-reactive protein reduction and cardiovascular risk reduction. Placebo-controlled studies where low-density lipoprotein and triglyceride data were available were also included in a meta-regression to assess the influence of these established risk factors on the efficacy of treatment when compared to C-reactive protein.
Results
Fifteen studies met the criteria for inclusion in this review, of which six were active comparator studies and nine were placebo controlled. Six placebo-controlled studies had data available for meta-regression. Eight studies demonstrated a reduction in events that could be explained by changes in lipid levels, whereas the results of five studies suggested that the association between C-reactive protein reduction and event rates cannot be explained by changes in lipid levels alone. No correlation was found between magnitude of C-reactive protein reduction and cardiovascular risk reduction. A strong correlation was found between C-reactive protein and low-density lipoprotein reduction (adjusted r2 = 0.8).
Conclusions
Targeting C-reactive protein does not offer additional benefit over targeting low-density lipoprotein across the general population in terms of cardiovascular risk reduction. However, there is value in targeting C-reactive protein in patients at high residual inflammatory risk despite non-elevated lipid levels or use of lipid-lowering therapy.
Keywords: Risk factors, atherosclerosis, cardiology, inflammation
Introduction
Atherosclerosis is now considered to be primarily a progressive inflammatory disease. Innate immune responses are activated during the development of atherosclerotic plaques, leading to the secretion of interleukin (IL)-1α and the activation of the inflammasome complex, which produces activated cytokines IL-1β and IL-18.1 Levels of IL-1β in atherosclerosis have been correlated to disease severity, with circulating IL-1β producing increases in reactive oxygen species, matrix metalloproteinases,2 inflammatory cytokines and activated T-cells, resulting in disruption of the extracellular matrix.
Although atherosclerosis involves chronic inflammation at every stage, there are no approved therapies to treat this inflammatory component. Canakinumab is a monoclonal antibody against IL-1β approved for auto-inflammatory disorders, which was evaluated in CANTOS.3 CANTOS showed that canakinumab reduces the risk of secondary cardiovascular events, representing conclusive evidence that targeting the inflammatory processes of atherosclerosis alone improves outcomes. An increase in serious and fatal infections contributed to the regulatory rejection of canakinumab for use in cardiovascular disease.
Statin medications, which inhibit cholesterol synthesis, have also been shown to possess immunomodulatory and anti-inflammatory properties. Statins have anti-inflammatory effects on the vascular endothelium via upregulation of endothelial nitric oxide synthase, increasing nitric oxide (NO) production. Statins also decrease the production of superoxide radicals and other reactive oxygen species via reduction of reduced nicotinamide adenine dinucleotide phosphate oxidase activity.4
Established biomarkers used to assess cardiovascular risk include total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglycerides. Plasma levels of apolipoprotein B (ApoB), which is present in very low density lipoprotein, LDL and IDL particles, can also be measured to provide an indication of atherogenic cholesterol. The ratio of ApoB to ApoA1 can be used as a risk marker,5 as this ratio reflects the balance between pro- and anti-atherogenic lipoproteins.
C-reactive protein (CRP) is the most widely investigated inflammatory marker examined in the context of atherosclerotic disease. It has been adopted as a biomarker of inflammation with applications in cancer, infections, chronic inflammatory diseases, diabetes and cardiovascular diseases. It is primarily produced by hepatocytes in response to secretion of IL-6 via upregulation of transcription factors CCAAT/enhancer-binding protein β (C/EBPβ) and C/EBPδ; however, it is also produced by endothelial cells, lymphocytes, smooth muscle cells and macrophages in atherosclerotic lesions.6 IL-1β and tumour necrosis factor can also increase the transcription rate of genes encoding CRP.7
An individual participant meta-analysis of 54 studies involving 160,309 subjects without cardiovascular disease(CVD) showed that CRP levels were linearly associated with coronary heart disease, ischaemic stroke and cardiovascular mortality.8 However, correction for conventional risk factors and baseline fibrinogen levels considerably weakened the association between CRP levels and coronary heart disease and eliminated the association between CRP levels and stroke. This suggests that CRP should not be used to assess risk independent of validated risk factors but should be used as an additional tool to identify those with prevalent atherosclerosis.
To date, a comprehensive evaluation of the association between changes in CRP levels following anti-inflammatory treatment and cardiovascular outcomes has not been conducted. This research aimed to characterise the relationship between achieved CRP and incidence of major adverse cardiovascular events (MACEs).
Methods
Literature search strategy
Inclusion criteria for the systematic review and meta-analysis were randomised controlled trials published in English that measured CRP levels before and after treatment with an anti-inflammatory therapy for at least 24 weeks and reported MACE (Table 1). Studies were excluded where the change in CRP could not be determined, the chosen therapy did not exert anti-inflammatory effects and significant comorbidities were present, such as heart or kidney failure. Placebo-controlled study designs were included in the meta-analysis, with studies comparing two or more interventions included in a narrative synthesis. Placebo-controlled studies that also recorded changes in LDL and triglycerides were included in a prespecified meta-regression.
Table 1.
PICOS eligibility criteria.
| Population | Those at risk of cardiovascular disease |
| Intervention | Statin or anti-inflammatory therapy that decreases CRP |
| Comparison | Placebo or statin/ anti-inflammatory comparator |
| Outcome | Major adverse cardiovascular events |
| Study design | Randomised controlled trials |
PubMed, Medline, Embase and Google Scholar were searched using a combination of the following keywords: (statin or anti-inflammatory) and (cardiovascular disease or atherosclerosis) and (CRP or hsCRP or C-reactive protein) and (Trial or RCT) and (MACE or cardiovascular events or mortality or outcomes or stroke or angina or myocardial infarction). The search was carried out between the 21 and 23 April 2019. The reference lists of each selected study were also consulted to identify further studies. One reviewer screened and selected articles for further review. The titles and abstracts were reviewed to assess whether the study met the prespecified criteria, with relevant articles then selected for a full-text review.
Quality and risk-of-bias assessments
The quality of each study selected for inclusion was assessed using the quality assessment tool for controlled intervention studies published by the National Heart, Lung, and Blood Institute.9 The final rating was determined by an overall judgement of the relevance of any issues to the validity of the results, and each study was given a grade of ‘good’, ‘fair’ or ‘poor’, which also took into account factors such as the applicability of the question to the study being assessed, and the impact of the quality of reporting on the information available for the quality assessment. Studies receiving a rating of ‘poor’ were to be excluded from the meta-analysis. The revised Cochrane risk-of-bias tool (RoB2) for randomised trials was used to assess the risk of bias of each study selected for inclusion.
Data extraction
The median baseline CRP, median achieved CRP, median change from baseline (%) and the percentage difference in CRP change from baseline between treatment groups reported in each study publication were collated. Incidence of MACE, which was defined as nonfatal myocardial infarction, nonfatal stroke, cardiovascular mortality, unstable angina requiring hospitalisation and urgent revascularisation, was also extracted for both the control and intervention groups and divided by the number of participants in each group to find the event rate. The between-group percentage differences in MACE incidence and follow-up duration were also recorded.
In order to carry out a meta-regression examining the influence of conventional risk factors LDL and triglycerides on cardiovascular outcomes, mean baseline LDL, mean achieved LDL, percentage change from baseline and between-group difference in achieved LDL were recorded. The same values were recorded for the triglyceride measurements.
Data synthesis
Due to methodological differences in the studies included in this review, the data collected were synthesised narratively, with a subset of the studies included in a meta-analysis. The data collected were assessed in turn in order to determine the extent of a relationship between a reduction in CRP and event risk. For the meta-analysis, an initial descriptive analysis of effect size, a forest plot illustrating the effect summary and a scatter plot of percentage change in CRP vs. percentage change in MACE were created using Microsoft Excel version 14.7.7. To calculate the effect summary and to graph the forest plot in Excel, the method described by Neyeloff et al. was employed.10 Heterogeneity between studies was measured using the I2 statistic (I2 = 100% × (Q –df)/Q), where Q is Cochran’s heterogeneity statistic. Due to high heterogeneity in the study populations and treatments (I2 = 81%), a random-effects model was applied. A meta-regression to examine the influence of LDL and triglyceride reduction on MACE, and the association between LDL and CRP reduction, was carried out using the metareg command in Stata version 15.1.
Results
Literature search
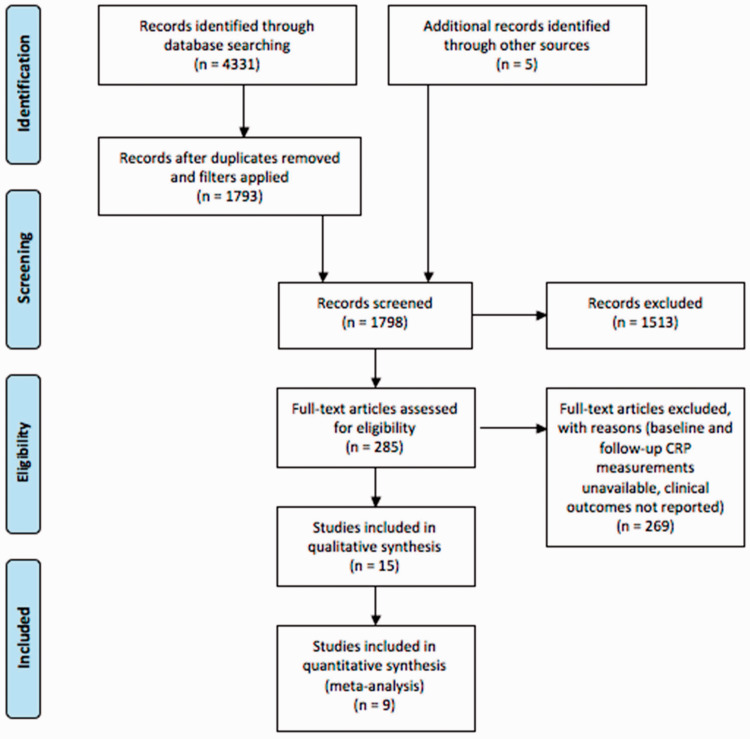
A total of 4331 articles were retrieved from PubMed, Medline, Embase and Google Scholar (Figure 1). After removing duplicates (Medline/Embase) and applying filters for the English language and clinical trials (PubMed and Medline/Embase), 1793 titles and abstracts were reviewed. Five articles were identified from reference lists. Of 285 full-text articles assessed, 269 were excluded, as they did not measure CRP before and after treatment, MACE incidence was not recorded or another aspect of the eligibility criteria was not met. Ultimately, 15 studies were eligible for inclusion in the narrative review, nine studies were included in the meta-analysis and six studies met the criteria for the meta-regression.
Figure 1.
PRISMA flow diagram: the literature search and screening process.
Quality and risk-of-bias assessments
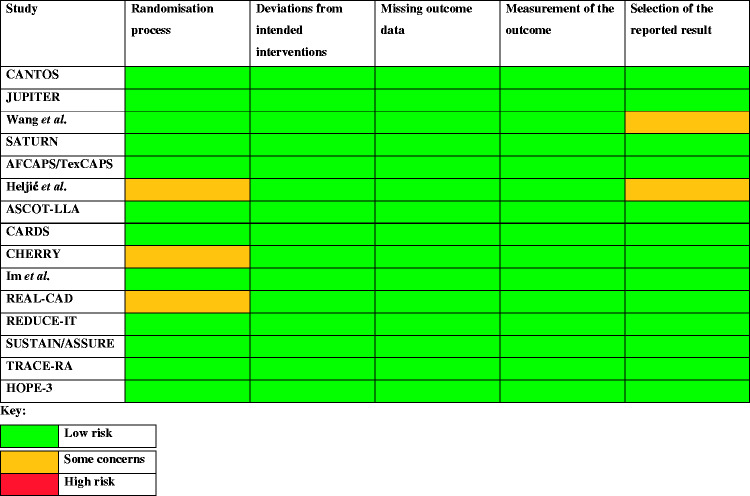
Of the 15 studies included in this review, 12 received a ‘good’ rating and 3 received a ‘fair’ rating. No studies received a ‘poor’ rating (Appendix 1). No studies were associated with a high risk of bias in any domain of the Cochrane risk-of-bias tool (Appendix 2).
Characteristics of the included studies
Of the 15 studies included, six studies used an active comparator study design. Wang et al. compared ezetimibe 10 mg/day and rosuvastatin 10 mg/day to rosuvastatin 10 mg/day alone;11 the SATURN study compared atorvastatin 80 mg to rosuvastatin 40 mg;12 the CHERRY study compared pitavastatin 4 mg and eicosapentaenoic acid 1800 mg to pitavastatin 4 mg alone;13 Im et al. compared atorvastatin 40 mg to pravastatin 20 mg;14 the REAL-CAD study compared pitavastatin 4 mg to pitavastatin 1 mg;15 the REDUCE-IT study evaluated icosapent ethyl in addition to statin therapy in comparison to statin therapy alone.16
The remaining nine studies compared a single intervention to placebo, with seven studies evaluating statin therapy,17–23 one study evaluating canakinumab3 and the SUSTAIN/ASSURE studies evaluating RVX-208, which is an inhibitor of bromo- and extra-terminal proteins that regulate ApoA1.24 Six placebo-controlled studies17,19–23 were included in the meta-regression, with TRACE-RA and HOPE-3 excluded due to missing LDL and triglyceride data, and CANTOS excluded as canakinumab has no effect on lipid levels (Appendix 3).
Association between CRP reduction and cardiovascular outcomes
Active comparator studies
Association is dependent on lipid lowering: In Wang et al., CRP decreased by 66.4% in the rosuvastatin group and by 78.2% in the rosuvastatin and ezetemibe group at 12 months.11 MACE incidence was 12.5% in the rosuvastatin group and 4% in the rosuvastatin and ezetimibe group. As ezetemibe does not typically decrease CRP alone,25,26 the additional reduction in CRP is likely to be related to the additional reduction in LDL observed in the rosuvastatin and ezetemibe group, when compared to the rosuvastatin group (62% vs. 47%, respectively). In the REAL-CAD study, CRP levels were 14% lower in the pitavastatin 4 mg group vs. the 1 mg group, with no change from baseline observed in the 1 mg group.15 A reduction in LDL of 16% from baseline in the 4 mg group and no change in the 1 mg group is likely to contribute to the difference in event rates (4.3% in the 4 mg group vs. 5.4% in the 1 mg group).
During REDUCE-IT, which evaluated the addition of icosapent ethyl 4 g/day to statin therapy, median CRP increased from 2.1 to 2.8 mg/l in the statin-only group.16 In the icosapent ethyl group, median CRP decreased from 2.2 to 1.8 mg/l. MACE occurred in 22% of the statin-only group and 17.2% of the icosapent ethyl group. Icosapent ethyl has been proven to reduce triglyceride levels, which are a validated risk factor for cardiovascular disease. Specifically, follow-up triglyceride levels were 20% lower in the intervention group, which may account for the decrease in events observed.
The reduction in MACE is proportional to the reduction in CRP achieved in these studies. However, there is no evidence to suggest that the risk reduction observed in these studies is mainly due to anti-inflammatory factors, as the extent of reduction in lipid levels also correlates to clinical outcome. The results from these studies support the view that additional CRP reduction in the intervention group may be a by-product of an improvement in the patients’ conditions, rather than a causal factor.
Association is independent of lipid lowering: Im et al. found that pravastatin 20 mg did not decrease CRP and was associated with a MACE incidence of 3.6%, whereas atorvastatin 40 mg decreased CRP by 22.2% from baseline and was associated with an event rate of 2%.14 Changes in LDL were minimal, although some patients were allocated to a less intensive treatment than their typical regimen. Nevertheless, the reduction in event rates achieved in this study suggests that anti-inflammatory pleiotropic effects of statin treatment are partly attributable.
No association between CRP reduction and cardiovascular outcomes: In SATURN and CHERRY, no associations were found between on-treatment CRP levels and event risk. In SATURN, rosuvastatin 40 mg was significantly more effective at lowering CRP vs. atorvastatin 80 mg; however, no significant differences in MACE were found.12 As SATURN was not powered to detect differences in MACE, conclusions cannot be drawn as to the presence of an association between CRP reduction and outcomes. In CHERRY, no significant between-group differences were observed for CRP reduction or MACE incidence.13 Although it could be argued that a lack of additional CRP reduction is associated with a lack of additional cardiovascular risk reduction in these studies, conventional risk factors LDL and triglyceride levels also showed little change after treatment in both SATURN and CHERRY. This suggests that aspects of the design and methodology of the studies, such as prior statin treatment in both study populations and lack of statistical power, hinder the ability to rationalise the lack of between-group differences.
Placebo-controlled studies
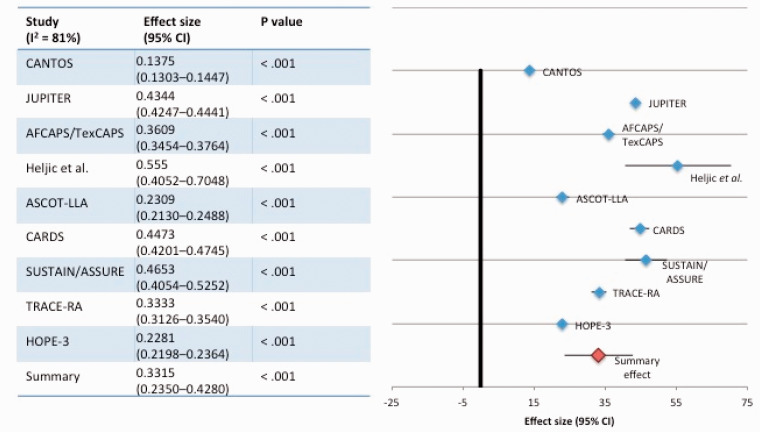
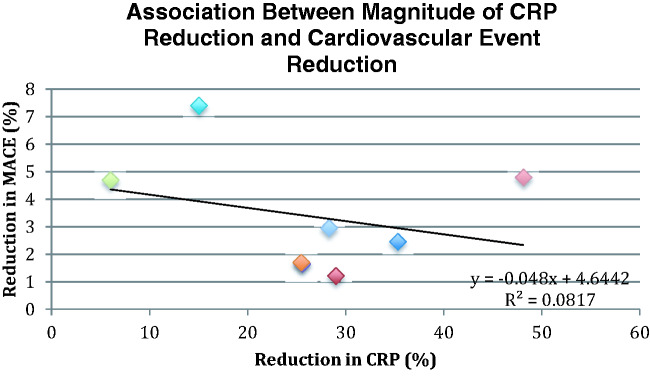
Treatment with statins, canakinumab and RVX-208 decreased the incidence of MACE in every placebo-controlled study (Figure 2). Each therapy decreased CRP; however, there was no correlation between the magnitude of CRP reduction achieved and the decrease in cardiovascular events between the groups in these studies (R2 = 0.082, p < 0.49) (Figure 3).
Figure 2.
Effect of treatment on cardiovascular event rates (placebo-controlled studies).
Figure 3.
Association between magnitude of CRP reduction and cardiovascular event reduction (p < 0.49).
Association is dependent on lipid lowering: Heljić et al. evaluated simvastatin in patients with type II diabetes mellitus without CVD.17 CRP decreased by 4% in the placebo group and 19% in the simvastatin 40 mg group. Moreover, 40% of patients receiving placebo experienced an event vs. 17.8% receiving statin therapy. LDL reduction was proportional to CRP reduction. This study reported the greatest between-group difference in event rates. This may be related to its small size (n = 95); additionally, it is not stated whether this study was powered to detect differences in clinical outcomes. In CARDS, atorvastatin 10 mg reduced event risk by 37%, CRP by 32% and LDL by 42% vs. placebo.22 No significant association was found between baseline, on-treatment or change in CRP and cardiovascular risk. No evidence was found that achieving a target CRP level in addition to a target LDL level further reduced risk.
ASCOT-LLA found that statin therapy decreased MACE by 22.81% vs. placebo in hypertensive patients without elevated lipids, with CRP 25% lower in the atorvastatin group vs. placebo.21 LDL levels decreased by 29% and triglycerides by 13%. Those who achieved CRP levels below the median did not have a reduced event risk when compared to those who did not achieve CRP levels below the median [OR 0.86, 95% CI: 0.49–1.51]. In contrast, event risk was significantly reduced in those achieving LDL below the median [OR 0.41, 95% CI: 0.22–0.75]. HOPE-3 also evaluated statin therapy in patients at intermediate risk without CVD.23 Although a subgroup analysis based on achieved CRP was not carried out, this study found that rosuvastatin possessed similar efficacy regardless of baseline CRP level. Small reductions in CRP were achieved when compared to the 26.5% reduction in LDL (log-transformed mean difference: 0.19 mg/l). The results of these four studies suggest that in primary prevention settings, achieving lower CRP does not provide a substantial benefit on top of achieving lipid targets.
TRACE-RA evaluated statin therapy in rheumatoid arthritis (RA) patients without pre-existing CVD.18 LDL and CRP levels were 34% and 28% lower in the atorvastatin 40 mg group vs. placebo, respectively. A non-significant 34% reduction in MACE was recorded. It appears that statins maintain their lipid-lowering effect in this population despite active inflammation, and although LDL levels are typically lower in RA patients (baseline LDL: 3.2 mmol/l), the 35% reduction achieved indicates that reduction of events was achieved primarily through lipid lowering.
Association is independent of lipid lowering: AFCAPs/TexCAPS evaluated lovastatin 20 mg vs. placebo in participants without established CVD and average LDL levels.20 Lovastatin decreased CRP by 14.8% vs. no reduction, with LDL levels reduced by 25%. The reduction in CRP observed with lovastatin was not correlated to LDL or triglyceride reduction. MACE occurred in 2.94% of those receiving lovastatin vs. 4.6% receiving placebo, with stratified analyses showing that lovastatin was effective in participants who had higher than average CRP and lower than average LDL, but not in those with both lower than average LDL and CRP.
JUPITER evaluated statin therapy in those with normal LDL cholesterol levels (<3.4 mmol/l) but elevated CRP (>2 mg/l).19 Rosuvastatin reduced CRP by 37% and LDL by 50%, which was associated with a 44% reduction in MACE. For those who achieved LDL < 1.8 mmol/l and CRP < 2 mg/l, MACE incidence was reduced by 65% vs. 33% in those who achieved one or neither targets.27 This points towards a predictive role for achieved CRP in relation to cardiovascular risk. In CANTOS, which evaluated anti-IL-1β antibody canakinumab in participants with normal LDL, 35–40% reductions in IL-6 and CRP were observed in the 150 and 300 mg canakinumab groups with no changes in cholesterol levels, which was predictive of clinical efficacy. The extent of reduction in CRP following canakinumab was strongly correlated to cardiovascular risk reduction.3
SUSTAIN/ASSURE suggested an association between CRP reduction and clinical outcomes that cannot be explained by changes in lipid levels.24 Treatment with RVX-208 and placebo produced no clinically significant changes in LDL and reduced CRP by 28.4% and 22.4%, respectively. MACE incidence was 5.4% in the treatment group vs. 10.1% with placebo. A significant but modest between-group increase (7.69%) in HDL levels was observed, which could not account for the reduction in events seen in this study.24 Anti-inflammatory mechanisms of RVX-208 have been proposed, which are supported by reductions in chemokine CCL18 and IL-18, by 22% and 10%, respectively, in groups treated with RVX-208.24 These four studies support the view that on-treatment reductions in inflammatory markers are predictive of clinical efficacy. Although they do not demonstrate a clear relationship between magnitude of reduction in CRP and reduction in cardiovascular risk, differences in study populations and treatment regimens complicate direct comparisons.
Meta-regression
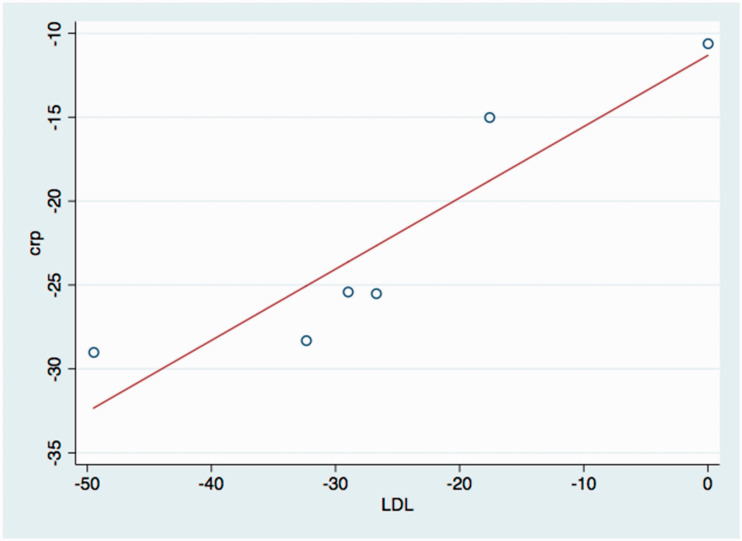
A meta-regression of six placebo-controlled studies showed a strong correlation (adjusted r2 = 0.8, p < 0.011) between changes in LDL levels and CRP levels (Figure 4). When the influence of changes in LDL and triglyceride levels on the effect sizes in each study was analysed, it was found that changes in CRP accounted for 54.25% of between-study differences in event rates and that LDL and triglyceride reduction accounted for 68.35% of between-study differences in event rates, which is accordance with the fact that CRP reduction is strongly correlated to LDL reduction. CRP, LDL and triglyceride reduction accounted for 80% of between-study differences in event rates.
Figure 4.
Association between LDL and CRP reduction.
Discussion
This systematic review evaluated the extent of reduction in CRP achieved by treatments for CVD and whether this correlated with improvements in outcomes in all trials that measured CRP before and after treatment and recorded incidence of adverse cardiac events. This research found that CRP reduction occurred alongside a reduction in events; however, a greater magnitude of reduction did not signify better clinical outcomes. The balance of the evidence supports the view that improvements in clinical outcomes can largely be accounted for by reduction in LDL, with CRP reduction occurring as a by-product to LDL reduction. These findings are in accordance with a meta-analysis conducted by Kinlay in 2007, which assessed the extent of the association between changes in CRP and LDL after treatment with a cholesterol-lowering therapy.28 This study found a variance-adjusted correlation between change in CRP and LDL of 0.80 (p < 0.001) and showed that 89–98% of CRP reduction was related to LDL reduction, with 2–11% of changes in CRP levels related to the anti-inflammatory effects of statins. This is in accordance with the strong correlation found in this review between CRP and LDL reduction (Figure 4).
The conflicting evidence provided by the included studies is likely to result from differences in study populations and baseline characteristics, supporting the view that targeting CRP in entire patient populations is less effective than stratifying based on baseline CRP. In studies with no restrictions as to baseline CRP, baseline CRP is lower (e.g. 1.4 mg/l in CARDS,22 2.7 mg/l in ASCOT-LLA21 and 2.0 mg/l in HOPE-3,23 compared to 4.3 mg/l in JUPITER19), which may explain the lack of evidence supporting cardiovascular risk reduction mediated by changes in CRP in these studies.
The CANTOS trial demonstrated that on-treatment CRP levels are effective in predicting the clinical response from anti-inflammatory therapies such as canakinumab. This reflects the fact that canakinumab treatment is intended for those with high residual inflammatory risk that is not adequately controlled by cholesterol-lowering therapies, whereas statin therapy is indicated for patients at risk of CVD regardless of their inflammation status.
Based on the results of this review, achieved CRP cannot be used as a reliable method of determining the efficacy of existing treatments for CVD. In the case of treatment strategies that solely target inflammation, the existence of a dose–response relationship for CRP reduction in CANTOS (26%, 37% and 41% in the 50, 150 and 300 mg treatment groups, respectively) did not directly translate to improved risk reduction (heart rate compared to placebo: 0.93, 0.80 and 0.85 in the 50, 150 and 300 mg groups, respectively).3 However, there is a clear relationship between reductions in inflammation and improved outcomes, as canakinumab exhibits no pharmacological effects that are not anti-inflammatory.
Ultimately, multiple processes contribute to the progression of atherosclerosis and the development of CVD. Effective treatment is likely to involve early and aggressive lipid-lowering and anti-inflammatory therapies, with CRP levels used to assess who is most likely to benefit from anti-inflammatory treatment. This research has shown for the first time that the magnitude of reduction in CRP achieved with therapies for atherosclerosis does not directly correlate to cardiovascular event reduction across clinical studies; however, there is a lack of data on treatments that reduce inflammation independent of cholesterol lowering. Further clinical studies of novel anti-inflammatory agents are necessary to expand on the efficacy observed during CANTOS.
In 2018, Ridker et al. reported that on-treatment CRP reduction after the first dose of canakinumab was predictive of clinical benefit during CANTOS when adjusted for baseline CRP and LDL cholesterol, but that baseline characteristics did not reliably predict efficacy.29 This may support a future use of achieved CRP levels in the clinic to assess whether anti-inflammatory treatment should be continued, which is particularly relevant from a cost-effectiveness perspective. However, this research does not support the routine use of on-treatment CRP to assess response to current therapies.
One strength of this review is the inclusion of studies that measured hard clinical endpoints. Another related strength is the quality of the studies, with 80% receiving the highest quality grade, and no studies receiving the lowest grade. There are several important limitations. First, one reviewer screened and selected studies. The meta-analysis also included both primary and secondary prevention studies, which may have affected the outcome due to differing baseline characteristics. Inconsistent duration of follow-up may also have introduced variation.
Conclusion
This systematic review suggests that the magnitude of CRP reduction does not correlate with cardiovascular outcomes following treatment with therapies for atherosclerosis. Established risk factors LDL and triglycerides had a greater influence on changes in event rates than changes in CRP, with CRP reduction strongly associated with LDL reduction. Lipid targets should remain the primary focus for cardiovascular risk reduction, with on-treatment CRP most useful for patients with high levels of inflammation characteristic of severe atherosclerosis.
Acknowledgements
We thank Emily Robinson (Research Fellow in Medical Statistics at King's College London) for statistical advice.
Appendix 1. Quality assessment
Y: yes; N: no; NR: not reported; CD: cannot be determined; N/A: not applicable.
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CANTOS | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| JUPITER | Y | Y | Y | Y | Y | Y | NR | NR | Y | Y | Y | Y | Y | Y | Good |
| Wang et al.11 | Y | Y | Y | NR | NR | Y | Y | N | Y | NR | Y | N | CD | CD | Fair |
| SATURN | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Good |
| AFCAPS/TexCAPS | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Good |
| Heljić et al.17 | Y | NR | NR | Y | Y | Y | NR | NR | Y | Y | Y | N | CD | CD | Fair |
| ASCOT-LLA | Y | Y | Y | N | Y | Y | Y | Y | NR | Y | Y | Y | Y | Y | Good |
| CARDS | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| CHERRY | Y | NR | NR | N | NR | Y | Y | Y | Y | Y | Y | N | Y | Y | Fair |
| Im et al.14 | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| REAL-CAD | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| REDUCE-IT | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y | Y | Y | Y | Good |
| SUSTAIN/ASSURE | Y | NR | NR | Y | Y | Y | NR | NR | NR | Y | Y | Y | Y | Y | Good |
| TRACE-RA | Y | Y | Y | Y | Y | Y | NR | NR | N | Y | Y | Y | Y | Y | Good |
| HOPE-3 | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Good |
Questions:
Was the study described as randomised, a randomised trial, a randomised clinical trial or an RCT?
Was the method of randomisation adequate (i.e. use of randomly generated assignment)?
Was the treatment allocation concealed (so that assignments could not be predicted)?
Were study participants and providers blinded to treatment group assignment?
Were the people assessing the outcomes blinded to the participants’ group assignments?
Were the groups similar at baseline on important characteristics that could affect outcomes (e.g. demographics, risk factors and co-morbid conditions)?
Was the overall drop-out rate from the study at endpoint 20% or lower of the number allocated to treatment?
Was the differential drop-out rate (between treatment groups) at endpoint 15 percentage points or lower?
Was there high adherence to the intervention protocols for each treatment group?
Were other interventions avoided or similar in the groups (e.g. similar background treatments)?
Were outcomes assessed using valid and reliable measures, implemented consistently across all study participants?
Did the authors report that the sample size was sufficiently large to be able to detect a difference in the main outcome between groups with at least 80% power?
Were outcomes reported or subgroups analysed prespecified (i.e. identified before analyses were conducted)?
Were all randomised participants analysed in the group to which they were originally assigned, i.e. did they use an intention-to-treat analysis?
Appendix 2. Cochrane risk-of-bias tool (RoB2)
| ||||||||
| Ridker et al.,3 CANTOS | 2017 | 10,061 | dbRCT | Previous MI, hsCRP ≥ 2 mg/l | Canakinumab 50, 100 and 150 mg | Placebo | Nonfatal MI, nonfatal stroke and CV mortality | |
| Ridker et al.,19 JUPITER | 2008 | 17,802 | dbRCT | No CVD history, LDL-C < 3.4 mmol/l and hsCRP ≥ 2 mg/l | Rosuvastatin 20 mg | Placebo | First MACE | |
| Wang et al.11 | 2016 | 106 | RCT | CHD + hyperlipidemia | Ezetimibe 10 mg + rosuvastatin 10 mg | Rosuvastatin 10 mg | New/recurrent MI, unstable angina, CV mortality and stroke | |
| Nicholls et al.,12 SATURN | 2011 | 1039 | dbRCT | One vessel with >20% stenosis + target vessel <50% obstructed | Atorvastatin 80 mg | Rosuvastatin 40 mg | Percent change in atheroma volume | Also measured MACE |
| Ridker et al.,20 AFCAPS/TexCAPS (substudy) | 2001 | 5742 | dbRCT | Average TC and LDL, below average HDL | Lovastatin 20 mg, adjusted to 40 mg as necessary | Placebo | First acute coronary event | |
| Heljić et al.17 | 2009 | 95 | RCT | T2DM without CHD | Simvastatin 40 mg | Placebo | Acute MI, revascularisation and stroke | |
| Sever et al.,21 ASCOT-LLA (substudy) | 2013 | 2772 | Randomised open-label, blinded-endpoint | Hypertensive patients with 3+ CVD risk factors but no MI or angina history | Atorvastatin 10 mg | Placebo | CV mortality, nonfatal MI, revascularisation and stroke | |
| Yusuf et al.,23 HOPE-3 | 2016 | 12,705 | dbRCT | At least one CVD risk factor (men) or at least two (women) | Rosuvastatin 10 mg | Placebo | CV mortality, nonfatal MI, nonfatal stroke, cardiac arrest, HF, revascularisation | |
| Soedamah-Muthu et al.,22 Collaborative Atorvastatin Diabetes Study | 2015 | 2322 | RCT | T2DM | Atorvastatin 10 mg | Placebo | MACE | |
| Watanabe et al.,13 CHERRY | 2017 | 193 | Non-blinded RCT | Stable angina, PCI | Eicosapentaenoic acid 1800 mg + pitavastatin 4 mg | Pitavastatin 4 mg | Change in coronary plaque tissue characteristics | Also measured MACE |
| Im et al.14 | 2018 | 2000 | dbRCT | PCI within 12 months, aspirin monotherapy | Atorvastatin 40 mg | Pravastatin 20 mg | MACE | |
| Kitas et al.,18 TRACE-RA | 2019 | 3002 | dbRCT | RA | Atorvastatin 40 mg | Placebo | MACE | |
| Taguchi et al.,15 REAL-CAD | 2018 | 13,054 | Randomised open-label, blinded-endpoint | Stable CAD | Pitavastatin 4 mg | Pitavastatin 1 mg | CV mortality, nonfatal stroke, nonfatal MI, unstable angina | |
| Bhatt et al.,16 REDUCE-IT | 2019 | 8179 | dbRCT | Established CVD, diabetes or other risk factors | Icosapent ethyl 2 g twice daily | Placebo | MACE | |
| Gilham et al.,24 SUSTAIN/ASSURE | 2016 | 499 | dbRCT | Stable CAD (SUSTAIN), coronary angiography (ASSURE) | RVX-208 100 mg twice daily + SOC | Placebo + SOC | MACE | |
Appendix 3. Characteristics of the included studies
| Study (authors/title) | Year published | Number of participants | Type of study | Inclusion criteria | Intervention | Comparator | Primary endpoints | Notes |
|---|---|---|---|---|---|---|---|---|
| Ridker et al.,3 CANTOS | 2017 | 10,061 | dbRCT | Previous MI, hsCRP ≥2 mg/l | Canakinumab 50, 100 and 150 mg | Placebo | Nonfatal MI, nonfatal stroke and CV mortality | |
| Ridker et al.,19 JUPITER | 2008 | 17,802 | dbRCT | No CVD history, LDL-C < 3.4 mmol/l and hsCRP ≥ 2 mg/l | Rosuvastatin 20 mg | Placebo | First MACE | |
| Wang et al.11 | 2016 | 106 | RCT | CHD + hyperlipidemia | Ezetimibe 10 mg + rosuvastatin 10 mg | Rosuvastatin 10 mg | New/recurrent MI, unstable angina, CV mortality and stroke | |
| Nicholls et al.,12 SATURN | 2011 | 1039 | dbRCT | One vessel with >20% stenosis + target vessel <50% obstructed | Atorvastatin 80 mg | Rosuvastatin 40 mg | Percent change in atheroma volume | Also measured MACE |
| Ridker et al.,20 AFCAPS/TexCAPS (substudy) | 2001 | 5742 | dbRCT | Average TC and LDL, below average HDL | Lovastatin 20 mg, adjusted to 40 mg as necessary | Placebo | First acute coronary event | |
| Heljić et al.17 | 2009 | 95 | RCT | T2DM without CHD | Simvastatin 40 mg | Placebo | Acute MI, revascularisation and stroke | |
| Sever et al.,21 ASCOT-LLA (substudy) | 2013 | 2772 | Randomised open-label, blinded-endpoint | Hypertensive patients with 3+ CVD risk factors but no MI or angina history | Atorvastatin 10 mg | Placebo | CV mortality, nonfatal MI, revascularisation and stroke | |
| Yusuf et al.,23 HOPE-3 | 2016 | 12,705 | dbRCT | At least one CVD risk factor (men) or at least two (women) | Rosuvastatin 10 mg | Placebo | CV mortality, nonfatal MI, nonfatal stroke, cardiac arrest, HF, revascularisation | |
| Soedamah-Muthu et al.,22 Collaborative Atorvastatin Diabetes Study | 2015 | 2322 | RCT | T2DM | Atorvastatin 10 mg | Placebo | MACE | |
| Watanabe et al.,13 CHERRY | 2017 | 193 | Non-blinded RCT | Stable angina, PCI | Eicosapentaenoic acid 1800 mg + pitavastatin 4 mg | Pitavastatin 4 mg | Change in coronary plaque tissue characteristics | Also measured MACE |
| Im et al.14 | 2018 | 2000 | dbRCT | PCI within 12 months, aspirin monotherapy | Atorvastatin 40 mg | Pravastatin 20 mg | MACE | |
| Kitas et al.,18 TRACE-RA | 2019 | 3002 | dbRCT | RA | Atorvastatin 40 mg | Placebo | MACE | |
| Taguchi et al.,15 REAL-CAD | 2018 | 13,054 | Randomised open-label, blinded-endpoint | Stable CAD | Pitavastatin 4 mg | Pitavastatin 1 mg | CV mortality, nonfatal stroke, nonfatal MI, unstable angina | |
| Bhatt et al.,16 REDUCE-IT | 2019 | 8179 | dbRCT | Established CVD, diabetes or other risk factors | Icosapent ethyl 2 g twice daily | Placebo | MACE | |
| Gilham et al.,24 SUSTAIN/ASSURE | 2016 | 499 | dbRCT | Stable CAD (SUSTAIN), coronary angiography (ASSURE) | RVX-208 100 mg twice daily + SOC | Placebo + SOC | MACE |
Contributorship
AB performed the searches and analyses. AB and AF wrote the paper.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
All primary data were collected from previously published clinical studies that obtained informed consent from participants. No further ethics approval was required to collect and analyse these data. This research was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor
Both AB and AF act as guarantors for the paper.
ORCID iD
Albert Ferro https://orcid.org/0000-0002-5486-9145
References
- 1.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol 2013; 13: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo XG, Uzui H, Mizuguchi T, et al. Imidaprilat inhibits matrix metalloproteinase-2 activity in human cardiac fibroblasts induced by interleukin-1beta via NO-dependent pathway. Int J Cardiol 2008; 126: 414–420. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 4.Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res 2017; 120: 713–735. [DOI] [PubMed] [Google Scholar]
- 5.Florvall G, Basu S, Larsson A. Apolipoprotein A1 is a stronger prognostic marker than are HDL and LDL cholesterol for cardiovascular disease and mortality in elderly men. J Gerontol A Biol Sci Med Sci 2006; 61: 1262–1266. [DOI] [PubMed] [Google Scholar]
- 6.Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem 2004; 279: 48487–48490. [DOI] [PubMed] [Google Scholar]
- 7.Nanri A, Moore MA, Kono S. Impact of C-reactive protein on disease risk and its relation to dietary factors. Asian Pac J Cancer Prev 2007; 8: 167–177. [PubMed] [Google Scholar]
- 8.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010; 375: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Heart, Lung, and Blood Institute. Study quality assessment tools, https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (2019, accessed 10 June 2019).
- 10.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes 2012; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Zhao X, Li L, et al. Effects of combination of ezetimibe and rosuvastatin on coronary artery plaque in patients with coronary heart disease. Heart Lung Circ 2016; 25: 459–465. [DOI] [PubMed] [Google Scholar]
- 12.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011; 365: 2078–2087. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T, Ando K, Daidoji H, et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol 2017; 70: 537–544. [DOI] [PubMed] [Google Scholar]
- 14.Im E, Cho YH, Suh Y, et al. High-intensity statin treatments in clinically stable patients on aspirin monotherapy 12 months after drug-eluting stent implantation: a randomized study. Rev Esp Cardiol (Engl Ed) 2018; 71: 423–431. [DOI] [PubMed] [Google Scholar]
- 15.Taguchi I, Iimuro S, Iwata H, et al. High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD): a randomized superiority trial. Circulation 2018; 137: 1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019; 380: 11–22. [DOI] [PubMed] [Google Scholar]
- 17.Heljić B, Velija-Asimi Z, Kulic M. The statins in prevention of coronary heart diseases in type 2 diabetics. Bosn J Basic Med Sci 2009; 9: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitas GD, Nightingale P, Armitage J, et al. A multicenter, randomized, placebo-controlled trial of atorvastatin for the primary prevention of cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheumatol 2019; 71: 1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359: 2195–2207. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001; 344: 1959–1965. [DOI] [PubMed] [Google Scholar]
- 21.Sever PS, Poulter NR, Chang CL, et al. Evaluation of C-reactive protein before and on-treatment as a predictor of benefit of atorvastatin: a cohort analysis from the Anglo-Scandinavian Cardiac Outcomes Trial lipid-lowering arm. J Am Coll Cardiol 2013; 62: 717–729. [DOI] [PubMed] [Google Scholar]
- 22.Soedamah-Muthu SS, Livingstone SJ, Charlton-Menys V, et al. Effect of atorvastatin on C-reactive protein and benefits for cardiovascular disease in patients with type 2 diabetes: analyses from the Collaborative Atorvastatin Diabetes Trial. Diabetologia 2015; 58: 1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374: 2021–2031. [DOI] [PubMed] [Google Scholar]
- 24.Gilham D, Wasiak S, Tsujikawa LM, et al. RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease. Atherosclerosis 2016; 247: 48–57. [DOI] [PubMed] [Google Scholar]
- 25.Tobaru T, Seki A, Asano R, et al. Lipid-lowering and anti-inflammatory effect of ezetimibe in hyperlipidemic patients with coronary artery disease. Heart Vessels 2013; 28: 39–45. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki D, Ishida M, Watanabe H, et al. Comparison of anti-inflammatory effects and high-density lipoprotein cholesterol levels between therapy with quadruple-dose rosuvastatin and rosuvastatin combined with ezetimibe. Lipids Health Dis 2013; 12: 9–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 2009; 373: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 28.Kinlay S. Low-density lipoprotein-dependent and -independent effects of cholesterol-lowering therapies on C-reactive protein: a meta-analysis. J Am Coll Cardiol 2007; 49: 2003–2009. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, MacFadyen JG, Everett BM, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 2018; 391: 319–328. [DOI] [PubMed] [Google Scholar]