Summary
Spinal subarachnoid haemorrhage is a rare complication of spinal anaesthesia, especially following atraumatic lumbar puncture and in the absence of coagulopathies. The initial presentation of spinal subarachnoid haemorrhage is variable and paraplegia with full recovery within a few hours is rare. Bleeding can extend into the intracranial subarachnoid space, but there are only a few reports of symptomatic intracranial and spinal subarachnoid haemorrhage after spinal anaesthesia. We report co‐existing spinal subarachnoid haemorrhage and intracranial subarachnoid haemorrhage after atraumatic spinal anaesthesia in a 69‐year‐old woman without a coagulopathy. The day after surgery she developed flaccid paraplegia that spontaneously resolved in a few hours. Magnetic resonance imaging demonstrated subarachnoid high signal intensity from T11–S2, consistent with spinal subarachnoid haemorrhage. On the same day the patient complained of severe headache which was later followed by diplopia. Neurological imaging studies revealed diffuse distribution of blood in the subarachnoid space but no intracranial vascular malformations. At the time of diagnosis spontaneous recovery of spinal symptoms had already begun and the clinical manifestations eventually resolved with conservative management. The possibility of an intracranial haemorrhage should always be considered when spinal subarachnoid haemorrhage is identified, even in cases of uncomplicated spinal anaesthesia in patients with no known risk factors for spinal haemorrhage.
Keywords: paraplegia, spinal anaesthesia, spinal hematoma, subarachnoid haemorrhage
Introduction
Co‐existence of both intracranial and spinal subarachnoid haemorrhage after spinal anaesthesia is rare [1, 2, 3, 4]. Intracranial subarachnoid haemorrhage can develop when the initial complication of spinal anaesthesia is a spinal subarachnoid haemorrhage. Due to the continuity between the spinal and intracranial subarachnoid compartments, redistribution or migration of the spinal subarachnoid haemorrhage can occur. This complication is manifest clinically by cerebral symptoms following the spinal symptoms and is diagnosed by neurological imaging demonstrating diffuse distribution of blood in the subarachnoid space without intracranial vascular malformations. The few published reports of co‐existence of symptomatic spinal and cranial subarachnoid haemorrhage after spinal anaesthesia were associated with factors that contributed to spinal haemorrhage, including the presence of a magnetic resonance imaging (MRI) detectable spinal vascular malformation, the recommencing of antiplatelet therapy early after surgery and technically difficult lumbar puncture [1, 2, 3, 4].
We report a case with co‐existing intracranial and spinal subarachnoid haemorrhage after atraumatic spinal anaesthesia in a woman without coagulopathy or any demonstrable intracranial or spinal vascular malformation. Our patient presented with transient, but complete, bilateral flaccid lower limb paralysis and urinary retention. At the time of diagnosis spontaneous recovery of spinal symptoms had started and the case resolved with conservative management.
Report
A 69‐year‐old woman, weighing 59 kg and 160 cm tall (body mass index; 23 kg.m‐2), was scheduled for elective trans‐vaginal resection of a localised rectal stromal tumour. Her past surgical history included a breast lumpectomy under general anaesthesia 24 years previously and rectal biopsies that were performed under spinal anaesthesia without complication three weeks previously. Her medical history was otherwise unremarkable. Pre‐operative platelet count and coagulation tests were normal. In the operating theatre, following application of recommended Association of Anaesthetists’ monitoring and insertion of a peripheral venous cannula, spinal anaesthesia was performed through a single atraumatic puncture at the L2–3 interspace using a 25‐Gauge Sprotte needle. No paraesthesia was elicited and the cerebrospinal fluid (CSF) was clear. Complete motor block of lower extremities and a cephalad T8 level of pin‐prick sensory blockade was achieved within 10 min after injection of 2.5 ml hyperbaric bupivacaine 0.5%. The surgical procedure was uneventful and the block had completely worn off 3 h postoperatively.
The following day the patient was mobilising and had normal bladder function. Whilst in the upright position she experienced nausea and mild leg pain, which prompted her to lay supine in bed, and approximately 30 min later she reported numbness, complete paralysis of both legs and an inability to void urine. Neurological examination revealed an almost complete flaccid paralysis of the lower limbs, except for dorsiflexion of the left foot, with a T12 level of sensory loss by pin‐prick testing. Emergency spinal MRI findings were consistent with an intradural lumbosacral posterior subarachnoid haemorrhage from T11–12 to S2 with involvement of the cauda equina and a concomitant level of normal CSF in the anterior segment of the dural sac (Fig. 1a). A similar appearance of normal CSF stratified on subarachnoid blood was also evident in a radicular pocket at the thoracic level. Approximately 1 h after the onset of symptoms the motor and sensitive deficits completely subsided in the right leg, while the left leg had normal sensation but reduced strength. Recovery was complete 3 h later.
Figure 1.
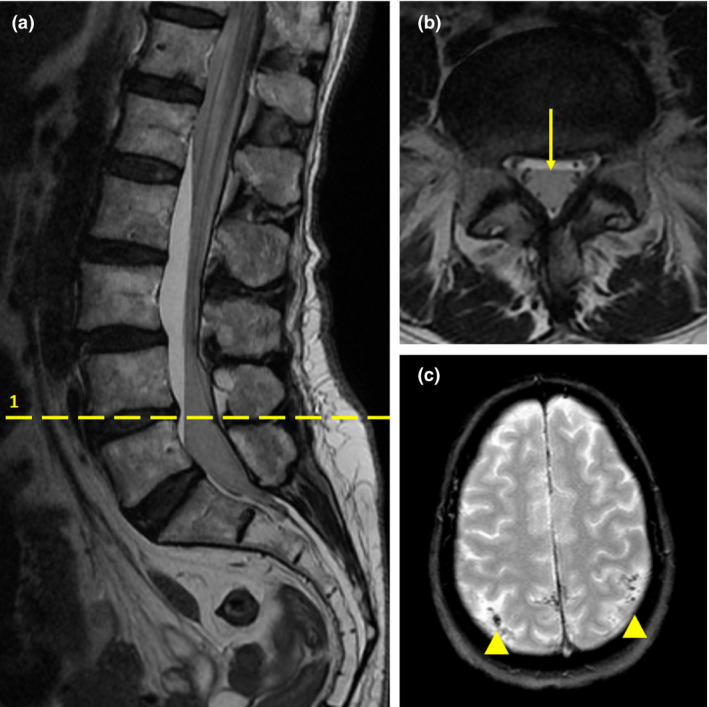
(a) Sagittal T2‐weighted lumbosacral magnetic resonance image (MRI) showing a linear high signal intensity mass, dorsally located in subarachnoid space with a concomitant level of normal cerebrospinal fluid (CSF) in the anterior part of the sac. This finding was compatible with spinal subarachnoid haemorrhage. (b) Axial T2‐weighted lumbosacral MRI scan (corresponding with dashed line 1 in image (a) showing the hypo‐intense signal area within the posterior subarachnoid space indicative of blood and anterior hyperintense signal congruous with displaced CSF (arrow). (c) T2‐weighted axial brain MRI performed 6 days after the onset of cranial symptoms revealing subtle subarachnoid haemorrhage as highlighted by low signals along posterior cortical sulci (arrowheads).
The patient was monitored closely, kept on bed rest and treated with intravenous dexamethasone 8 mg.day‐1 and mannitol. Later that day the patient developed intense frontal headache, which was only partially controlled with paracetamol 3 g.day‐1 and oxycodone 10 mg.day‐1 and intensified over the next three days, during which the patient developed blurred vision and photophobia. Neurological examination confirmed a mild bilateral sixth cranial nerve palsy. A cranial computed tomography (CT)‐angiography scan was performed 72 h after the onset of intracranial symptoms and revealed subtle subarachnoid haemorrhage in both parieto‐occipital sulci with evidence of a small blood level in the right posterior ventricular horn. No intracranial vascular malformations were detected. A brain MRI performed three days later confirmed the presence of blood in parieto‐occipital sulci (Fig. 1b). The headache and associated symptoms resolved over the following four days. Brain CT and spinal MRI showed no residual subarachnoid blood five days later.
The patient subsequently underwent repair of a rectovaginal fistula under general anaesthesia without complication and was discharged home without any neurological sequelae after 20 days in hospital.
Discussion
Although a rare occurrence, clinically significant spinal subarachnoid haemorrhage has been reported as a complication of spinal anaesthesia even when spinal puncture is performed atraumatically using pencil point needles in patients without coagulopathy, as in our patient [5,6]. Usually, iatrogenic puncture of radicular vessels in the subarachnoid space is the source of bleeding, while factors other than coagulopathy may be difficult to identify as the cause for significant subarachnoid blood accumulation after spinal anaesthesia. Advanced age and female sex were the only predisposing factors for spinal bleeding in our patient. Postoperative management for the first 24 h did not include thromboprophylaxis or anti‐inflammatory drugs other than the selective cyclo‐oxygenase‐2 (COX‐2) inhibitor parecoxib, which has no direct effect on platelet function. The lack of MRI signs of old haemorrhage makes it unlikely that subarachnoid blood had accumulated following uncomplicated spinal anaesthesia performed 3 weeks previously. It remains speculative whether the site of spinal puncture, which was the L2‐L3 interspace in the present case and L3‐L4 in the previous spinal procedure, resulted in different degrees of laceration of the small radicular vessels in the subarachnoid space by the spinal needle due to location‐dependent variability in vasculature.
Two features of the present case are especially unusual: the initial presentation of spinal subarachnoid haemorrhage was sudden transient paraplegia; and the clinical course was dominated by the co‐existence of subarachnoid intracranial haemorrhage in the absence of cerebral vascular malformations.
Spinal subarachnoid haemorrhage complicating spinal or epidural anaesthesia clinically manifests within 24 h of the procedure in most patients, and this was the case in our patient. It may be difficult to diagnose this complication clinically because its presentation may be variable, overlapping and even limited to minor symptoms that might be misdiagnosed as transient neurological symptoms.
Paraplegia was the main initial symptom of spinal subarachnoid haemorrhage in our patient, and its full recovery within hours after the onset reflected the lack of irreversible neurological damage. There are few reports indicating that spinal haematoma may initially manifest as hemiplegia or paraplegia that spontaneously resolves in less than a few hours. In contrast to our patient, manifestations in previously reported cases of spontaneous spinal epidural haematomas were relapsing and their spontaneous full resolution was attributed to the intermittent decompression that resulted from dissection of the haematoma within the epidural space [7,8]. We found only a few reports of temporary paraparesis secondary to subarachnoid haemorrhage following spinal lumbar puncture. However, in those cases, the paraparesis did not progress to paraplegia and required at least two days to spontaneously resolve [9].
In our patient, the sudden presentation of spinal symptoms was presumably due to acute spinal cord hypoperfusion, which was likely related to the posterior location of the haemorrhage. Data suggest that the clinical manifestations of spinal haemorrhage are influenced both by longitudinal and by cross‐sectional locations of the bleeding. In particular, posteriorly located spinal haemorrhages may have higher ischaemic potential than those located anteriorly and hence may produce more severe symptoms.
The mechanism responsible for the rapid regression of paraplegia within a few hours remains unknown. Spinal subarachnoid haemorrhage rarely presents as a haematoma because of the diluting and redistributing effect of the CSF, unless the bleeding is severe enough to block CSF flow. In our patient, the volume of blood was presumably not sufficient to negate the clearing process by CSF circulation, as indirectly evidenced by the lack of a fixed spinal cord compression. The symptoms developed soon after a change in position from standing to supine decubitus, and we postulate that this postural change may have played a role in facilitating the longitudinal extension of subarachnoid blood up into the cranial subarachnoid compartment. Intracranial symptoms caused by migration of spinal blood occur later than the spinal symptoms, with a time delay ranging from a few hours, as in our patient, to days, and include headache, impaired level of consciousness and even coma [1, 2, 3, 4]. Headache, nausea, photophobia and diplopia ensuing two days later were the clinical manifestations of intracranial bleeding in our patient. Diplopia is a known rare complication of spinal anaesthesia and is attributed to cranial nerve traction produced by the CSF hypotension secondary to CSF leakage through the dural defect [10]. Positional headache typically precedes the onset of diplopia, as in our patient. Although the enforced bed rest of our patient prevented us from clearly evidencing the orthostatic nature of the headache, cranial manifestations appear attributable more to the possible presence of intracranial CSF hypotension than to meningeal irritation caused by subarachnoid blood. In addition, the co‐existence of intracranial subarachnoid bleeding in our patient could not be entirely due to blood migration from the spine. An additional contributing factor could be related to vessel damage that results from changes in transmural wall pressure, possibly related to intracranial CSF hypotension [4].
Intracranial imaging is not considered mandatory when the source of spinal bleeding is identified and cranial symptoms are absent. However, in agreement with other authors, we recommend the radiological study of both the spinal cord and the brain when spinal haematoma after spinal anaesthesia is suspected, even in the absence of intracranial symptoms [2, 3].
Conservative management of spinal subarachnoid haemorrhage is indicated in cases with minimal or rapidly improving neurological deficits, as in our patient. This report supports evidence that co‐existence of spinal and intracranial haemorrhage after spinal anaesthesia does not, in itself, determine treatment [1, 2, 3, 4].
In conclusion, even though extremely rare, the possibility of an intracranial haemorrhage after spinal anaesthesia should always be considered when spinal subarachnoid haemorrhage is identified, even in uncomplicated procedures in patients without coagulopathy or known risk‐factors for an increased incidence of spinal haemorrhage.
Acknowledgements
Published with the written consent of the patient. No external funding or competing interests declared.
References
- 1. Hans GA, Senard M, Ledoux D et al. Cerebral subarachnoid blood migration consecutive to a lumbar haematoma after spinal anaesthesia. Acta Anaesthesiologica Scandinavica 2008; 52: 1021–3. [DOI] [PubMed] [Google Scholar]
- 2. Rocchi R, Lombardi C, Marradi I, Di Paolo M, Cerase A. Intracranial and intraspinal hemorrhage following spinal anesthesia. Neurological Sciences 2009; 30: 393–6. [DOI] [PubMed] [Google Scholar]
- 3. Liu WH, Lin JH, Lin JC, Ma HI. Severe intracranial and intraspinal subarachnoid hemorrhage after lumbar puncture: a rare case report. American Journal of Emergency Medicine 2008; 26: 633. [DOI] [PubMed] [Google Scholar]
- 4. Espinosa‐Aguilar M, Hernandez‐Palazon J, Fuentes‐Garcia D. Intraspinal and intracranial subarachnoid haemorrhage with severe cerebral vasospasm after spinal anaesthesia for assisted delivery. British Journal of Anaesthesia 2012; 108: 885–6. [DOI] [PubMed] [Google Scholar]
- 5. Park JH, Shin KM, Hong SJ, Kim IIS, Nam SK. Subacute spinal subarachnoid hematoma after spinal anesthesia that causes mild neurologic deterioration. Anesthesiology 2007; 107: 846–8. [DOI] [PubMed] [Google Scholar]
- 6. Vidal M, Strzelecki A, Houadec M, Krikken IR, Danielli A, Souza Neto EP. Spinal subarachnoid haematoma after spinal anaesthesia: case report. Brazilian Journal of Anesthesiology 2016; 66: 533–5. [DOI] [PubMed] [Google Scholar]
- 7. Li C, He R, Li X, Zhong Y, Ling L, Li F. Spontaneous spinal epidural hematoma mimicking transient ischemic attack. Medicine 2017; 96: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernandez D, Vinuela F, Feasby RTE. Recurrent paraplegia with total recovery from spontaneous spinal epidural hematoma. Annals of Neurology 1982; 11: 623–4. [DOI] [PubMed] [Google Scholar]
- 9. Gerancher JC, Waterer R, Middleton J. Transient paraparesis after postdural puncture spinal hematoma in a patient receiving ketorolac. Anesthesiology 1997; 86: 490–4. [DOI] [PubMed] [Google Scholar]
- 10. Basaranoglu G, Saidoglu L. Isolated transient diplopia and nystagmus after spinal anaesthesia. Journal of Anesthesia 2013; 4: 643–4. [DOI] [PubMed] [Google Scholar]


