Abstract
Background and Aims
Anti‐Tumor Necrosis Factor (TNF)‐induced lupus (ATIL) is a distinct clinical entity, increasingly recognized in patients with inflammatory bowel disease treated with anti‐TNF therapy. Our aims were to evaluate the incidence and clinical and serological markers of ATIL in this population.
Methods
This observational cohort study reviewed 454 patient treatment courses with anti‐TNF therapy (300 infliximab and 154 adalimumab). A diagnosis of ATIL was based on the most widely accepted diagnostic criteria: (i) a temporal relationship between symptoms and anti‐TNF therapy and resolution of symptoms following cessation of the offending medication; (ii) at least one serologic American College of Rheumatology (ACR) criterion of Systemic Lupus Erythematosus (SLE); and (iii) at least one nonserological criterion such as arthritis, serositis, or rash. Clinical, demographic, and serological predictors were evaluated.
Results
The incidence rate of ATIL was 5.7% for infliximab and 0.6% for adalimumab, which are much higher than previously reported postmarketing estimates. The median duration to diagnosis following commencement of anti‐TNF therapy was 15 months (3–62 months). ATIL occurs more commonly patients that commence therapy at an older age (46.47 years ± 13.79 years vs. 38.85 years ± 14.75 years, P = 0.033).
Conclusions
ATIL is a significant complication of anti‐TNF therapy, affecting 1 in every 20 patients who commence infliximab. A panel of serological markers is useful to confirm the diagnosis and exclude other conditions that may mimic ATIL. Clinicians using anti‐TNF medications should counsel patients about this potential risk and monitor for clinical manifestations of lupus during routine follow up.
Keywords: adalimumab, anti‐tumor necrosis factor, anti‐TNF‐induced lupus, inflammatory bowel disease, infliximab, lupus
This is a retrospective study that looks at anti‐TNF‐induced lupus, a distinct clinical entity increasingly identified in patients with inflammatory bowel disease on anti‐TNF therapy. ATIL occurs more commonly than previously reported and is more common in those who commence therapy at an older age. The combination of clinical symptoms, the temporal relationship to anti‐TNF therapy, and a panel of serological markers can assist clinicians in confirming a diagnosis.
Introduction
The introduction of anti‐tumor necrosis factor alpha (anti‐TNF) medications, including infliximab and adalimumab, has revolutionized the management of patients with inflammatory bowel disease (IBD). These agents have demonstrated efficacy, are well tolerated, and have a relatively good safety profile.1 Pivotal clinical trials and postmarketing surveillance studies have identified some drug‐related adverse events, including infusion reactions, infections, and malignancy, as well as a number of immune‐mediated phenomena.2, 3, 4
Anti‐TNF medications have been demonstrated to induce autoimmunity in the form of antinuclear antibodies (ANAs) and anti‐double‐stranded deoxyribonucleic acid (anti‐dsDNA) antibodies. 5, 6 Pooled analysis of the initial open‐labeled clinical trials for infliximab in rheumatoid arthritis demonstrated an increase in ANA positivity from 29% pretreatment to 53% posttreatment.6 In this analysis, 14% of patients also developed anti‐dsDNA antibodies. Some patients may also develop clinical symptoms that mimic idiopathic lupus, and this has been recognized as a distinct clinical entity called anti‐TNF‐induced lupus (ATIL).7 The most widely accepted diagnostic criteria for ATIL are (i) a temporal relationship between symptoms and anti‐TNF therapy and resolution of symptoms following cessation of the offending medication; (ii) at least one serologic American College of Rheumatology (ACR) criterion of SLE, being either a positive ANA or anti‐dsDNA; and (iii) at least one nonserological criterion such as arthritis, serositis, or rash.
The estimated reported incidence of ATIL based on postmarketing studies is 0.19–0.22% for infliximab and 0.10% for adalimumab.8 The actual incidence, however, is difficult to determine due to lack of prospective studies, lack of recognition of the condition, and the overlap in clinical symptoms with the extraintestinal manifestations of IBD. Given the widespread use of anti‐TNF agents in the management of IBD, our aims were to evaluate the incidence and clinical and serological markers of ATIL in this population.
Materials and methods
Study design and participants
This study was a retrospective observational cohort study based on a prospectively collected registry of all IBD patients who were commenced on anti‐TNF therapy at Royal Perth Hospital, Western Australia between January 1st 2008 and December 31st 2017. The study population included all patients with a confirmed diagnosis of IBD, who were commenced on a course of anti‐TNF therapy, (infliximab or adalimumab), and had completed induction and at least one maintenance dose of therapy. Patient follow up occurred till a census date of 31st March 2018.
Patients were retrospectively defined as having ATIL based on the aforementioned diagnostic criteria: (i) a temporal relationship between symptoms and anti‐TNF therapy and resolution of symptoms following cessation of the offending medication (within 3 months of discontinuation); (ii) at least one serologic ACR criterion of SLE, being either a positive ANA or anti‐dsDNA; and (iii) at least one nonserological criterion such as arthritis, serositis, or rash.7 The incidence of ATIL and time to diagnosis were calculated for both infliximab and adalimumab. Demographic and clinical parameters, including age, gender, type of IBD, and use of concurrent immunomodulatory therapy, were obtained from electronic records.
Age, gender, and IBD type were evaluated as predictors in the development of ATIL. The results of several serological markers, if performed, including ANA, anti‐ds‐DNA, antihistone antibodies, anti‐Smith antibodies (anti‐Sm), anticyclic citrullinated peptide (anti‐CCP), and rheumatoid factor (RF), were also collected.
Statistical analysis
Statistical analyses were performed using Stata (StataCorp 2015. Release 14) For the clinical and demographic predictors, continuous variables were analyzed using a two‐sided t‐test and categorical variables using a Fishers exact test or Pearson's χ 2 test. The threshold for statistical significance was defined as a P value < 0.05.
Results
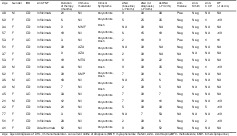
A total of 454 patient treatment courses with anti‐TNF therapy were included, comprising 300 with infliximab and 154 with adalimumab. Seventeen (5.7%) patients who received infliximab developed ATIL, and one patient (0.6%) on adalimumab developed ATIL. The clinical, demographic, and serological characteristics of each case of ATIL are summarized in Table 1. Twelve patients received glucocorticoids at the time of symptom manifestation. Patients who developed ATIL were more likely to be older at the time of commencement of anti‐TNF medication (46.47 years ± 13.79 years vs 38.85 years ± 14.75 years, P = 0.033) (Table 2). There was a trend that female gender was a risk factor for ATIL (72.2% vs 47.0%, P = 0.052). The median duration of therapy till development of ATIL was 15 months (range of 3–62 months).
Table 1.
Sociodemographic, clinical, and serological characteristics of individual patients diagnosed with anti‐TNF‐induced lupus
| Age | Gender | IBD | Anti‐TNF | Duration of therapy (months) | Immunomodulator | Clinical Symptoms | ANA (baseline) (<7 IU/mL) | ANA (at diagnosis) (<7 IU/mL) | dsDNA (<7 IU/mL) | Anti‐histone | Anti‐Smith | Anti‐CCP | RF (<14k U/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 | M | CD | Infliximab | 21 | Nil | Polyarthritis | 0 | 30 | 38 | Pos | Neg | NA | NA |
| 69 | F | CD | Infliximab | 6 | Nil | Polyarthritis | 5 | 25 | 35 | Neg | Neg | 1 | <10 |
| 34 | F | CD | Infliximab | 9 | MMF | Rash | NA | 30 | NA | Neg | Neg | NA | NA |
| 57 | F | CD | Infliximab | 10 | Nil | Polyarthritis, | 6 | 15 | 10 | Neg | Neg | NA | <10 |
| 53 | F | UC | Infliximab | 3 | Nil | Polyarthritis, Rash | 2 | 10 | 0 | Pos | Neg | 1 | 11 |
| 51 | F | CD | Infliximab | 30 | AZA | Polyarthritis | NA | 30 | NA | Neg | Neg | NA | NA |
| 37 | F | CD | Infliximab | 9 | AZA | Polyarthritis | 2 | 30 | NA | NA | NA | NA | NA |
| 53 | F | CD | Infliximab | 10 | MTX | Polyarthritis | 0 | 30 | 22 | Neg | Neg | NA | NA |
| 39 | M | CD | Infliximab | 44 | Nil | Rash | 0 | 30 | 35 | Neg | Neg | 1 | <10 |
| 62 | F | CD | Infliximab | 30 | 6MP | Polyarthritis, | 7 | 30 | 6 | Neg | Neg | NA | NA |
| 36 | M | UC | Infliximab | 18 | Nil | Rash | NA | 25 | 6 | Neg | Neg | NA | NA |
| 49 | M | CD | Infliximab | 7 | Nil | Polyarthritis | 2 | 30 | 5 | NA | NA | NA | NA |
| 45 | F | UC | Infliximab | 33 | Nil | Rash | 7 | 30 | 7 | Neg | Neg | NA | NA |
| 21 | M | CD | Infliximab | 12 | Nil | Polyarthritis | 7 | 30 | 11 | Neg | Neg | NA | <10 |
| 42 | F | CD | Infliximab | 21 | Nil | Polyarthritis | 5 | 30 | 39 | Neg | Neg | 5 | <10 |
| 77 | F | CD | Infliximab | 3 | Nil | Polyarthritis | NA | 7 | 53 | NA | NA | NA | <10 |
| 51 | F | CD | Infliximab | 28 | Nil | Polyarthritis | 2 | 30 | 6 | Neg | Neg | 2 | <10 |
| 41 | F | CD | Adalimumab | 62 | Nil | Polyarthritis Polyarthritis | 0 | 30 | 52 | Neg | Neg | NA | NA |
Age–age at diagnosis of ATIL, Immunomodulator—concurrent at time of diagnosis.
AZA, Azathioprine; MMF, mycophenolate mofetil; MTX, methotrexate; 6MP, 6 mercaptopurine.
Table 2.
Differences in characteristics of between ATIL and non‐ATIL
| Non‐ATIL (n = 436) | ATIL (n = 18) | P value | |
|---|---|---|---|
| Male/female | 231/205 | 5/13 | 0.052 |
| Age | |||
| Mean ± SD (years) | 38.85 ± 14.75 | 46.47 ± 13.70 | 0.033 |
| IBD type | |||
| CD | 312 (71.6%) | 15 (83.3%) | 0.209 |
| UC | 119 (27.3%) | 3 (16.7%) | 0.241 |
| IBD‐U | 5 (1.1%) | 0 (0%) | 0.816 |
All patients with a diagnosis of ATIL demonstrated an elevated ANA with results ranging from 7 to 30 IU/mL. In those patients who had a baseline ANA performed prior to commencing anti‐TNF therapy, the ANA level increased compared with their baseline level. Additional serological markers that were positive in patients diagnosed with ATIL included anti‐ds‐DNA in 10 of 15 (67%) and antihistone antibodies in 2 of 15 (13%.) Serological testing to exclude other inflammatory conditions, including idiopathic SLE and seropositive arthropathies, were performed in a subset of the patients (Table 3).
Table 3.
Serological profile of patients diagnosed with ATIL
| Autoantibody | Number (%) |
|---|---|
| ANA | 18/18 (100%) |
| dsDNA | 10/15 (66.7%) |
| Anti‐Histone | 2/13 (15.4%) |
| Anti‐Smith | 0/15 (0%) |
| Anti RF | 0/8 (0%) |
| Anti‐CCP | 0/5 (0%) |
Five patients were on concurrent immunomodulator therapy at time of ATIL diagnosis. Ten patients on infliximab who developed ATIL were subsequently switched to adalimumab, and none of these developed ATIL on adalimumab, with a median follow‐up period of 29.3 months.
Discussion
ATIL is a distinct clinical entity increasingly recognized and described in the rheumatologic literature. Data remain limited in the IBD population due to lack of recognition of the condition, as well as difficulty making a diagnosis due to significant overlap in symptoms with extraintestinal manifestations of IBD and other autoimmune diseases. Our study demonstrates an incidence rate of 5.7% for infliximab and 0.6% for adalimumab, which are much higher than postmarketing studies.8 This corresponds to a retrospective study in an IBD population, which identified 20 of 289 patients (6.9%) on anti‐TNF therapy who developed a lupus‐like reaction.9
This is a significant finding, given the widespread and increasing utilization of anti‐TNF therapy in IBD patients, as our results suggest that 1 in 20 patients who commence infliximab will develop ATIL. Infliximab is considered more immunogenic compared with adalimumab based on its chimeric structure and is also thought to reach higher tissue concentrations.10 Clinicians should counsel patients about the potential risk prior to commencing therapy and should monitor for clinical manifestations of lupus, which include rash, photosensitivity, and arthritis, as well as rare systemic manifestations, including pericarditis and neurological and hematological disorders, during routine follow up. 11 The extraintestinal manifestations of IBD include some of these symptoms, which make the diagnosis of ATIL more challenging. Peripheral arthritis can occur in both ulcerative colitis and Crohn's disease. It typically manifests as a nonerosive seronegative arthritis and occurs in between 5 and 10% of patients with ulcerative colitis and 10–20% of patients with Crohn's disease.12 Various cutaneous manifestations have also been associated with IBD and can occur in up to 15% of patients.12 There are various other autoimmune conditions, including the seropositive arthropathies and idiopathic SLE, which may occur in patients with IBD and also share clinical manifestations with ATIL. It is very difficult to differentiate ATIL and these various conditions based on clinical symptoms alone, and further information, including the temporal relationship with anti‐TNF therapy and serological markers is required.
Anti‐TNF therapies have been demonstrated to induce autoantibodies; however, no specific tests have been validated for ATIL. The most widely recognized diagnostic criteria require a positive ANA or ds‐DNA.6 All the patients with confirmed ATIL in our cohort had a positive ANA at the time of diagnosis, with an increase in the titer from baseline level. Anti‐ds‐DNA levels were elevated in 66.7% of patients tested. Anti‐Sm antibodies are part of the serological diagnostic criteria for SLE. The presence of these antibodies is almost exclusive to idiopathic SLE, and it is rarely found in drug‐induced SLE. 13 Antihistone antibodies have been found to occur in 95% of cases of drug‐induced SLE; however, this antibody is not pathognomonic as they are also found in 75% of patients with idiopathic SLE.14 The presence of antihistone antibodies in studies of patients with ATIL, however, report much lower rates, ranging from 17% to 57%.13 Only 15.4% of patients diagnosed with ATIL demonstrated positive antihistone antibodies in our cohort. A full panel of autoantibodies, including ANA, anti‐ds‐DNA, anti‐CCP, RF, antihistone antibodies, and anti‐Sm antibodies, should be performed in all patients with a clinical suspicion for ATIL.
We were unable to establish whether concurrent immunomodulatory therapy was a factor in the development or prevention of ATIL as patients in the cohort who did not develop ATIL were on both varying doses and durations of immunomodulator therapy. It has been postulated that concurrent immunomodulatory therapy may decrease the immunogenicity of a biologic therapy.15 Use of an immunomodulator, in the form of a thiopurine or methotrexate, when used together with a biologic agent has been associated with decreased formation of antidrug antibodies16 Further studies are required to assess whether concurrent immunomodulatory therapy reduces the development of ATIL. Based on small numbers, our study does suggest that it is safe to switch to adalimumab if a patient develops ATIL on infliximab.
Our study had several limitations. It's retrospective nature makes it prone to data entry error. As patients were retrospectively diagnosed with ATIL, the true incidence is likely underestimated as serology, which is a component of the diagnostic criteria, would only be performed if a diagnosis was suspected at the time, at the discretion of the treating physician.
ATIL is a complication of anti‐TNF therapy that is more common than previously reported. Diagnosis in an IBD population is often difficult due to lack of recognition of the condition and shared clinical symptoms with both the extraintestinal manifestations of IBDs and other autoimmune conditions. The combination of clinical symptoms, the temporal relationship to anti‐TNF therapy, and a panel of serological markers can assist clinicians in confirming a diagnosis.
Acknowledgment
S.P. has received speaker fees from Pfizer and Takeda.
Declaration of conflict of interest: There are no conflicts of interest to disclose from any of the authors with regard to this manuscript.
Author Contribution: S.P. designed the study and performed statistical analyses. S.P., K.S., and K.V. all participated in the writing and review of the final manuscript.
Conference Presentations: Oral Presentation—Australian Gastroenterology Week (AGW), Brisbane, September 2018. Published in Abstract form in JGH. Poster of Excellence and Poster Champion Presentation—United European Gastroenterology Week, Vienna, October 2018.
References
- 1. Hoentijen F, Van Bodegraven A. Safety of anti‐tumor necrosis factor therapy in inflammatory bowel disease. World J. Gastroenterol. 2009; 15: 2067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanauer SB, Feagan BG, Lichtenstein GR et al Maintenance Infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359: 1541–9. [DOI] [PubMed] [Google Scholar]
- 3. Colombel JF, Sandborn WJ, Rutgeerts P et al Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007; 132: 52–65. [DOI] [PubMed] [Google Scholar]
- 4. Wiens A, Venson R, Correr CJ et al Meta‐analysis of the efficacy and safety of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis. Pharmacotherapy. 2010; 30: 339–53. [DOI] [PubMed] [Google Scholar]
- 5. Ramos‐Casals M, Brito‐Zerón P, Muñoz S et al Autoimmune disease induced by TNF‐ targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007; 86: 242–51. [DOI] [PubMed] [Google Scholar]
- 6. Charles PJ, Smeenk RJ, De Jong J et al Assessment of antibodies to double‐stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: findings in open‐label and randomized placebo‐controlled trials. Arthritis Rheum. 2000; 43: 2383–90. [DOI] [PubMed] [Google Scholar]
- 7. Williams EL, Gadola S, Edwards CJ. Anti‐TNF induced lupus. Rheumatology. 2009; 48: 716–20. [DOI] [PubMed] [Google Scholar]
- 8. Almoallim H, Al‐Ghamdi Y, Almaghrabi H et al Anti tumor necrosis factor—a induced systemic lupus erythematosus. Open Rheumatol. J. 2012; 6: 315–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yanai H, Shuster D, Clalbrese E et al The incidence and predictors of lupus like reaction in patients with IBD treated with anti‐TNF therapies. Inflamm. Bowel Dis. 2013; 19: 2778–86. [DOI] [PubMed] [Google Scholar]
- 10. Kocharla L, Mongey AB. Is the development of drug‐related lupus a contraindication for switching from one TNF alpha inhibitor to another? Lupus. 2009; 18: 169–71. [DOI] [PubMed] [Google Scholar]
- 11. Hocehberg MC, Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology . Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- 12. Vavricka SR, Schoepfer A, Scharl M et al Extraintestinal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2015; 21: 1982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katz U, Zandman‐Goddard G. Drug‐induced lupus: an update. Autoimmun. Rev. 2010; 10: 46–50. [DOI] [PubMed] [Google Scholar]
- 14. De Bandt M, Sibilia J, Le Loet X et al Systemic lupus erythematosus induced by anti‐tumour necrosis factor alpha therapy: a French national survey. Arthritis Res. Ther. 2005; 7: 545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kothari MM, Nguyen DL, Parekh NK. Strategies for overcoming anti‐tumor necrosis factor drug antibodies in inflammatory bowel disease: case series and review of literature. World J. Gastrointest. Pharmacol. Ther. 2017; 8: 155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vermeire S, Noman M, Van Assche G et al Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. 2007; 56: 1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


