Abstract
Background and Aim
Looping is a major problem in colonoscopy, and it prolongs procedure time. We evaluated the efficacy and safety of an external abdominal compression device (back brace support belt; Maxbelt) with respect to cecal insertion time and other outcomes.
Methods
We performed a prospective study on outpatients undergoing elective colonoscopy in Toyoshima Endoscopy Clinic. Subjects were randomly assigned to groups and were subjected to either Maxbelt (n = 39) or no device (control, n = 38) during colonoscopy. The colonoscopist was blinded to the study. The primary outcome that was observed was insertion time.
Results
The intubation time of the Maxbelt group was shorter than that of the no device group, but the difference was not significant (3.29 vs 4.49 min, P = 0.069). After stratifying by age, the use of Maxbelt significantly decreased cecal intubation time in elderly participants (age ≥ 45) compared to no device group (3.27 vs 5.00 min, P = 0.032). The use of the Maxbelt significantly decreased insertion difficulty encountered by the colonoscopist (P = 0.01). There was no difference in adenoma detection rate, manual pressure, position change, and adverse event.
Conclusions
The use of a back brace support belt could be a viable approach for colonoscopy in elderly patients. (University Hospital Medical Information Network: UMIN000029361).
Keywords: back brace support belt; colonoscopy; randomized, prospective trial
A back brace support belt could keep the colonoscope straight in the sigmoid colon and avoid looping throughout the examination and facilitates more comfortable insertion. This randomized prospective trial showed a significantly shorter cecal intubation time for elderly participants.

Introduction
Colorectal cancer is the third most common cancer worldwide.1 Colonoscopy is widely recommended for colorectal cancer screening due to its effectiveness in reducing colorectal cancer incidence and related mortality.2, 3, 4, 5 Although the technology of colonoscopy equipment and endoscopists' skills are continuously progressing, colonoscopy can be a difficult and painful procedure for some patients. The looping of the sigmoid or transverse colon is one of the well‐known difficulties encountered during insertion, which poses a challenge in reaching the cecum. Looping stretches the colonic mesentery and leads to intense pain and difficulty in advancing the colonoscope. To avoid loop formation, abdominal pressure and position change are often used.6, 7 However, these maneuvers require one or more assistants to change the patient's position when he or she is sedated. Besides the need for uninterrupted assistance, manual compression may result in uncontrolled pressure. Rupture of an abdominal aortic aneurysm after colonoscopy has been previously reported,8, 9 and it is better to avoid manual compression if possible. Therefore, we used an external abdominal compression device (back brace support belt; Maxbelt). It could be effective, especially for patients in whom the insertion of colonoscope seems difficult. Unlike manual abdominal pressure, this belt exerts effective pressure for the entire duration of the procedure. Three previously published studies have evaluated the effects of abdominal binders on colonoscopy performance with contradictory results.10, 11, 12
Therefore, we hypothesized that a back brace support belt could facilitate faster and more effective colonoscopic insertion in patients for whom insertion could be problematic. In this randomized trial, we evaluated the effect of a back brace support belt on colonoscopic performance and safety.
Methods
Study overview
The trial protocol was approved by the Ethical Review Committee of the Hattori Clinic on 7 September 2017. This trial was conducted in accordance with the principles of the most recent version of the Declaration of Helsinki. All the patients provided written informed consent before participation. The trial was performed at Toyoshima Endoscopy Clinic, an outpatient clinic specializing in endoscopy. The study was registered prior to initiation (Clinical Trials number: UMIN000029361). This article is reported in accordance with the CONSORT guideline.13
Patients
Patients were recruited from December 2017 to September 2018. Having a lower or higher body mass index (BMI) was reported to be indicative of longer cecal intubation time (CIT).14, 15 Eligible patients aged 20–80 years with BMI ≥25 kg/m2 or <18.5 kg/m2 and/or previous CIT ≥8 min were enrolled. The indication for the need of colonoscopy included the evaluation of symptoms, screening, or polyp surveillance. Patients with the following conditions were excluded in this study: pregnancy, breastfeeding, previous colorectal resection, past colorectal cancer or inflammatory bowel disease, latex allergy, and severe concomitant illness.
Study design
The back brace support belt used in this study was the Maxbelt (Nippon Sigmax Co., Tokyo, Japan; Fig. 1). Its price is ¥1829 ($16.2) per belt. Patients were randomized to either the Maxbelt group (with Maxbelt attached to the abdomen) or the control group (without Maxbelt) before colonoscopy. Randomization was performed by using the web‐based system of Mujinwari (Iruka System Ltd., Tokyo, Japan). Eligible patients were randomly assigned to the groups in a 1:1 ratio. The colonoscopist was blinded to the study group assignments. Prior to this study, the colonoscopist had conducted 50 colonoscopies in patients using the Maxbelt.
Figure 1.

The back brace support belt used in this study (Maxbelt).
Colonoscopic procedure
Patients involved in this study underwent colonic preparation using 2 L of polyethylene glycol solution administered orally 5 h before the procedure. Polyethylene glycol solution or magnesium citrate was added when the stool did not become clear liquid.16 In this study, all colonoscopy procedures were performed by an endoscopist who had performed more than 10 000 colonoscopies.
For the Maxbelt group, the belt was wrapped around the circumference of the abdomen by the assistant. Once wrapped, participants were asked if the wrap was fastened tightly but not uncomfortably. The patients wore a gown over the belt for examination so that it was not visible to the operator. The assistant removed the belt after all colonoscopic procedures were completed.
We performed colonoscopy using CF‐HQ290ZI or PCF‐H290ZI (Evis Lucera Elite system; Olympus, Tokyo, Japan). PCF‐H290ZI was used for patients aged 70 years or older who underwent a previous abdominal surgery. Sedation with midazolam and/or pethidine was performed based on the patient's willingness. Colonoscope insertion in the cecum was accomplished using standard maneuvers. Small shaking, jiggling, and right turn‐shortening maneuvers have been frequently used for insertion. We commenced the colonoscopy with the patients in the left lateral position. Then, we placed the patients in the supine position after observation of the lower rectum. Abdominal compression or postural change was performed when needed during insertion. Carbon dioxide insufflation and a new‐generation flushing pump (OFP‐2; Olympus) were used during colonoscopy.17, 18 In the absence of contraindications, when the colonoscope reached the cecum, we administered scopolamine butylbromide.19 Quality of the bowel preparation was graded as A (all colon segments empty and clean or minor amount of fluid in the gut that was easily removed by suction), B (at least one colon segment with residual amounts of brown liquid or semi‐solid stool that could be easily removed or displaced), or C (at least one colon segment with only partially removable stool preventing complete visualization of mucosa).
Outcomes
The primary outcome for this study was CIT (the time needed to reach cecum from anus).
Secondary outcomes included insertion difficulty, excessive looping, intraprocedural and postprocedural discomfort reported by patients, adenoma detection, manual pressure on patient's abdomen by the assistant, patient position change during colonoscopic insertion, colonoscopy completion, and the dosage of midazolam and pethidine. Any adverse events were also recorded. Insertion difficulty and excessive looping were measured according to a 3‐point scale.11, 20 Intra‐ and postprocedural discomfort were measured according to a 4‐point scale.11
Planned priori subgroup analyses included stratification according to age, gender, BMI, body height, colonoscope, and previous insertion time. The American Cancer Society recommends that adults should undergo screening for colorectal cancer beginning from 45 years of age,21 and age was classified into two subgroups (age: n < 45 and 45 ≤ n). Patients were classified according to BMI for analysis in accordance with the international standard provided by World Health Organization (BMI < 18.5, 18.5 ≤ BMI < 25, and 25 ≤ BMI).22
Statistical analysis and sample size
We evaluated that we needed 70 patients (35 per group) to detect a 2‐min difference in CIT (standard deviation 3 min), with 80% power and a two‐sided alpha of 0.05. Taking into account the loss to follow up and dropout rates, we expanded the sample by 10%, which required 39 patients in each group. Based on this sample size calculation, a total of 78 patients were finally recruited in this study.
The primary outcome was analyzed by Welch's t‐test. Differences between the two groups of patients (Maxbelt group vs control group) were detected using Student's t‐test or Welch's t‐test for continuous data. Categorical secondary outcomes were compared using chi‐square or Fisher's exact test. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the Stat Mate IV software (Atoms, Tokyo, Japan) and R version 3.5.1 (R Core Team 2018, Vienna, Austria).
Results
Participants and procedure characteristics
A total of 78 patients were recruited in this study, and 1 patient, in whom the Maxbelt was taken off (device failure), was excluded for the final analysis. Finally, 77 patients were included in analyses, of whom 39 were allocated to the Maxbelt group and 38 to the control group (Fig. 2).
Figure 2.
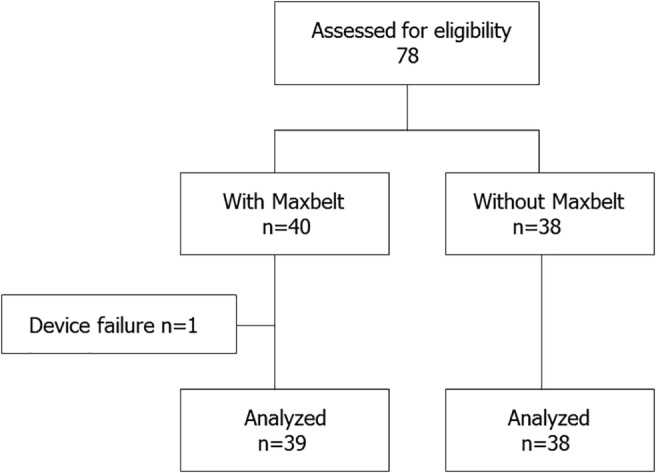
Flow chart showing flow of participants throughout trial.
The characteristics of the trial groups are shown in Table 1. In the Maxbelt group, mean age was significantly younger (P = 0.049). Other characteristics of the trial groups were similar.
Table 1.
The characteristics of the trial groups
| Characteristics | None | Maxbelt |
|---|---|---|
| Number | 38 | 39 |
| Age | ||
| Mean ± SD, years | 56.24 ± 11.47 | 51.31 ± 10.13 |
| ≥45 years | 30 (78.9%) | 32 (82.1%) |
| Gender, male | 25 (65.8%) | 24 (61.5%) |
| Body height, mean ± SD, cm | 166.15 ± 9.75 | 166.51 ± 8.86 |
| Body weight, mean ± SD, kg | 68.93 ± 18.8 | 72.85 ± 16.13 |
| Body mass index | 24.68 ± 5.33 | 26.06 ± 4.36 |
| Mean ± SD, kg/m2 | ||
| <18.5 | 9 (23.7%) | 6 (15.4%) |
| 18.5–25 | 5 (13.2%) | 2 (5.1%) |
| ≥25 | 24 (63.1%) | 31 (79.5%) |
| Colonoscopy indication | ||
| Evaluation of symptoms | 9 (23.7%) | 14 (35.9%) |
| Screening | 21 (55.3%) | 19 (48.7%) |
| Polyp surveillance | 8 (21.0%) | 6 (15.4%) |
| Type of colonoscope | ||
| CF‐HQ290 | 32 (84.2%) | 36 (92.3%) |
| PCF‐H290H | 6 (15.8%) | 3 (7.7%) |
| Preparation | ||
| A | 21 (55.3%) | 19 (48.7%) |
| B | 15 (39.5%) | 17 (43.6%) |
| C | 2 (5.2%) | 3 (7.7%) |
| Previous insertion time ≥ 8 min | 5 | 4 |
Main outcomes
A decreasing trend was observed for CIT in the Maxbelt group, but this was not statistically significant (Maxbelt group: 3.29 min; control group: 4.49 min; P = 0.069; Table 2).
Table 2.
Effect of Maxbelt on cecal intubation time and the subgroup analyses by age, gander, BMI, body height, colonoscope, and previous insertion time
| None | Maxbelt | P value | |
|---|---|---|---|
| Main outcomes | |||
| Cecal intubation time, min | |||
| Mean ± SD | 4.49 ± 3.73 | 3.29 ± 1.41 | 0.069 |
| Range | 1.42–19.75 | 1.1–7.98 | |
| Subgroup analyses | |||
| Age stratification | |||
| ≥45 years | 5.00 ± 4.05 | 3.27 ± 1.32 | 0.032 |
| <45 years | 2.58 ± 0.46 | 3.38 ± 1.89 | 0.311 |
| Gender stratification | |||
| Male | 5.02 ± 4.39 | 3.15 ± 1.53 | 0.053 |
| Female | 3.46 ± 1.58 | 3.51 ± 1.21 | 0.926 |
| BMI stratification, kg/m2 | |||
| <18.5 | 3.22 ± 1.37 | 3.45 ± 0.79 | 0.729 |
| 18.5–25 | 5.84 ± 3.99 | 4.02 ± 2.1 | 0.58 |
| ≧25 | 4.68 ± 4.23 | 3.21 ± 0.99 | 0.115 |
| Body height stratification, cm | |||
| <165 | 4.35 ± 4.35 | 3.47 ± 1.28 | 0.448 |
| ≧165 | 4.58 ± 3.3 | 3.19 ± 1.5 | 0.078 |
| Colonoscope stratification | |||
| CF‐HQ290 | 4.04 ± 3.4 | 3.14 ± 1.22 | 0.163 |
| PCF‐H290H | 6.88 ± 4.77 | 5.11 ± 2.56 | 0.575 |
| Previous insertion time stratification | |||
| Previous insertion time ≥8 min | 5.84 ± 3.99 | 5.07 ± 2.29 | 0.743 |
BMI, body mass index.
Subgroup analyses
After stratifying by age, the use of Maxbelt was associated with a significantly shorter CIT in elderly participants (age ≥ 45 years) compared to the control group (3.27 vs 5.00 min, P = 0.032, Table 2). In subgroup analyses, results were similar after stratification by gender, BMI, body height, colonoscope, and previous insertion times.
Secondary outcomes
Significantly less difficulty in insertion was reported by the colonoscopist in the Maxbelt group (P = 0.01, Table 3). The colonoscopist reported no significant reduction in excessive looping. There was no significant reduction in the discomfort reported by patients. Adenoma detection rate of the Maxbelt group was higher than that of control group, but the difference was not significant (69.2 and 52.6%, respectively). There was no significant reduction in the manual pressure and position change required. There were no statistically significant differences in colonoscopy completion, the dosage requirement of midazolam and pethidine, or incidence of adverse events (Table 3).
Table 3.
Effect of Maxbelt on secondary outcomes
| Secondary outcomes | None | Maxbelt | P value |
|---|---|---|---|
| Colonoscopist‐assessed insertion difficulty | 0.01 | ||
| None | 27 | 36 | |
| Mild | 7 | 0 | |
| Severe | 4 | 3 | |
| Colonoscopist‐assessed excessive looping | 0.235 | ||
| None | 14 | 15 | |
| Mild | 9 | 15 | |
| Severe | 15 | 9 | |
| Patient‐reported intraprocedural discomfort (1–4 scale) | 0.695 | ||
| No discomfort | 23 | 28 | |
| Slight discomfort | 6 | 5 | |
| Uncomfortable | 8 | 6 | |
| Unacceptable | 1 | 0 | |
| Patient‐reported discomfort after procedure (1–4 scale) | 0.431 | ||
| No discomfort | 34 | 37 | |
| Slight discomfort | 4 | 2 | |
| Uncomfortable | 0 | 0 | |
| Unacceptable | 0 | 0 | |
| Adenoma detection rate, % | 52.6 | 69.2 | 0.135 |
| Manual pressure | 1 | 0 | 0.308 |
| Change in position | 4 | 3 | 0.665 |
| Incomplete colonoscopy | 0 | 0 | |
| Adverse event | 0 | 0 | |
| Sedation | 35 | 38 | 0.292 |
| Midazolam, mean ± SD, mg | 3.34 ± 1.19 | 3.87 ± 1.34 | 0.071 |
| Pethidine, mean ± SD, mg | 9.90 ± 10.4 | 12.1 ± 14.6 | 0.444 |
Discussion
In this prospective, randomized trial, we found that the use of a back brace support belt resulted in shorter CIT for elderly participants. Furthermore, the back brace support belt decreased the insertion difficulty faced by a colonoscopist in the Maxbelt group compared with that in control.
A back brace support belt could keep the colonoscope straight in the sigmoid colon and avoid looping throughout the examination and could facilitate a more comfortable insertion because loop formation stretches the colonic mesentery and causes pain. Unlike manual abdominal pressure or position change, the belt generally provides effective pressure that facilitates insertion without the help of assistants or patients themselves.
In the subgroup analysis, use of a back brace support belt significantly decreased CIT in elderly participants compared to the control group. Khashab et al. investigated the effect of age on colorectal length using computed tomography colonography.23 The transverse colon was significantly longer in elderly adults. Our results suggested that abdominal pressure generated by the belt could be more effective in the case of redundant colon in older patients.
Adenoma detection rates were 69.2% in the Maxbelt group and 52.6% in the control group. These data indicate that the use of a back brace support belt does not interfere with adenoma detection.
There are some limitations to our study. As this study used only a single experienced endoscopist, it is difficult to apply these findings directly to other endoscopists. Although randomization and concealed allocation were performed, there was an imbalance among groups with respect to age, which might have led to some confounding.
In conclusion, we demonstrated that a back brace support belt facilitated the insertion of a colonoscope in elderly patients, which may be beneficial for both patients and endoscopists.
Acknowledgments
The authors are grateful to StaGen Co., Ltd. for the review and certification of statistical methods.
Declaration of conflict of interest: All authors have no conflicts of interest or financial ties to disclose.
Author contribution: Osamu Toyoshima and Toshihiro Nishizawa designed the study, recruited patients, analyzed data, and wrote the manuscript. Kosuke Sakitani, Shuntaro Yoshida, Kazushi Fukagawa, and Keisuke Hata critically revised the manuscript. Tadahiro Yamakawa collected the data and maintained study database. Soichiro Ishihara and Hidekazu Suzuki supervised the study.
References
- 1. Bevan R, Rutter MD. Colorectal cancer screening‐who, how, and when? Clin. Endosc. 2018; 51: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N. Engl. J. Med. 2014; 370: 2541. [DOI] [PubMed] [Google Scholar]
- 3. Toyoshima O, Hata K, Yoshida S, Arita M. New‐generation chromoendoscopy may increase confidence in the DISCARD2 study. Gut. 2018; 67: 1742–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yee YK, Tan VP, Chan P, Hung IF, Pang R, Wong BC. Epidemiology of colorectal cancer in Asia. J. Gastroenterol. Hepatol. 2009; 24: 1810–16. [DOI] [PubMed] [Google Scholar]
- 5. Nishizawa T, Yahagi N. Endoscopic mucosal resection and endoscopic submucosal dissection: technique and new directions. Curr. Opin. Gastroenterol. 2017; 33: 315–19. [DOI] [PubMed] [Google Scholar]
- 6. Catalano F, Catanzaro R, Branciforte G et al Colonoscopy technique with an external straightener. Gastrointest. Endosc. 2000; 51: 600–4. [DOI] [PubMed] [Google Scholar]
- 7. Tsutsumi S, Fukushima H, Kuwano H. Colonoscopy using an abdominal bandage. Hepatogastroenterology. 2007; 54: 1983–4. [PubMed] [Google Scholar]
- 8. Lalak N, Englund R, Hanel KC. Incidence of rupture of aortic aneurysms after coincidental operation. Cardiovasc. Surg. 1995; 3: 30–4. [DOI] [PubMed] [Google Scholar]
- 9. Souto‐Ruzo J, Yanez‐Lopez J, Martinez‐Ares D, Liz‐Lois Palomares MT, Vazquez‐Iglesias JL. Rupture of an aneurysm of the right external iliac artery as a complication of colonoscopy. Am. J. Gastroenterol. 2003; 98: 709–10. [DOI] [PubMed] [Google Scholar]
- 10. Toros AB, Ersoz F, Ozcan O. Does a fitted abdominal corset makes colonoscopy more tolerable? Dig. Endosc. 2012; 24: 164–7. [DOI] [PubMed] [Google Scholar]
- 11. Crockett SD, Cirri HO, Kelapure R, Galanko JA, Martin CF, Dellon ES. Use of an abdominal compression device in colonoscopy: a randomized, sham‐controlled trial. Clin. Gastroenterol. Hepatol. 2016; 14: 850–7 e853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu GQ, Huang XM, Li HY et al Use of an abdominal obstetric binder in colonoscopy: a randomized, prospective trial. J. Gastroenterol. Hepatol. 2018; 33: 1365–9. [DOI] [PubMed] [Google Scholar]
- 13. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010; 152: 726–32. [DOI] [PubMed] [Google Scholar]
- 14. Moon SY, Kim BC, Sohn DK et al Predictors for difficult cecal insertion in colonoscopy: the impact of obesity indices. World J. Gastroenterol. 2017; 23: 2346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsieh YH, Kuo CS, Tseng KC, Lin HJ. Factors that predict cecal insertion time during sedated colonoscopy: the role of waist circumference. J. Gastroenterol. Hepatol. 2008; 23: 215–17. [DOI] [PubMed] [Google Scholar]
- 16. Toyoshima O, Yoshida S, Nishizawa T et al CF290 for pancolonic chromoendoscopy improved sessile serrated polyp detection and procedure time: a propensity score‐matching study. Endosc. Int. Open. 2019; 7: E987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen SW, Hui CK, Chang JJ et al Carbon dioxide insufflation during colonoscopy can significantly decrease post‐interventional abdominal discomfort in deeply sedated patients: a prospective, randomized, double‐blinded, controlled trial. J. Gastroenterol. Hepatol. 2016; 31: 808–13. [DOI] [PubMed] [Google Scholar]
- 18. Nishizawa T, Banno S, Kinoshita S et al Feasibility of endoscopic mucosa‐submucosa clip closure method (with video). Endosc. Int. Open. 2018; 6: E1070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corte C, Dahlenburg L, Selby W et al Hyoscine butylbromide administered at the cecum increases polyp detection: a randomized double‐blind placebo‐controlled trial. Endoscopy. 2012; 44: 917–22. [DOI] [PubMed] [Google Scholar]
- 20. Ell C, Fischbach W, Bronisch HJ et al Randomized trial of low‐volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am. J. Gastroenterol. 2008; 103: 883–93. [DOI] [PubMed] [Google Scholar]
- 21. Wolf AMD, Fontham ETH, Church TR et al Colorectal cancer screening for average‐risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018; 68: 250–81. [DOI] [PubMed] [Google Scholar]
- 22. James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes. Res. 2001; 9 (Suppl. 4): 228S–33S. [DOI] [PubMed] [Google Scholar]
- 23. Khashab MA, Pickhardt PJ, Kim DH, Rex DK. Colorectal anatomy in adults at computed tomography colonography: normal distribution and the effect of age, sex, and body mass index. Endoscopy. 2009; 41: 674–8. [DOI] [PubMed] [Google Scholar]


