Abstract
Background
Currently, the data on the relationship between obesity and gastroesophageal reflux disease (GERD) in Asian populations are scarce.
Methods
The aim of this study is to investigate the prevalence of reflux esophagitis (RE) among obese Japanese patients in each body mass index (BMI) range group. In addition, we aim to investigate the risk factors for RE in obese Japanese patients. The present retrospective cohort study included 674 obese Japanese patients who underwent bariatric surgery between January 2003 and April 2016. The patients were stratified into five groups based on BMI range.
Results
The mean BMI was 42.7 ± 9.24 kg/m2. The prevalence of RE among each of the groups was as follows: Group 1 (BMI 30–34.9) = 20.7%; Group 2 (BMI 35–39.9) = 24.0%; Group 3 (BMI 40–44.9) = 25.2%; Group 4 (BMI 45–49.9) = 26.7%; and Group 5 (BMI ≥50) = 24.8%. Overall, the prevalence of RE was 24.2% in our study. Furthermore, no significant difference in BMI was noted between the RE and non‐RE groups (43.4 ± 9.3 kg/m2 and 42.5 ± 10.2 kg/m2, respectively; p = 0.24). According to the multivariate logistic regression model, gender, Helicobacter pylori infection status, GERD‐related symptoms, and hiatal hernia were significantly correlated with RE.
Conclusion
Our study shows that the prevalence of RE in severely obese Japanese patients was significantly higher than the average prevalence of RE in Japan. However, the prevalence of RE did not increase with BMI in our cohort.
Keywords: bariatric surgery, gastroesophageal reflux disease, obesity
Our study is the first to report the prevalence of RE in severely obese Japanese patients. Although it has certain limitations, it can still provide valuable reference data in epidemiology.
Introduction
Gastroesophageal reflux disease (GERD) is a disease characterized by a series of troublesome symptoms with complications that arise from the reflux of gastric contents into the esophagus.1 Earlier reports have shown that the prevalence of GERD has been estimated to be between 10 and 20% in the United States and Europe, with a lower frequency in Asia.2 A recent systematic review estimated the prevalence of GERD in the United States at 18.1–27.8%.3 El‐Serag reported that its incidence had significantly increased in the last two decades in North America and Europe but not in Asia.4 On the contrary, Fujiwara reported that the prevalence of GERD is increasing in Japan, as well as in the West.5
Obesity is a known risk factor for GERD or reflux esophagitis (RE). In earlier studies, several pathophysiological mechanisms linking the two conditions were reported.6, 7 Several studies have demonstrated a higher prevalence of GERD in obese individuals compared with the nonobese population.8, 9 Moreover, numerous studies from the United States have shown that increased levels of obesity have been associated with a higher likelihood of GERD.1, 10, 11 The same relationship between obesity and GERD has been observed in Europe.12, 13, 14, 15 However, data regarding the relationship between obesity and GERD in Asia have been scarce. One plausible reason for this is the relatively smaller population of severely obese patients among Asian populations. Kang et al. studied 2457 participants who underwent upper gastrointestinal (GI) endoscopy in Korea.16 The prevalence rates of RE were 5.6, 8.1, and 15.5% for the body mass index (BMI) groups of <25, 25–30, and > 30 kg/m2, respectively. Consequently, it appears that a higher BMI is proportional to the occurrence of RE. Currently, there is insufficient data regarding the relationship between obesity and GERD or RE in other Asian countries. In particular, such a relationship in severely obese Japanese patients has not been reported despite the growing severely obese population in Japan.17 This study demonstrates that GERD or RE is prevalent in many severely obese Japanese individuals, which can serve as valuable reference data in epidemiologic studies.
Here, we aim to investigate the prevalence of RE in obese Japanese patients who have undergone bariatric surgery. To the best of our knowledge, this is the first study on this topic.
Methods
Patients
This retrospective cohort study included 674 consecutive obese Japanese patients (295 females and 379 males) who underwent bariatric surgery between January 2003 and April 2016 at Yotsuya Medical Cube, Tokyo, Japan. All patients provided written informed consent. The inclusion criteria for laparoscopic bariatric surgery were based on the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Guidelines for Clinical Application of Laparoscopic Bariatric Surgery (medically uncontrolled, with ages between 18 and 65 years, and a BMI greater than 30 kg/m2 with obesity‐related comorbidities), which was approved by the Institutional Review Board. Each patient was preoperatively screened and evaluated by our multidisciplinary team.18 The following parameters were evaluated prior to surgery: patient characteristics (gender, age, height, weight, and BMI), visceral fat area, visceral/subcutaneous fat ratio, usage of proton pump inhibitor (PPI) treatment, status of Helicobacter pylori (H. pylori) infection, GERD‐related symptoms, Barrett's esophagus (BE), and hiatal hernia. Abdominal computed tomography scans were routinely performed to assess visceral/subcutaneous fat measured on one cross‐sectional scan obtained at the level of the umbilicus.
In our cohort, all the patients underwent gastroscopy before bariatric surgery. Regarding the status of H. pylori infection, patients with a history of H. pylori infection that had already been eradicated were also categorized into the H. pylori‐negative group in this study. Prior to the upper GI endoscopy, the participants were interviewed regarding their main complaints and symptoms (chest pain, dyspepsia, and dysmotility). The severity of RE observed on gastroscopy was graded from A to D and was based on the Los Angeles classification system.19 On upper GI endoscopy, a hiatal hernia was considered if the diaphragmatic indentation was >2 cm distal to the Z‐line and proximal margins of the gastric mucosal folds, which were observed with considerable air insufflation during inspiration. The distance was measured using the centimeter markings during the upper GI endoscopy.20, 21
The patients were divided into five groups according to their BMI: Group 1 (30–34.9 kg/m2), Group 2 (35–39.9 kg/m2), Group 3 (BMI 40–44.9 kg/m2), Group 4 (BMI 45–49.9 kg/m2), and Group 5 (≥50 kg/m2). Then, the prevalence of RE in each group was determined. The associations between the visceral fat ratio and BMI were analyzed using Spearman's correlation coefficient. In addition, it was the researcher's aim to investigate the risk factors for RE that were associated with obese Japanese patients.
The present study was approved by the Institutional Review Board to ensure the protection of patient privacy and confidentiality, and it was performed in accordance with the ethical standards of the World Medical Association's Declaration of Helsinki.
Statistical analysis
Statistical analysis was performed using JMP® 11 (SAS Institute Inc., Cary, NC, USA). The results were expressed as the mean ± standard deviation and percentage. Moreover, the grouped data were expressed as the median (range), and nonparametric methods were used. A univariate analysis was performed using the chi‐square test or Fisher's exact test to identify the associations between variables. Furthermore, Cox proportional hazards regression was performed to analyze gender, the status of H. pylori infection, GERD‐related symptoms, and hiatal hernia. A probability (P) value <0.05 was considered statistically significant. A correlation analysis was also performed using Spearman's correlation coefficient. The study by Guilford (1956) was used to interpret and compare the correlation coefficients. The correlation coefficients he described are discussed below. Here, <0.20: “slight almost negligible relationships”; 0.20–0.40: “low correlation”; 0.40–0.70: “moderate correlation”; 0.70–0.90: “high correlation, marked relationship”; and > 0.90: “very high correlation, very dependable relationship.”
Results
Regarding patient characteristics, the mean BMI, body weight, and age were 42.7 ± 9.24 kg/m2, 117.7 ± 29.4 kg, and 41.2 ± 10.3 years, respectively. On examination, H. pylori infection was found in 29 patients (4.3%). The mean visceral fat ratio was 0.39 ± 0.19. GERD‐related symptoms were also noted in 41% of patients. Among the 674 patients, Grades A, B, C, and D were present in 114 cases (16.9%), 37 cases (5.5%), 11 cases (1.5%), and 1 case (0.2%), respectively. In all, the prevalence of RE was 24.2% in our study. Approximately 40% of patients who underwent surgery at our institution had hiatal hernia, and 1.6% had BE. Prior to surgery, 8.9% of patients had already taken PPI medication. Other patient characteristics are shown in Table 1. The prevalence rates of RE in all the groups were as follows: Group 1, 20.7% @@@(n = 27/130); Group 2, 24.0% (n = 43/179); Group 3, 25.2% (n = 35/139); Group 4, 26.7% (n = 27/101); and Group 5, 24.8% (n = 31/125) (Table 2).
Table 1.
Patients characteristics
| Variables | n = 674 |
|---|---|
| Gender, n (%) | |
| Male | 379 (56.3) |
| Female | 295 (43.7) |
| Age (years), mean ± SD | 41.2 ± 10.3 |
| BMI | 42.7 ± 9.24 |
| Helicobacter pylori, n (%) | |
| Positive | 29 (4.3) |
| Negative | 645 (95.7) |
| Visceral/subcutaneous fat ratio, mean ± SD | 0.39 ± 0.19 |
| Visceral fat area (cm2), mean ± SD | 189.40 ± 141.50 |
| GERD‐related symptoms, n (%) | |
| Positive | 276 (41.0) |
| Negative | 398 (59.0) |
| LA classification, n (%) | |
| N | 316 (46.9) |
| M | 195 (28.9) |
| A | 114 (16.9) |
| B | 37 (5.5) |
| C | 11 (1.5) |
| D | 1 (0.2) |
| Barret esophagus, n (%) | |
| Positive | 11 (1.6) |
| Negative | 663 (98.4) |
| Hiatal hernia | |
| Positive | 268 (39.8) |
| Negative | 406 (60.2) |
| Medication (PPI), n (%) | |
| Positive | 60 (8.9) |
| Negative | 614 (91.1) |
BMI, body mass index; GERD, gastroesophageal reflux disease; LA, Los Angeles; PPI, proton pump inhibitor; RE, reflux esophagitis; SD, standard deviation.
Table 2.
The prevalence of RE in each group
| BMI (kg/m2) | 30< | 35< | 40< | 45< | 50< |
| Age (years), mean ± SD | 45.5 ± 9.3 | 41.1 ± 11.4 | 40.5 ± 10.4 | 40.2 ± 9.9 | 38.1 ± 8.3 |
| Number of patients | 27/130 | 43/179 | 35/139 | 27/101 | 31/125 |
| Ratio of patients (%) | 20.7 | 24 | 25.2 | 26.7 | 24.8 |
BMI, body mass index; RE, reflux esophagitis; SD, standard deviation.
In the univariate analysis, no significant difference in BMI was noted between the RE and non‐RE groups (43.4 ± 9.3 and 42.5 ± 10.2 kg/m2, respectively; P = 0.24) (Table 3). Furthermore, no significant correlation was observed between the visceral fat ratio and BMI (0.39 ± 0.19 and 42.7 ± 9.23, respectively; P < 0.0001; Spearman's correlation coefficient) (Fig. 1). No significant differences were noted in age, visceral/subcutaneous fat ratio, BE, and usage of PPI therapy between the RE and non‐RE groups. Male gender was more prevalent in the RE group (P < 0.0001). The proportion of patients who experienced GERD‐related symptoms and hiatal hernia was significantly higher in the RE group compared with the non‐RE group (P < 0.0001 and P < 0.0001, respectively, for the RE group and non‐RE group). On the contrary, the proportion of patients with H. pylori infection was significantly lower in the RE group (P < 0.02) (Table 4). According to the multivariate logistic regression model, gender, status of H. pylori infection, GERD‐related symptoms, and hiatal hernia were significantly correlated with RE (Table 5).
Table 3.
The associations between RE and BMI
| LA classification | M, N | A, B, C, D | Univariate analysis (P) |
|---|---|---|---|
| Number of patients | 163 | 511 | 0.24 |
| BMI (kg/m2) | 43.4 ± 9.3 | 42.5 ± 10.2 |
BMI, body mass index; RE, reflux esophagitis.
Figure 1.
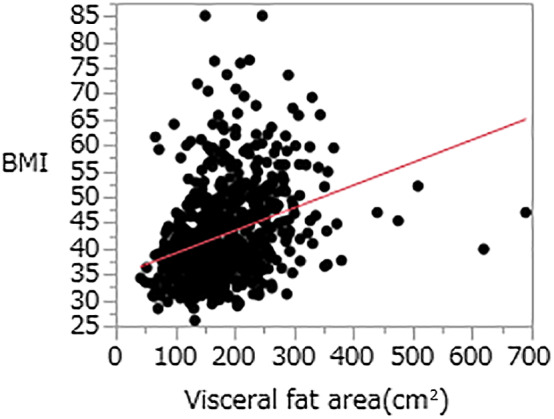
The associations between the visceral fat area and body mass index (BMI) (Spearman's correlation coefficient). No significant correlation between the visceral fat area and BMI was noted (0.39 ± 0.19 and 42.7 ± 9.23, respectively; P < 0.0001).
Table 4.
The risk factors for RE in obese Japanese patients
| Variables | RE (+) | RE (−) | Univariate analysis (P) |
|---|---|---|---|
| Gender, n (%) | <0.0001 | ||
| Male | 95 (14.1) | 200 (29.7) | |
| Female | 68 (10.1) | 311 (46.1) | |
| Age (years), mean ± SD | 41.8 ± 9.5 | 40.9 ± 10.5 | 0.22 |
| Helicobacter pylori, n (%) | <0.02 | ||
| Positive | 2 (0.3) | 27 (4.0) | |
| Negative | 161 (23.9) | 484 (71.8) | |
| Visceral/subcutaneous fat ratio, mean ± SD | 0.4 ± 0.17 | 0.38 ± 0.19 | 0.11 |
| Visceral fat area (cm2), mean ± SD | 195.07 ± 74.35 | 187.59 ± 157.01 | 0.55 |
| GERD‐related symptoms, n (%) | <0.0001 | ||
| Positive | 88 (13.1) | 188 (27.9) | |
| Negative | 75 (11.1) | 323 (47.9) | |
| Barret esophagus, n (%) | 0.34 | ||
| Positive | 4 (0.6) | 7 (1.0) | |
| Negative | 159 (23.6) | 504 (74.8) | |
| Hiatal hernia, n (%) | <0.0001 | ||
| Positive | 104 (15.4) | 164 (24.3) | |
| Negative | 59 (8.8) | 347 (51.5) | |
| Medication (PPI), n (%) | 0.08 | ||
| Positive | 9 (1.3) | 51 (7.6) | |
| Negative | 154 (22.9) | 460 (68.2) |
GERD, gastroesophageal reflux disease; PPI, proton pump inhibitor; RE, reflux esophagitis; SD, standard deviation.
Table 5.
Multivariate logistic regression model associated with RE
| Univariate | Adjusted OR | 95% CI | Multivariate analysis (p‐value) |
|---|---|---|---|
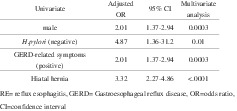
| Male | 2.01 | 1.37–2.94 | 0.0003 |
| Helicobacter pylori (negative) | 4.87 | 1.36–31.2 | 0.01 |
| GERD‐related symptoms (positive) | 2.01 | 1.37–2.94 | 0.0003 |
| Hiatal hernia | 3.32 | 2.27–4.86 | <0.0001 |
CI, confidence interval; GERD, gastroesophageal reflux disease; OR, odds ratio; RE, reflux esophagitis.
Discussion
The aim of this study is to evaluate the prevalence of RE in severely obese Japanese patients stratified according different ranges of BMI. In this study, only severely obese Japanese patients with a BMI greater than 30 were included. To date, no data of this scale with this demographic are available in literature reviews. We believe that the information gained from this study could demonstrate the relationship between obesity and RE among Asian populations, particularly among obese Japanese.
Obesity has increased globally, and more than 30% of adults are considered to have a BMI value exceeding 25.0 kg/m2.22 Although the obesity rate in Japan is the lowest among the Organization for Economic Co‐operation and Development‐affiliated countries, this rate has increased among men and the elderly. In a national health and nutrition survey conducted in 2017, 30.7% of adult males and 21.9% of adult females in Japan had a BMI exceeding 25 kg/m2. Theoretically, the prevalence of GERD or RE will eventually increase with the increase in obesity rates. A recent study showed that the prevalence rates of GERD were 6.2%, 2.5–4.8%, and 3.5% in China, Hong Kong, and Korea, respectively.23, 24, 25, 26, 27 Based on earlier studies on Asian populations, the prevalence of GERD or RE in Asia remains lower than that reported in the West.2, 3, 8, 9, 28, 29 Although the percentage of obese Asians may be low, epidemiological studies regarding GERD or RE complicate the comparison because of the differences in its prevalence caused by environmental factors and genetic variations in the populations in Asia and the West.30 Accordingly, investigations on the prevalence of GERD or RE in Asia and in severe obese individuals would be extremely useful as only a few studies on this topic have been reported.16, 26, 31 A recent Japanese epidemiological study investigated the prevalence of GERD between normal‐weight and overweight or obese patients.32 This study showed that the prevalence rates of RE were significantly higher in overweight (25.8%) (odds ratio [OR], 2.27; 95% confidence interval [CI], 1.82–2.82) and obese (35.9%) (OR, 3.65; 95% CI, 2.40–5.57) patients compared with normal‐weight patients. Although related studies about the prevalence of GERD or RE based on differences in body weight have been previously reported,32 this is the first study that compares the prevalence of RE in severely obese Japanese patients according to their BMI.
In general, the prevalence of RE was 6.1–13.7% in Japan.5, 33, 34, 35, 36 The present study shows that the prevalence of RE in severely obese Japanese patients was significantly higher than the average prevalence of RE in Japan. Obesity may increase the risk of RE, which can be attributed to mechanical factors.6, 7, 37, 38, 39 From a physiologic standpoint, abdominal obesity increases intra‐abdominal pressure.40, 41 If the intra‐abdominal pressure increases, both gastric and esophageal pressures also increase, and the lower esophageal sphincter (LES) may be displaced in a cephalad fashion.42 Therefore, an increased intra‐abdominal pressure in obese individuals may cause RE. The previous statement is supported by Wilson's observation: a higher prevalence of hiatal hernia in obese patients may contribute to an increased rate of RE in the same population.43 Fisher and Mercer, respectively, noted that the gastroesophageal pressure gradient and the frequency of transient LES relaxation were significantly higher in obese patients.6, 44 Theoretically, with an increase in BMI, the abdominal pressure was predicted to be high, thereby increasing the prevalence of RE. However, the prevalence of RE did not increase with BMI in our cohort.
The possible causes of the increase in BMI are not necessarily an increase in visceral fat but an increase in subcutaneous fat. In this study, no correlation between the BMI and visceral fat ratio was noted. Therefore, the prevalence of RE in obese patients was higher than that in normal‐weight patients, but RE presumably did not increase with increasing BMI among severely obese patients.
Several reports comparing normal‐weight and obese patients have identified a strong association between an increased BMI and GERD‐related symptoms, although all these differ in their observations.43, 45, 46 Furthermore, the prevalence of GERD in obese patients was higher than that in normal‐weight patients in the West, but it did not necessarily increase with the BMI.8, 47
An earlier Japanese study demonstrated that the proportions of participants with GERD‐related symptoms were 23.3, 26.7, and 50% for the BMI groups of <25, 25–30, and > 30 kg/m2, respectively, and that the corresponding prevalence rates of RE were 12.5, 29.8, and 26.9%.32 In a recent report comparing the prevalence of RE in Japanese normal‐weight patients compared with obese patients, obesity was significantly different when considering the increased prevalence of RE, but it was not significantly different in GERD.32 In addition, a comprehensive assessment of the data from earlier studies suggests that the prevalence of GERD or RE can be significantly increased in obese patients compared with normal‐weight patients.43, 45, 46 However, the data obtained in this study suggest that a sole relationship between obesity grade and prevalence of RE among obese patients cannot be established.
Recently, esophageal hiatal hernia, H. pylori infection, and gender have been reported as risk factors for RE in Europe, United States, and Asia.30, 48, 49, 50, 51 According to this study, similar results were obtained for severely obese Japanese patients.
This study has some limitations that are inherent to observational studies that should be addressed. First, Grade M of GERD is a classification unique to Japan; its comparison with grades in other foreign studies is difficult. Bias cannot be totally excluded when the diagnosis of Grade M is considered, especially in the presence of an enforcer. As multiple enforcers were involved in this examination, the introduction of a bias is highly possible. Second, because this study examined patients who underwent bariatric surgery, the population was isolated. Inevitably, this study was focused on a group of young patients with several comorbid obesity‐related diseases, and therefore, such a group may not, in general, correctly reflect severely obese Japanese in the general population. Nonetheless, a relatively large number of cases was involved, and endoscopic examinations were performed in all the cases as a preoperative evaluation step. This is a retrospective study, but it may be highly reliable because it is consecutive and has no selective bias. Finally, the present study evaluated the prevalence of RE and did not accurately evaluate nonerosive reflux disease (NERD). Technically, endoscopy and 24‐h pH monitoring impedance testing are required to properly diagnose NERD. However, pH monitoring and impedance testing are practically difficult to conduct in all severely obese patients who have GERD‐related symptoms but who do not primarily intend to undergo treatment for RE. In addition, 8.9% patients had already undergone PPI therapy before surgery, which may have caused bias.
Conclusion
Our study is the first to report the prevalence of RE in severely obese Japanese patients. We show that the prevalence of RE in severely obese Japanese patients was significantly higher than the average prevalence of RE in Japan. However, no significant difference was observed in BMI between the RE and non‐RE groups. Although our study has certain limitations, these findings can still provide valuable reference data in the epidemiology field. In the future, a well‐designed, large‐sample, multicenter study is required to confirm these findings.
Declaration of conflict of interest: None
Author contribution: Yosuke Seki and Kodai Takahashi were responsible for the conception and design of the study, acquisition analysis and interpretation of data, drafting the text, and revision of the intellectual content. Kazunori Kasama, Manabu Amiki, Satoshi Baba, Masayoshi Ito, Tatsuro Tanaka, and Eiji Kanehira were responsible for the revision of the intellectual content of the text. All authors read and approved the final manuscript.
References
- 1. Hampel H, Abraham NS, El‐Serag HB. Meta‐analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann. Intern. Med. 2005; 143: 199–211. [DOI] [PubMed] [Google Scholar]
- 2. Dent J, El‐Serag HB, Wallander MA, Johansson S. Epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut. 2005; 54: 710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El‐Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut. 2014; 63: 871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El‐Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin. Gastroenterol. Hepatol. 2007; 5: 17–26. [DOI] [PubMed] [Google Scholar]
- 5. Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J. Gastroenterol. 2009; 44: 518–34. [DOI] [PubMed] [Google Scholar]
- 6. Fisher BL, Pennathur A, Mutnick JL, Little AG. Obesity correlates with gastroesophageal reflux. Dig. Dis. Sci. 1999; 44: 2290–4. [DOI] [PubMed] [Google Scholar]
- 7. O'Brien TF Jr. Lower esophageal sphincter pressure (LESP) and esophageal function in obese humans. J. Clin. Gastroenterol. 1980; 2: 145–8. [PubMed] [Google Scholar]
- 8. Corley DA, Kubo A, Zhao W. Abdominal obesity, ethnicity and gastro‐oesophageal reflux symptoms. Gut. 2007; 56: 756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA Jr. Body‐mass index and symptoms of gastroesophageal reflux in women. N. Engl. J. Med. 2006; 354: 2340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El‐Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am. J. Gastroenterol. 2005; 100: 1243–50. [DOI] [PubMed] [Google Scholar]
- 11. Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat. Rev. Gastroenterol. Hepatol. 2011; 8: 340–7. [DOI] [PubMed] [Google Scholar]
- 12. Diaz‐Rubio M, Moreno‐Elola‐Olaso C, Rey E, Locke GR 3rd, Rodriguez‐Artalejo F. Symptoms of gastro‐oesophageal reflux: prevalence, severity, duration and associated factors in a Spanish population. Aliment. Pharmacol. Ther. 2004; 19: 95–105. [DOI] [PubMed] [Google Scholar]
- 13. Murray L, Johnston B, Lane A et al Relationship between body mass and gastro‐oesophageal reflux symptoms: The Bristol Helicobacter Project. Int. J. Epidemiol. 2003; 32: 645–50. [DOI] [PubMed] [Google Scholar]
- 14. Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA. 2003; 290: 66–72. [DOI] [PubMed] [Google Scholar]
- 15. Nocon M, Labenz J, Willich SN. Lifestyle factors and symptoms of gastro‐oesophageal reflux – a population‐based study. Aliment. Pharmacol. Ther. 2006; 23: 169–74. [DOI] [PubMed] [Google Scholar]
- 16. Kang MS, Park DI, Oh SY et al Abdominal obesity is an independent risk factor for erosive esophagitis in a Korean population. J. Gastroenterol. Hepatol. 2007; 22: 1656–61. [DOI] [PubMed] [Google Scholar]
- 17. Sakaguchi M, Oka H, Hashimoto T et al Obesity as a risk factor for GERD in Japan. J. Gastroenterol. 2008; 43: 57–62. [DOI] [PubMed] [Google Scholar]
- 18. SAGES Guidelines Committee . SAGES guideline for clinical application of laparoscopic bariatric surgery. Surg. Endosc. 2008; 22: 2281–300. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong D, Bennett JR, Blum AL et al The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996; 111: 85–92. [DOI] [PubMed] [Google Scholar]
- 20. Johnson DA, Younes Z, Hogan WJ. Endoscopic assessment of hiatal hernia repair. Gastrointest. Endosc. 2000; 52: 650–9. [DOI] [PubMed] [Google Scholar]
- 21. Mittal RK. Hiatal hernia: myth or reality? Am. J. Med. 1997; 103: 33s–9s. [DOI] [PubMed] [Google Scholar]
- 22. GBD 2015 Maternal Mortality Collaborators . Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016; 388: 1775–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen M, Xiong L, Chen H, Xu A, He L, Hu P. Prevalence, risk factors and impact of gastroesophageal reflux disease symptoms: a population‐based study in South China. Scand. J. Gastroenterol. 2005; 40: 759–67. [DOI] [PubMed] [Google Scholar]
- 24. Cheung TK, Lam KF, Hu WH et al Positive association between gastro‐oesophageal reflux disease and irritable bowel syndrome in a Chinese population. Aliment. Pharmacol. Ther. 2007; 25: 1099–104. [DOI] [PubMed] [Google Scholar]
- 25. Cho YS, Choi MG, Jeong JJ et al Prevalence and clinical spectrum of gastroesophageal reflux: a population‐based study in Asan‐si, Korea. Am. J. Gastroenterol. 2005; 100: 747–53. [DOI] [PubMed] [Google Scholar]
- 26. Wong WM, Lai KC, Lam KF et al Prevalence, clinical spectrum and health care utilization of gastro‐oesophageal reflux disease in a Chinese population: a population‐based study. Aliment. Pharmacol. Ther. 2003; 18: 595–604. [DOI] [PubMed] [Google Scholar]
- 27. Wong WM, Lai KC, Lam KF et al Onset and disappearance of reflux symptoms in a Chinese population: a 1‐year follow‐up study. Aliment. Pharmacol. Ther. 2004; 20: 803–12. [DOI] [PubMed] [Google Scholar]
- 28. Cremonini F, Locke GR 3rd, Schleck CD, Zinsmeister AR, Talley NJ. Relationship between upper gastrointestinal symptoms and changes in body weight in a population‐based cohort. Neurogastroenterol. Motil. 2006; 18: 987–94. [DOI] [PubMed] [Google Scholar]
- 29. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999‐2010. JAMA. 2012; 307: 491–7. [DOI] [PubMed] [Google Scholar]
- 30. Wong BC, Kinoshita Y. Systematic review on epidemiology of gastroesophageal reflux disease in Asia. Clin. Gastroenterol. Hepatol. 2006; 4: 398–407. [DOI] [PubMed] [Google Scholar]
- 31. Ma XQ, Cao Y, Wang R et al Prevalence of, and factors associated with, gastroesophageal reflux disease: a population‐based study in Shanghai, China. Dis Esophagus. 2009; 22: 317–22. [DOI] [PubMed] [Google Scholar]
- 32. Sakaguchi M, Amano M, Takao M et al The correlation between gastroesophageal reflux disease with obesity and lifestyle related diseases. Ther. Res. 2016; 37: 367–77. [Google Scholar]
- 33. Adachi K, Mishiro T, Tanaka S, Yoshikawa H, Kinoshita YA. A Study on the relationship between reflux esophagitis and periodontitis. Intern. Med. 2016; 55: 2523–8. [DOI] [PubMed] [Google Scholar]
- 34. Kusano M, Kouzu T, Kawano T, Ohara S. Nationwide epidemiological study on gastroesophageal reflux disease and sleep disorders in the Japanese population. J. Gastroenterol. 2008; 43: 833–41. [DOI] [PubMed] [Google Scholar]
- 35. Takeshita E, Sakata Y, Hara M et al Higher frequency of reflux symptoms and acid‐related dyspepsia in women than men regardless of endoscopic esophagitis: analysis of 3,505 Japanese subjects undergoing medical health checkups. Digestion. 2016; 93: 266–71. [DOI] [PubMed] [Google Scholar]
- 36. Yamagishi H, Koike T, Ohara S et al Clinical characteristics of gastroesophageal reflux disease in Japan. Hepatogastroenterology. 2009; 56: 1032–4. [PubMed] [Google Scholar]
- 37. Dodds WJ, Hogan WJ, Miller WN, Stef JJ, Arndorfer RC, Lydon SB. Effect of increased intraabdominal pressure on lower esophageal sphincter pressure. Am. J. Dig. Dis. 1975; 20: 298–308. [DOI] [PubMed] [Google Scholar]
- 38. Mittal RK, Lange RC, McCallum RW. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology. 1987; 92: 130–5. [DOI] [PubMed] [Google Scholar]
- 39. Nandurkar S, Locke GR 3rd, Fett S, Zinsmeister AR, Cameron AJ, Talley NJ. Relationship between body mass index, diet, exercise and gastro‐oesophageal reflux symptoms in a community. Aliment. Pharmacol. Ther. 2004; 20: 497–505. [DOI] [PubMed] [Google Scholar]
- 40. Sugerman HJ. Increased intra‐abdominal pressure in obesity. Int. J. Obes. Relat. Metab. Disord. 1998; 22: 1138. [DOI] [PubMed] [Google Scholar]
- 41. Sugerman HJ, DeMaria EJ, Felton WL 3rd, Nakatsuka M, Sismanis A. Increased intra‐abdominal pressure and cardiac filling pressures in obesity‐associated pseudotumor cerebri. Neurology. 1997; 49: 507–11. [DOI] [PubMed] [Google Scholar]
- 42. Crowell MD, Bradley A, Hansel S et al Obesity is associated with increased 48‐h esophageal acid exposure in patients with symptomatic gastroesophageal reflux. Am. J. Gastroenterol. 2009; 104: 553–9. [DOI] [PubMed] [Google Scholar]
- 43. Wilson LJ, Ma W, Hirschowitz BI. Association of obesity with hiatal hernia and esophagitis. Am. J. Gastroenterol. 1999; 94: 2840–4. [DOI] [PubMed] [Google Scholar]
- 44. Mercer CD, Wren SF, DaCosta LR, Beck IT. Lower esophageal sphincter pressure and gastroesophageal pressure gradients in excessively obese patients. J. Med. 1987; 18: 135–46. [PubMed] [Google Scholar]
- 45. Lagergren J, Bergstrom R, Nyren O. No relation between body mass and gastro‐oesophageal reflux symptoms in a Swedish population based study. Gut. 2000; 47: 26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Risk factors associated with symptoms of gastroesophageal reflux. Am. J. Med. 1999; 106: 642–9. [DOI] [PubMed] [Google Scholar]
- 47. El‐Serag HB, Ergun GA, Pandolfino J, Fitzgerald S, Tran T, Kramer JR. Obesity increases oesophageal acid exposure. Gut. 2007; 56: 749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goh KL, Chang CS, Fock KM, Ke M, Park HJ, Lam SK. Gastro‐oesophageal reflux disease in Asia. J. Gastroenterol. Hepatol. 2000; 15: 230–8. [DOI] [PubMed] [Google Scholar]
- 49. Labenz J, Jaspersen D, Kulig M et al Risk factors for erosive esophagitis: a multivariate analysis based on the ProGERD study initiative. Am. J. Gastroenterol. 2004; 99: 1652–6. [DOI] [PubMed] [Google Scholar]
- 50. Wang JH, Luo JY, Dong L, Gong J, Tong M. Epidemiology of gastroesophageal reflux disease: a general population‐based study in Xi'an of Northwest China. World J. Gastroenterol. 2004; 10: 1647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu JC, Sung JJ, Ng EK et al Prevalence and distribution of Helicobacter pylori in gastroesophageal reflux disease: a study from the East. Am. J. Gastroenterol. 1999; 94: 1790–4. [DOI] [PubMed] [Google Scholar]


