Abstract
Background
Triptans are the most commonly prescribed acute treatments for migraine; however, not all triptan users experience adequate response. Information on real-world resource use and costs associated with triptan insufficient response are limited.
Methods
A retrospective claims analysis using US commercial health plan data between 2012 and 2015 assessed healthcare resource use and costs in adults with a migraine diagnosis newly initiating triptans. Patients who either did not refill triptans but used other non-triptan medications or refilled triptans but also filled non-triptan medications over a 24-month follow-up period were designated as potential triptan insufficient responders. Patients who continued filling only triptans (i.e. triptan-only continuers) were designated as potential adequate responders. All-cause and migraine-related resource use and total (medical and pharmacy) costs over months 1–12 and months 13–24 were compared between triptan-only continuers and potential triptan insufficient responders.
Results
Among 10,509 new triptan users, 4371 (41%) were triptan-only continuers, 3102 (30%) were potential triptan insufficient responders, and 3036 (29%) did not refill their index triptan or fill non-triptan medications over 24 months’ follow-up. Opioids were the most commonly used non-triptan treatment (68%) among potential triptan insufficient responders over 24 months of follow-up. Adjusted mean all-cause and migraine-related total costs were $5449 and $2905 higher, respectively, among potential triptan insufficient responders versus triptan-only continuers over the first 12 months.
Conclusions
In a US commercial health plan, almost one-third of new triptan users were potential triptan insufficient responders and the majority filled opioid prescriptions. Potential triptan insufficient responder patients had significantly higher all-cause and migraine-related healthcare utilization and costs than triptan-only continuers.
Keywords: Migraine, migraine medication, refill patterns, claims data, burden of illness, direct costs, payer costs, resource utilization
Introduction
Migraine is a chronic disease with episodic attacks characterized by neurological symptoms that are often incapacitating, including pain, nausea, and sensitivity to light and sound (1). Migraine has a substantial impact on many aspects of life, including social, emotional, workplace, family, functional disability, and health-related quality of life (2–4). Attacks can be debilitating; approximately half of those experiencing migraine attacks report severe impairment or the need for bed rest (4). Migraine is a leading cause of disability worldwide, with approximately 65% of all disability attributed to neurological conditions caused by migraine (5). With peak prevalence between 30 and 39 years of age, migraine is the leading cause of disability for those aged 15 to 49 (6,7).
In addition, migraine has a high economic impact (including direct and indirect costs) to payers and employers (8–16). In the United States (US), the estimated mean annual direct all-cause healthcare costs for individuals with migraine is between $6575 and $9798 higher compared to those without migraine (9,11). Migraine is accountable for approximately $56 billion in annual US healthcare expenditures (15).
Effective acute treatment can reduce pain, symptoms, and disability associated with migraine attacks. In contrast, suboptimal acute treatment can lead to increased migraine-related disability, use of inappropriate treatments, risk of medication overuse, and risk of disease progression (17–20). Suboptimal acute treatment is common, with nearly 40% reporting dissatisfaction with acute treatment (21). More than half (56%) report inadequate response to acute treatment, as measured by pain freedom at two hours (22). Significant predictors of insufficient response to acute treatment include male sex, higher body mass index, and increasing migraine frequency and severity (22).
Triptans are recommended as the first-line treatment option for the acute treatment of migraine by the American Academy of Neurology (AAN) and the American Headache Society (AHS) (23,24). Although they are the most commonly used first-line prescription treatment for a migraine attack, observational studies have consistently demonstrated low adherence and persistence to triptans in the real world (25,26). Among those who discontinue triptans, the most commonly cited reasons are lack of efficacy and adverse events (26,27). The latest AHS Consensus Statement recommends trial of at least two oral triptans prior to switching to another medication class for acute treatment of migraine attacks (24). However, more than half of new triptan users do not refill their first triptan prescription; most switch to non-triptan migraine medications instead of refilling their triptan; and approximately one in four discontinue acute treatment entirely. Among new triptan users that switch medications, the overwhelming majority do not switch to another triptan, instead receiving barbiturates, non-steroidal anti-inflammatory drugs (NSAIDs), and opioids (25). These findings suggest that some new triptan users are receiving suboptimal treatment benefits with triptans for the acute treatment of migraine.
While the effects of migraine on direct medical costs have been studied, little is known about the impact of suboptimal response or poor tolerability of acute treatments on costs. Although insurance claims offer objective data well suited to studying care and costs patterns, research on the effect of acute treatment patterns on direct medical costs has been limited by the lack of information on patient-reported outcomes, including assessments of triptan effectiveness or tolerability. In this study, we utilize prescription fill patterns of triptans and other acute migraine medications among those newly initiating a triptan to provide a measure of treatment adequacy. We define patients as potential triptan insufficient responders (TIRs) based on patterns of triptan and non-triptan acute medication prescription fills. The objectives of this study were to determine the proportion of new triptan users who are categorized as potential TIRs based on prescription fill patterns and estimate their incremental costs and healthcare resource use compared to those who are potentially adequate responders to triptans.
Methods
Study design
This study was a retrospective cohort analysis conducted using commercial health plan data spanning between 2012 and 2015 from the Optum Clinformatics™ Data Mart (CDM). The CDM is a database comprised of administrative health claims data for a large national US insurer. Data from the CDM spans across all 50 states and contains a geographically diverse population. Claims were submitted for payment by providers and pharmacies and were verified, adjudicated, adjusted, and de-identified prior to inclusion in the CDM.
Study population
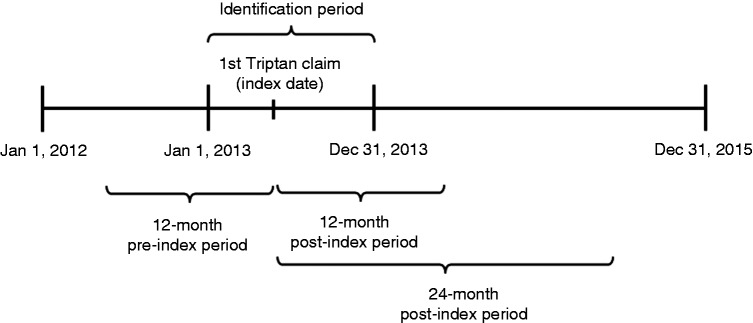
The analysis included commercially-insured adults aged 18 or older with at least one prescription claim for a triptan (the first triptan claim is designated the index triptan) with the first claim date (designated the index triptan date) occurring within the identification window of 1 January 2013 to 31 December 2013 (Figure 1). The triptans included almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan, sumatriptan combinations, and zolmitriptan. All patients were required to have continuous enrollment in the 12-month pre-index and 24-month post-index periods. Patients were also required to have at least one migraine diagnosis (ICD-9-CM code 346.XX) and no prior triptan claims during the 12-month pre-index to qualify as new triptan users. Patients were excluded if they had claims for multiple triptan prescriptions on the index date.
Figure 1.
Study design.
Next, the use of triptans and other acute treatments for migraine was identified in the 24-month post-index period. The inclusion of other acute treatments for migraine was based on a modified list of medications from an evidence review from the American Academy of Neurology (28,29). This list included oral formulations that were grouped into medication classes of acetaminophen, other guideline-listed triptans, butalbital combinations, ergots, NSAIDs, and opioids including opioid combinations (see Supplemental Table 1). Finally, an additional consideration was that opioids, NSAIDs, and butalbital combinations may be used for other indications and hence, a medical claim with a migraine diagnosis in the 15-day window including the prescription fill date and 14 days prior to that date was applied as an additional criteria for these agents to be classified as a migraine-related prescription. The 15-day requirement was similar to the definition used in prior research to identify non-migraine specific medications as being used for migraine (25).
Based on the triptan and other acute treatment fill patterns of new triptan users over the 24-month post-index period, different groups were identified for exclusion and inclusion from the sample. The group of patients who did not refill their index triptan or any other acute treatment for migraine over the 24-month post-index period were excluded. This group likely represented patients who were misdiagnosed with migraine or had low frequency or severity migraine attacks not requiring prescription treatments. Hence, at least one refill for a triptan or non-triptan acute treatment of migraine in the 24-month post-index period was required to select for patients with ongoing migraine attacks requiring acute treatment with prescription medications.
From the remainder of the sample, two groups were identified (Table 1). The first group was comprised of triptan-only continuers, which included patients with at least one refill of the index triptan or a new fill for a non-index triptan without any new fills for a non-triptan medication within the 24-month post-index period. This group of patients was labeled as potentially having adequate response to triptans. The second group attempted to capture potential TIR patients (Table 1). It included two types of patients: Triptan discontinuers using other non-triptan medications and triptan continuers using other acute medications. Triptan discontinuers using other non-triptan medications included patients without any refills of the index triptan or any new fills for a non-index triptan during the 24-month post-index period and at least one fill for ergots, butalbital combinations, NSAIDs, or opioids. These patients likely represented those with triptan tolerability issues or lack of response who still needed other non-triptan medications. Triptan continuers using other acute medications included patients with at least one refill of the index triptan or a new fill for a non-index triptan during the 24-month post-index period and at least one fill for a non-triptan acute treatment for migraine. These patients likely represented those with suboptimal response to triptans, needing additional non-triptan medications for treating their migraine attacks.
Table 1.
Study cohort definitions.
| Cohort names | Definition |
|---|---|
| Triptan-only continuers | Patients with ≥1 refill for their index triptan within 24 months without non-index triptan or other non-triptan migraine-related medications used over the same 24 months |
| Triptan discontinuers using other acute medications | Patients with no refills for index triptan or no fill of non-index triptan within 24 months plus use of other non-triptan medications for migraine over the same 24 months |
| Triptan continuers using other acute medications | Patients with ≥1 refill for index triptan or fill for non-index triptan within 24 months plus use of other non-triptan medications for migraine over the same 24 months |
| Potential triptan insufficient responders (TIR) | Combined triptan continuers and triptan discontinuers using other acute medications |
Outcomes
Outcomes of this study were healthcare resource use and costs over the first year (months 1–12) and second year (months 13–24) post-index, stratified by triptan-only continuers and potential TIR patients. Healthcare resource use measures included the percentage of patients and average number of inpatient visits and emergency department (ED) visits, and the average number of outpatient visits with a physician or neurologist. Healthcare costs (medical and prescription costs) were based on amounts paid on adjudicated claims including plan payments and patient out-of-pocket costs in the form of copayment, deductible, and coinsurance. Both resource use and costs were classified as either all-cause or migraine-related costs. Migraine and/or headache-related classification were based on the presence of a migraine or headache diagnosis with the medical claim. Migraine-related costs included prescription costs for oral acute medications (triptans, NSAIDs, ergots, butalbital combinations, and opioids) and oral preventive medications (antihypertensives, anticonvulsants, and antidepressants). A comprehensive list of acute medications can be found in Supplemental Table 1.
Statistical analysis
Sample demographic and clinical characteristics of potential TIR patients and triptan-only continuers (i.e. potentially adequate triptan responders) were assessed using descriptive statistics. Mean unadjusted healthcare resource use and cost outcomes were estimated in the two study groups. Multivariate regression analyses were conducted to estimate the differences in healthcare resource use and cost outcomes between both groups. Specifically, multivariate logistic regressions were estimated for the binary outcomes of hospitalization and ED visits. Multivariate generalized linear models (GLM) with links selected by use of the Pearson correlation test, the Pregibon link test, and the modified Hosmer and Lemeshow test and families selected by the modified Park test were estimated for the remaining healthcare resource use and cost outcomes (30) (see Supplemental Table 2). The adjusted estimates for the healthcare resource use and cost outcomes were calculated using the method of recycled predictions (30). Demographic characteristics included in the models were age, gender, geographic region, and type of insurance plan. Clinical covariates included Elixhauser comorbidities; relevant migraine-related comorbidities of anxiety and mood disorders, allergic rhinitis, pain (chronic pain, central pain, psychogenic pain, other pain, neck pain, back pain, fibromyalgia), and sleep disturbances; and cardiovascular comorbidities wherein triptans have warnings or contraindications (hypertension, hyperlipidemia, obesity, and ischemic disease including ischemic heart disease, cerebrovascular disease, and peripheral vascular disease). The regression models also included indicator variables identifying the specific index triptan agent. Additional covariates were included to serve as proxies of migraine severity and health status. These consisted of the number of distinct classes of acute medications used (i.e. butalbital combinations, ergots, NSAIDs, and opioids including opioid combinations), presence of chronic migraine diagnosis, any pre-index migraine related inpatient visits or ED visit, and total all-cause costs (categories based on quartiles) in the pre-index period.
Results
Patient characteristics
A total of 10,509 patients were identified as new triptan users (see Supplemental Figure 1). Of new triptan users, 3036 (29%) did not refill index triptan or fill any other non-index triptan or other acute treatments for migraine and were excluded from the analysis. Another 4371 (41%) were identified as triptan-only continuers and 3102 (30%) were classified as potential TIR patients.
By definition, the triptan-only continuer group was on triptan monotherapy and all patients in the potential TIR groups received a non-triptan acute treatment of migraine in the 24-month post-index period. The most common non-triptan acute medications filled in the post-index period by potential TIR patients were opioids and opioid combination prescriptions (53% and 68% in 12- and 24-month post-index periods, respectively). Other less commonly filled non-triptan acute medications among the potential TIR patients in the post-index period included NSAIDs (32%) and butalbital combination (27%) prescriptions. The use of ergots was very low (2%) and no patients were identified with prescription fills for acetaminophen in the 24-month post-index period.
Sample characteristics of the two study groups, namely the triptan-only continuer group and the potential TIR group are presented in Table 2. In both groups, most patients were female (>80%), between the ages of 35 and 64 (>60%), with a point of service plan (>75%). The most commonly reported index triptan by frequency was sumatriptan, rizatriptan, and eletriptan. Triptan-only continuers had fewer comorbidities, as measured by Elixhauser comorbidity count; fewer migraine-related comorbidities such as pain, mood disorders, and sleep disturbances; and fewer cardiovascular disease-related comorbidities, such as hypertension, than potential TIR patients. Compared to triptan-only continuers, potential TIR patients had higher use of non-triptan acute medication classes in the pre-index period. Nearly twice as many potential TIR patients had a migraine-related inpatient or ED visit (20.2% vs. 11.2%) and a higher proportion of potential TIR patients fell in the upper quartile of annual cost in the pre-index period relative to the triptan-only continuers. Supplemental Table 3 further presents the characteristics of the two types of patients comprising the potential TIR group, namely the triptan discontinuers using other acute medications and triptan continuers using other acute medications, and shows that these two subgroups were generally similar.
Table 2.
Patient demographic and clinical characteristics.
| Characteristic, n (%) | Triptan-only continuers n = 4371 |
Potential TIR patients n = 3102 |
|---|---|---|
| Age (years) | ||
| 18–24 | 494 (11.3) | 314 (10.1) |
| 25–34 | 865 (19.8) | 658 (21.2) |
| 35–44 | 1391 (31.8) | 996 (32.1) |
| 45+ | 1621 (37.1) | 1134 (36.6) |
| Gender | ||
| Female | 3583 (82.0) | 2592 (83.6) |
| Male | 788 (18.0) | 510 (16.4) |
| Region | ||
| Northeast | 333 (7.6) | 220 (7.1) |
| Midwest | 1332 (30.5) | 835 (26.9) |
| South | 1788 (40.9) | 1406 (45.3) |
| West | 918 (21.0) | 641 (20.7) |
| Plan type | ||
| Point of service | 3384 (77.4) | 2439 (78.6) |
| Health maintenance organization | 405 (9.3) | 280 (9.0) |
| Other* | 582 (13.3) | 383 (12.3) |
| Elixhauser comorbidity count | ||
| 0 | 1953 (44.7) | 1022 (32.9) |
| 1 | 1265 (28.9) | 894 (28.8) |
| 2–3 | 913 (20.9) | 815 (26.3) |
| 4+ | 240 (5.5) | 371 (12.0) |
| Migraine-related comorbidities | ||
| Pain | 970 (22.2) | 1125 (36.3) |
| Mood disorders | 864 (19.8) | 906 (29.2) |
| Rhinitis | 753 (17.2) | 611 (19.7) |
| Sleep disturbances | 404 (9.2) | 517 (16.7) |
| Cardiovascular disease-related comorbidities | ||
| Hyperlipidemia | 875 (20.0) | 729 (23.5) |
| Hypertension | 733 (16.8) | 708 (22.8) |
| Obesity | 327 (7.5) | 334 (10.8) |
| Ischemic disease (coronary heart disease, peripheral | 181 (4.1) | 235 (7.6) |
| vascular disease, and/or cerebrovascular disease) | ||
| Index triptan agent | ||
| Sumatriptan | 2702 (61.8) | 1972 (63.6) |
| Rizatriptan | 814 (18.6) | 557 (18.0) |
| Eletriptan | 437 (10.0) | 293 (9.4) |
| Zolmitriptan | 199 (4.6) | 115 (3.7) |
| Naratriptan | 88 (2.0) | 71 (2.3) |
| Frovatriptan | 73 (1.7) | 58 (1.9) |
| Almotriptan | 36 (0.8) | 18 (0.6) |
| Sumatriptan combination | 22 (0.5) | 18 (0.6) |
| Presence of chronic migraine diagnosis | 228 (5.2) | 286 (9.2) |
| Pre-index count of distinct medication classes for acute treatment of migraine** | ||
| 0 | 3359 (76.8) | 1711 (55.2) |
| 1 | 888 (20.3) | 1115 (35.9) |
| 2+ | 124 (2.8) | 276 (8.9) |
| Pre-index migraine-related inpatient or emergency department visit | 489 (11.2) | 626 (20.2) |
| Pre-index all-cause total costs | ||
| Quartile 1 (<$1287): | 1198 (27.4) | 520 (16.8) |
| Quartile 2 ($1287–$3817): | 1171 (26.8) | 629 (20.3) |
| Quartile 3 ($3819–$10,860): | 1071 (24.5) | 829 (26.7) |
| Quartile 4 ($10,865–$645,097) | 931 (21.3) | 1124 (36.2) |
Note: All data are n (%) unless otherwise indicated.
EPO: exclusive provider organization; NSAID: nonsteroidal anti-inflammatory drug; PPO: preferred provider organization; TIR: triptan insufficient responder.
*Plan type – ‘Other’ includes preferred provider organization, exclusive provider organization.
**Distinct medication classes for acute treatment of migraines includes butalbital combinations, ergots, NSAIDs, and opioids including opioid combinations.
Healthcare resource utilization
In the unadjusted analysis, potential TIR patients had significantly higher rates of migraine-related inpatient and ED visits as well as mean number of physician visits compared to triptan-only continuers (see Supplemental Table 4). In the adjusted analysis, a significantly higher proportion of potential TIR patients versus triptan-only continuers had at least one migraine-related inpatient visit in months 1–12 (+3.9%; p < 0.001) and months 13–24 (+3.2%; p < 0.001). Similarly, a significantly higher proportion of potential TIR patients had at least one migraine-related ED visit in months 1–12 (+10.8%; p < 0.001) and months 13–24 (+8.6%; p < 0.001) compared to triptan-only continuers. Compared to the triptan-only continuers, potential TIR patients had 1.2 and 0.8 (p < 0.001) additional migraine-related physician visits during months 1–12 and months 13–24 post-index, respectively.
Healthcare costs
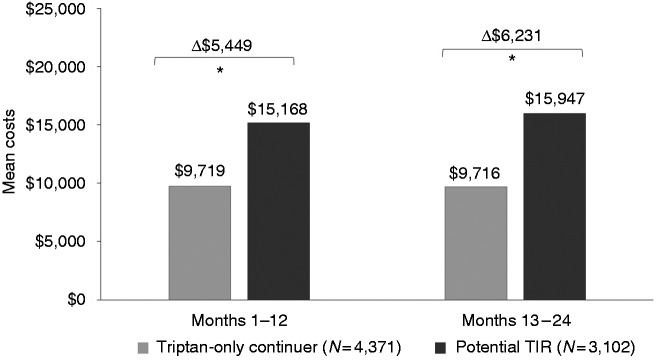
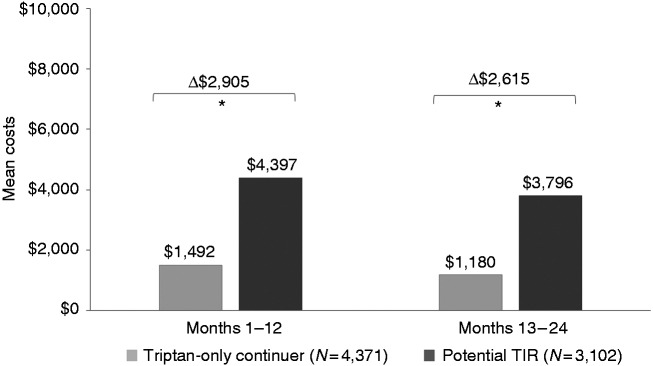
Unadjusted all-cause total costs, migraine-related total costs and migraine-specific medical costs were higher in the potential TIR group versus the triptan-only group (see Supplemental Table 5). After adjustment, mean annual all-cause costs remained significantly higher for potential TIR patients, with an incremental cost of $5449 in months 1–12 and $6231 in months 13–24 post-index (both p < 0.001) (Figure 2). For potential TIR patients, adjusted mean annual migraine-related costs were $2905 higher than triptan-only continuers in months 1–12 and $2615 higher in months 13–24 post-index (both p < 0.001) (Figure 3). Most migraine-related costs were medical, rather than pharmacy costs. Adjusted mean annual migraine-related medical costs for potential TIR patients were $2607 higher than triptan-only continuers in months 1–12 and $2422 higher in months 13–24 post-index (both p < 0.001).
Figure 2.
Adjusted all-cause total costs.
*p < 0.001.
TIR: triptan insufficient responder.
Figure 3.
Adjusted migraine-related total costs.
*p < 0.001.
TIR: triptan insufficient responder.
Discussion
To our knowledge, this claims-based study is the first attempt to identify potential TIR patients and quantify direct healthcare costs associated with triptan insufficient response in a geographically diverse population of a large national US commercial insurer. Given that clinical response and tolerability issues are not captured in insurance claims data, fill patterns for non-triptan acute treatments were used as a proxy to identify potential TIR patients. Our study has several important findings with potential implications for clinicians and payers. First, almost one in three (30%) new triptan users were potential TIR patients. Second, two-thirds (68%) of these potential TIR patients received a migraine-related opioid prescription over 24 months since initiating a new triptan and over half (53%) did so over 12 months. Third, potential TIR patients had significantly higher migraine-related healthcare resource use and costs than those who were potentially adequate responders, even after adjusting for sociodemographic and clinical characteristics.
Despite the use of a claims-based proxy for TIR status, our finding that almost one-third of the new triptan users were potential TIR patients is within the range of estimates reported from data sources wherein triptan response and tolerability issues could be measured, including results from the Adelphi Disease-Specific program (38%), the ACHIEVE I and II clinical trial program (23–24%), and reviews of triptan clinical studies (30–40%) (31–36). Furthermore, the high rate of migraine-related opioid prescription fills identified in these potential TIR patients during follow-up further lends support to our claims-based proxy of TIR status. It highlights that these patients had an unmet need requiring them to resort to potent medications such as opioids while discontinuing triptans or in addition to continuing their triptan prescription. Our data came from a population-based analysis of patients newly initiating triptans from a large US insurer, making our results more generalizable, and raise the need for both clinicians and payers to identify TIR patients in clinical practice and address their unmet needs.
Our finding on the high rates of opioid use among the potential TIR patients is surprising in light of the broad recommendations to avoid opioids as acute treatments for migraine (24,37,38). This may reflect the limited availability of well-tolerated and effective treatments for migraine attacks not adequately managed by triptans. Post-hoc analyses confirmed that the vast majority (70%) of the TIR patients using opioids during 12-month post-index follow-up did not have any opioid use prior to initiation of triptans. These findings are similar to another study, which reported that among those who switched from triptans to a new medication class after initial triptan fill, approximately half switched to an opioid (25). This suggests that real-world treatment patterns deviate from clinical guidelines including the AHS Consensus Statement of trying two oral triptans prior to switching medication classes (24). While switching between triptans has not demonstrated any clinical benefit or cost savings, augmenting treatment with opioids, barbiturates, and NSAIDs also does not improve headache-related disability (20,39,40). While our study results are based on US data, where opioid use for migraine is a well-recognized problem, opioids were reported to be the second most common treatment for migraine (after triptans) in a survey of self-diagnosed individuals with migraine across 17 European countries, suggesting that opioid use is a problem that extends beyond the US (16). Although several programs have been put in place to manage opioid use in the US, the findings reported here demonstrate the need to pay special attention to the care of migraine patients among other patients with pain conditions.
Our findings on significantly higher healthcare resource use and costs associated with triptan insufficient response have important implications for payers. Total all-cause costs were almost 1.5 times higher for potential TIRs and migraine-related costs were nearly three times higher compared to potentially adequate responders. This may still underestimate the true cost of suboptimal acute treatment, as we did not capture the patient cost of over-the-counter medications or cost of medications not covered by insurance. While published literature comparing resource use and costs among people adequately versus suboptimally managed with triptans is scarce, a 2004 study from the United Kingdom investigating medication and primary care costs of switching from sumatriptan to an alternative triptan resulted in a modest cost increase at follow-up (40). Our results on higher rates of migraine-related ED and hospital visits and ensuing costs among potential TIR patients further highlight that an unmet need exists among patients who either discontinue triptans potentially due to lack of clinical response or tolerability issues or those who continue with triptans despite possible suboptimal treatment response. As new acute treatments for migraine enter the market and the treatment paradigm shifts, payers will need to continuously evaluate choices and adequacy of treatments to validate continued use and potentially lower direct medical costs as a result of better management of migraine attacks.
There were several limitations in our study. As with any administrative claims study, coding and entry errors may exist. The analysis was limited to a commercially insured population, which may not be generalizable to other insurance plans. We could only observe prescriptions filling behavior, but we could not confirm how medications were taken. We assumed those who refilled their index triptan or filled another triptan without use of other prescription-strength acute medications for migraine were taking them properly and receiving adequate treatment benefits. We also did not have access to patient-reported outcomes data; hence, we could not directly determine their clinical response to triptans. A patient who switches to a second triptan that was also ineffective but had no use of non-triptan acute medications would be considered a triptan-only continuer (i.e. not a potential TIR patient). Hence, our definition may underestimate the true prevalence of TIR. Alternatively, our definition may overestimate the true prevalence of TIR by inclusion of patients who use both non-triptan medications such as NSAIDs in addition to triptans, despite an adequate response to triptans, to manage varying types or severity of migraine attacks. Similarly, we cannot determine why people did not refill a triptan. However, non-clinical reasons such as out-of-pocket costs are unlikely to be the reason for the lack of a triptan refill since the vast majority of patients in our study were using sumatriptan as their index triptan agent, which was available as a cheap generic drug during our study period. It is possible that those who did not refill their index triptan were not correctly diagnosed or did not continue to experience migraine attacks. However, this is unlikely since the analysis was limited to those with at least two fills of an acute treatment (index triptan and subsequent fill of triptan or non-triptan medication) to avoid including those who were misdiagnosed or no longer needed any acute prescription treatment for migraine. Non-specific acute treatments for migraine can be used to treat other conditions. To ensure acute medications were used for migraine attacks, fills for NSAIDs, butalbital combinations, and opioids were required to have occurred within 15 days of an encounter with a migraine diagnosis to be considered migraine related. Finally, potential TIR patients had higher healthcare resource use and cost in both the pre-index and post-index period. In adjusted models, we controlled for comorbidities, the presence of pre-index inpatient or ED visits, pre-index cost quartiles, and the presence of chronic migraine diagnosis to adjust for severity unrelated to acute treatment optimization. After adjustments, migraine-related healthcare resource use and cost remained higher for potential TIR patients. However, confounding factors such as higher migraine severity and/or frequency may remain due to unobserved or unmeasured artifacts that contribute to differences in both groups. Other systematic differences between study groups not captured by claims data could account for reported outcomes, despite adjusting for differences in demographic and clinical characteristics.
Conclusion
In people with migraine who receive a new prescription for a triptan, nearly a third (30%) are defined as potential TIR patients. Potential TIR patients have higher migraine-related healthcare resource utilization and costs than patients who are potentially adequate responders to triptans for acute treatment of migraine. The findings of this study suggest that more effective or better tolerated acute treatments are needed for the management of migraine attacks for some patients.
Article highlights
Results from this claims analysis suggest that 30% of patients who fill new triptan prescriptions may have had intolerability issues or an insufficient response to the triptan based on not refilling the triptan and/or based on the use of other acute medications.
Potential triptan insufficient responders incur greater migraine-related healthcare resource use and costs over the first year and second year of follow-up than patients who are potentially adequate responders.
The annual incremental migraine-related total costs of potential triptan insufficient responders are $2905 higher in the first 12 months.
Supplemental Material
Supplemental material, CEP915167 Supplemental Figure 1 for Comparison of healthcare resource utilization and costs among patients with migraine with potentially adequate and insufficient triptan response by Steven C Marcus, Anand R Shewale, Stephen D Silberstein, Richard B Lipton, William B Young, Hema N Viswanathan and Jalpa A Doshi in Cephalalgia
Supplemental material, CEP915167 Supplemental Tables for Comparison of healthcare resource utilization and costs among patients with migraine with potentially adequate and insufficient triptan response by Steven C Marcus, Anand R Shewale, Stephen D Silberstein, Richard B Lipton, William B Young, Hema N Viswanathan and Jalpa A Doshi in Cephalalgia
Acknowledgements
Editorial support for development of this manuscript was provided by Lisa M Bloudek and Victor B Nguyen, at Curta, Inc, (Seattle, WA), and funded by Allergan, plc (Dublin, Ireland). All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SCM is a consultant for Allergan; ASS is a full-time employee and shareholder at Allergan; SDS is a consultant and/or advisory panel member for and has received honoraria from Alder Biopharmaceuticals, Allergan, Amgen, Avanir, ElectroCore Medical, eNeura, Labrys Biologics, Medscape, Medtronic, Neuralieve, NINDS, Pfizer, and Teva. His employer receives research support from Allergan, Amgen, Cumberland Pharmaceuticals, ElectroCore Medical, Eli Lilly, Labrys Biologics, Merz, and Troy Healthcare.
RBL serves on the editorial boards of Neurology and Cephalalgia and as senior advisor to Headache. He has received research support from the NIH. He also receives support from the Migraine Research Foundation and the National Headache Foundation. He has reviewed for the NIA and NINDS and has served as consultant or advisory board member for or has received honoraria from Alder, Allergan, Amgen, Autonomic Technologies, Avanir, Biohaven, Biovision, Boston Scientific, Dr. Reddy’s, ElectroCore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Novartis, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, and Vedanta. He receives royalties from Wolff’s Headache (8th Edition, Oxford University Press), Informa, and Wiley. He holds stock options in eNeura Therapeutics and Biohaven.
WBY is a consultant or acts on an advisory board for Alder, Allergan, Avanir, and Supernus, is a speaker for Amgen, and has conducted research for Amgen, Autonomic Technologies, CoLucid, Cumberland, Dr. Reddy’s Laboratories, Eli Lilly, Novartis, PCORI, Scion, Teva, and Zosano; HNV is a full-time employee and shareholder at Allergan; JAD reports serving as a consultant and/or advisory board member for Allergan, Boehringer Ingelheim, Catabasis, Ironwood Pharmaceuticals, Janssen, Kite Pharma, MeiraGTx, Merck, Otsuka, Regeneron, Sage Therapeutics, Sanofi, Sarepta, The Medicines Company, and Vertex, and has received research funding from Abbvie, Biogen, Humana, Janssen, Novartis, Pfizer, PhRMA, Regeneron, Sanofi, and Valeant.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Allergan, plc. Dublin, Ireland.
ORCID iD
Stephen D Silberstein https://orcid.org/0000-0001-9467-5567
References
- 1.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Bigal ME, Kolodner K, et al. The family impact of migraine: Population-based studies in the USA and UK. Cephalalgia 2003; 23: 429–440. [DOI] [PubMed] [Google Scholar]
- 3.Buse DC, Scher AI, Dodick DW, et al. Impact of migraine on the family: Perspectives of people with migraine and their spouse/domestic partner in the CaMEO study. Mayo Clin Proc 2016; 91: 596–611. [DOI] [PubMed] [Google Scholar]
- 4.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68: 343–349. [DOI] [PubMed] [Google Scholar]
- 5.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2012. Lancet 2013; 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiner TJ, Stovner LJ, Vos T, et al. Migraine is first cause of disability in under 50s: Will health politicians now take notice? J Headache Pain 2018; 19: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017; 16: 76–87. [DOI] [PubMed] [Google Scholar]
- 8.Bloudek LM, Stokes M, Buse DC, et al. Cost of healthcare for patients with migraine in five European countries: Results from the International Burden of Migraine Study (IBMS). J Headache Pain 2012; 13: 361–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonafede M, Sapra S, Shah N, et al. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache 2018; 58: 700–714. [DOI] [PubMed] [Google Scholar]
- 10.Elston Lafata J, Moon C, Leotta C, et al. The medical care utilization and costs associated with migraine headache. J Gen Intern Med 2004; 19: 1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilligan AM, Foster SA, Sainski-Nguyen A, et al. Direct and indirect costs among United States insured employees with migraine. J Occup Environ Med 2018; 60: 1120–1127. [DOI] [PubMed] [Google Scholar]
- 12.Korolainen MA, Kurki S, Lassenius MI, et al. Burden of migraine in Finland: Health care resource use, sick-leaves and comorbidities in occupational health care. J Headache Pain 2019; 20: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: The Eurolight project. Eur J Neurol 2012; 19: 703–711. [DOI] [PubMed] [Google Scholar]
- 14.Osumili B, McCrone P, Cousins S, et al. The economic cost of patients with migraine headache referred to specialist clinics. Headache 2018; 58: 287–294. [DOI] [PubMed] [Google Scholar]
- 15.Raval AD, Shah A. National trends in direct health care expenditures among U.S. adults with migraine: 2004 to 2013. J Pain 2017; 18: 96–107. [DOI] [PubMed] [Google Scholar]
- 16.Vo P, Paris N, Bilitou A, et al. Burden of migraine in Europe using self-reported digital diary data from the Migraine Buddy© application. Neurol Ther 2018; 7: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipton RB, Fanning KM, Serrano D, et al. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology 2015; 84: 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May A, Schulte LH. Chronic migraine: Risk factors, mechanisms and treatment. Nat Rev Neurol 2016; 12: 455–464. [DOI] [PubMed] [Google Scholar]
- 19.Schwedt TJ, Alam A, Reed ML, et al. Factors associated with acute medication overuse in people with migraine: Results from the 2017 migraine in America symptoms and treatment (MAST) study. J Headache Pain 2018; 19: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serrano D, Buse DC, Kori SH, et al. Effects of switching acute treatment on disability in migraine patients using triptans. Headache 2013; 53: 1415–1429. [DOI] [PubMed] [Google Scholar]
- 21.Lipton RB, Buse DC, Serrano D, et al. Examination of unmet treatment needs among persons with episodic migraine: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2013; 53: 1300–1311. [DOI] [PubMed] [Google Scholar]
- 22.Lipton RB, Munjal S, Buse DC, et al. Predicting inadequate response to acute migraine medication: Results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2016; 56: 1635–1648. [DOI] [PubMed] [Google Scholar]
- 23.Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012; 78: 1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache 2019; 59: 1–18. [DOI] [PubMed] [Google Scholar]
- 25.Katić BJ, Rajagopalan S, Ho TW, et al. Triptan persistency among newly initiated users in a pharmacy claims database. Cephalalgia 2011; 31: 488–500. [DOI] [PubMed] [Google Scholar]
- 26.Alam A, Munjal S, Reed M, et al. Triptan use and discontinuation in a representative sample of persons with migraine: Results from Migraine in America Symptoms and Treatment (MAST) Study. Neurology 2019; 92(15 suppl): P4.10–019. [Google Scholar]
- 27.Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache 2014; 54: 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55: 3–20. [DOI] [PubMed] [Google Scholar]
- 29.Silberstein SD. Practice parameter: Evidence-based guidelines for migraine headache (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55: 754–762. [DOI] [PubMed] [Google Scholar]
- 30.Glick H, Doshi JA, Sonnad S, et al. Economic evaluations in clinical trials, 2nd edition Oxford, UK: Oxford University Press, 2007. [Google Scholar]
- 31.Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med 2019; 381: 2230–2241. [DOI] [PubMed] [Google Scholar]
- 32.Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: The ACHIEVE II randomized clinical trial. JAMA 2019; 322: 1887–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumenfeld AM, Goadsby PJ, Dodick DW, et al. Ubrogepant is effective for the acute treatment of migraine in patients for whom triptans are ineffective. Presented at: the 61st Annual Meeting of the American Headache Society, Philadelphia, PA, USA, 11–14 July 2019.
- 34.Silberstein SD, Shewale AR, Baradaran S, et al. Opioid use, rebound headache, and resource utilization among migraine patients with insufficient response to triptans based on real-world data (S59. 007). Poster presented at: American Academy of Neurology 2019 Annual Meeting, Philadelphia, PA, USA, 4–11 May, 2019.
- 35.Viana M, Genazzani AA, Terrazzino S, et al. Triptan nonresponders: Do they exist and who are they? Cephalalgia 2013; 33: 891–896. [DOI] [PubMed] [Google Scholar]
- 36.Rapoport AM, Tepper SJ, Bigal ME, et al. The triptan formulations. CNS Drugs 2003; 17: 431–447. [DOI] [PubMed] [Google Scholar]
- 37.American Academy of Neurology. Five things physicians and patients should question, http://www.choosingwisely.org/societies/american-academy-of-neurology (21 February 2013, accessed 22 October 2019).
- 38.Institute for Clinical and Economic Review. Controversies in migraine management: A technology assessment, final report, https://icer-review.org/wp-content/uploads/2016/01/CTAF_Migraine_Final_Report_081914-2.pdf (19 August 2014, accessed September 10, 2019).
- 39.Buse DC, Serrano D, Reed ML, et al. Adding additional acute medications to a triptan regimen for migraine and observed changes in headache-related disability: Results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2015; 55: 825–839. [DOI] [PubMed] [Google Scholar]
- 40.Savani N, Martin A, Browning D. Switching patients with migraine from sumatriptan to other triptans increases primary care costs. Int J Clin Pract 2004; 58: 758–763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CEP915167 Supplemental Figure 1 for Comparison of healthcare resource utilization and costs among patients with migraine with potentially adequate and insufficient triptan response by Steven C Marcus, Anand R Shewale, Stephen D Silberstein, Richard B Lipton, William B Young, Hema N Viswanathan and Jalpa A Doshi in Cephalalgia
Supplemental material, CEP915167 Supplemental Tables for Comparison of healthcare resource utilization and costs among patients with migraine with potentially adequate and insufficient triptan response by Steven C Marcus, Anand R Shewale, Stephen D Silberstein, Richard B Lipton, William B Young, Hema N Viswanathan and Jalpa A Doshi in Cephalalgia