Abstract
Purpose: Exercise could lower the risk of cancer recurrence and improve mortality, exercise capacity, physical and cardiovascular function, strength, and quality of life in patients with cancer. This systematic review and meta-analysis of randomized controlled trials (RCTs) aimed to determine the effects of exercise on mortality and recurrence in patients with cancer. Methods: We searched for articles published before May 2019 in MEDLINE, CINAHL, the Cochrane Library, Scopus, ProQuest, and PEDro. We included RCTs of exercise interventions, such as resistance exercise and aerobic exercise, in patients with cancer that evaluated the risk of mortality and recurrence. The standardized mean difference with 95% confidence intervals (CIs) was calculated for quantitative indices. The random-effect model was used as the pooling method. Results: Of 2868 retrieved articles, 8 RCTs were included in the meta-analysis, with a mean PEDro score of 4.50 (SD = 1.25). Exercise significantly reduced the risk of mortality in patients with cancer and in cancer survivors (risk ratio [RR] = 0.76, 95% CI = 0.40-0.93, I2 = 0%, P = .009). Exercise significantly reduced the risk of recurrence in cancer survivors (RR = 0.52, 95% CI = 0.29-0.92, I2 = 25%, P = .030). Conclusion: This study found that exercise has a favorable effect on mortality and recurrence in patients with cancer. However, the effect could not be fully determined due to data insufficiency.
Keywords: exercise, rehabilitation, cancer, mortality, recurrence, survival
Introduction
According to Global Cancer Statistics (2018), the global cancer burden has risen to 18.1 million new cases and 9.6 million cancer deaths.1 The most common symptoms that patients with cancer exhibit are pain and fatigue as a result of treatment.2 Additionally, in the general population, depression and anxiety affect up to 20% and 10% of patients with cancer, respectively, in contrast to 5% and 7%, respectively, in the past year.3 Muscle dysfunction is also a common phenomenon in cancer, where patients across a wide range of diagnoses are subject to impaired muscle function.4 Because patients with cancer experience various symptoms, their quality of life (QOL) is of major concern.5 Muscle dysfunction and sarcopenia have been associated with poor performance status, increased mortality risk, and greater prevalence of treatment side effects.6 However, as indicated by systematic reviews and meta-analyses, physical exercise has beneficial effects on QOL,7 muscle strength, muscle function, and aerobic fitness,8 and cancer-related fatigue in patients with cancer.9-11 Adults with cancer are advised that exercise can provide QOL and fitness benefits during or after treatment. Systematic reviews examining patients with all cancer types have shown the positive influence of physical exercise on QOL during the course of active treatment.12
Physical exercise can also be an effective method of improving the patient’s exercise capacity, physical and cardiovascular function, and strength. It can also lower the risk of cancer recurrence and improve the odds of mortality.13 Another review also showed that patients with cancer who performed physical exercise following their diagnosis were observed to have a lower relative risk of cancer mortality and recurrence and experienced fewer or less severe adverse effects. Therefore, it is safe to speculate that physical exercise is an important adjunct therapy for cancer.14 This systematic review and meta-analysis of randomized controlled trials (RCTs) aimed to determine how physical exercise, such as resistance exercise and aerobic exercise, affects mortality and recurrence in patients with cancer at all survival time.
Methods
Protocol
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.15 We used a prespecified protocol, registered with PROSPERO (CRD:42019140268).
Search Strategy
Literature searches were conducted in MEDLINE, CINAHL, the Cochrane Library, Scopus, ProQuest, and PEDro for articles published before May 2019. The search strategy was conducted on the aforementioned databases based on the following MeSH terms: “cancer,” “tumor,” “randomized controlled trial,” “exercise,” “rehabilitation,” and “physical therapy.” The phrase “disorder for cancer” was also used for the search (eg, lymphoma, hematopoietic malignancy, carcinosarcoma). In addition, the words “mortality,” “recurrence,” and “prevalence” were added to the search terms.
Selection Criteria
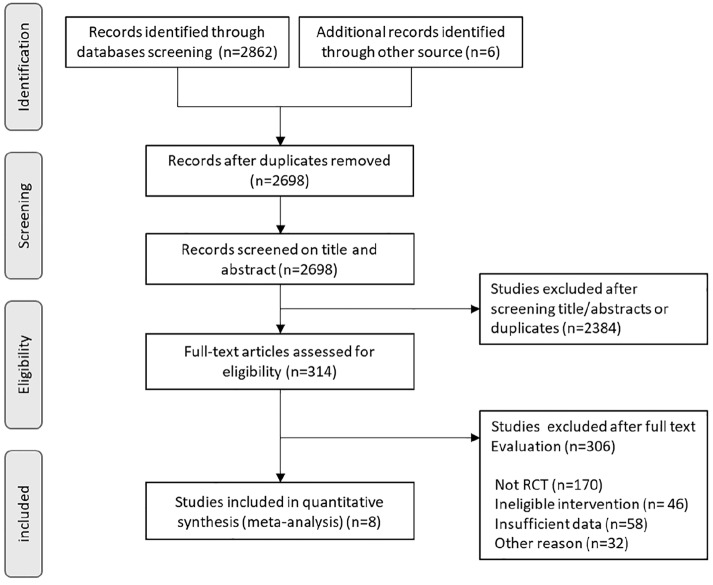
The inclusion criteria consisted of randomized controlled clinical trials that included patients with cancer and cancer survivors in any setting; the intervention group performed physical exercise and its effects on mortality were evaluated. Systematic reviews, editorials, cross-sectional studies, case reports, and case series were excluded. Exercise guidance was also excluded from the intervention. A control group, which did not receive any (major) exercise intervention, was used for comparisons. The different stages of study selection are shown in Figure 1. The titles and abstracts of all retrieved articles were screened by 5 independent reviewers to ensure their eligibility. Full-text articles were retrieved for review when there was an indication that they met the inclusion criteria or when there was insufficient information in the abstract and title to make a decision. To perform meta-analysis, data details were examined. Final inclusion of eligible RCTs was determined in consensus meetings in which all authors participated.
Figure 1.
Study flow diagram of the selection process.
Data Extraction
Two reviewers were responsible for data extraction. When the data in the full text of an article were deemed insufficient, the authors of the article were contacted by email for further information. The following data were extracted from each included study: first author’s last name, publication year, cancer type, sample size, type of exercise, duration and timing of exercise, and observation period. Mortality, recurrence, and prevalence data were selected for meta-analysis.
Quality Assessment
Assessment of the methodological quality of studies, including their risk of bias, was conducted using the Cochrane tool for assessing risk of bias.16 This tool includes the following 7 domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias. Two trained reviewers scored each item according to the criteria established by Higgins et al in 2011.16 Potential disagreements were resolved during consensus meetings with all authors present. The quality of studies was rated using the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) criteria.17
Data Analysis
All statistical analyses were conducted using the Review Manager software, version 5.1.18 We calculated the risk ratio (RR) for each report to investigate the effect of exercise on mortality and recurrence. The standardized mean difference with 95% confidence intervals (CIs) was calculated for quantitative indices. The random-effect model was used as the pooling method. We also assessed the statistical heterogeneity using the I2 statistic. Furthermore, we adopted the levels of I2 that were suggested by the Cochrane Handbook for Systematic Reviews of Interventions (I2 values of 0%, 25%, 50%, and 75% represented no, low, moderate, and high heterogeneity, respectively). The threshold for interpreting the I2 value can be misleading. Therefore, we determined the importance of the observed I2 value by assessing the magnitude and direction of the effect, as well as the strength of evidence for clinical heterogeneity.
Results
Study Selection
The database searches retrieved 2868 references, which were reduced to 2698 after the exclusion of duplicate articles. The remaining 2698 studies were screened by title and abstract, resulting in the exclusion of 2384 studies due to irrelevant study designs or discrepancies regarding the population or the intervention type. A full-text review was performed for 314 RCTs, and 306 RCTs were excluded. Figure 1 shows the outcome of the search and study selection process. The included studies were conducted in the following countries: 3 in Germany,19-21 2 in Australia,22,23 and 1 each in Canada,24 the United States,25 and Switzerland.26
Study Characteristics
Detailed characteristics of the 8 RCTs are shown in Table 1. Subjects in 2 of the RCTs23,24 were patients with breast cancer postsurgery. These studies showed that aerobic exercise and resistance exercise had a positive effect on the survival of cancer patients. In other RCTs, the subjects were patients who were diagnosed with lung cancer. In this study, aerobic exercise had a positive effect on the survival period.22 Patients in other RCTs26 received any intervention before surgery. This study showed that high-intensity interval training resulted in mortality reduction. One RCT was intended for patients who were candidates for allogeneic hematopoietic stem cell transplantation.21 The rest of the RCTs included patients with various cancer diagnoses,19,20 with one of the RCTs targeting patients with cancer who had heart failure.25 This study showed that aerobic exercise had an effect on the reduction of all-cause mortality.
Table 1.
Characteristics of the Included Studies.
| Author, Year | Cancer Type | Intervention | Participants (Gender, Number, Age) | Intervention | Duration and Timing of Exercise | Observation Period | Measure (Outcome) |
|---|---|---|---|---|---|---|---|
| Courneya et al,24 2014 | Breast | Ex1 = aerobic exercise Ex2 = resistance training vs Con = usual care |
% female = 100% Ex = 160 <50 years = 90 >50 years = 70 Con = 82 <50 years = 42 >50 years = 40 |
Aerobic exercise: 3 times/week, beginning at 60% of their
VO2max for 1-6 weeks and progressing to 70%
during 7-12 weeks and 80% beyond 12 weeks. Exercise duration
began at 15 minutes for 1-3 weeks and increased by 5 minutes
every 3 weeks until 45 minutes at 18
weeks. Resistance exercise (3 times/week): performing 2 sets of 8-12 repetitions of 9 different exercises at 60% to 70% of their estimated 1 repetition maximum. |
Duration of chemotherapy, beginning 1-2 weeks after starting chemotherapy and ending 3 weeks after completing chemotherapy (at least 18 weeks) | 8 years | DFS, mortality, DDFS, RFI |
| Dhillon et al,22 2017 | Lung | Ex = aerobic exercise vs Con = usual care |
% female = 45% Ex = 56 64 (38-80) years Con = 55 64 (34-76) years |
Increasing recreational physical activity (PA) by>3 MET h/week. Sessions lasted 1 hour; 45-minute PA; 15-minute behavior support. PA was predominantly aerobic, and home-based PA was encouraged. | Ambulatory treatment 8 weeks |
6 months | Mortality, PA, accelerometers (min/day) |
| Hayes et al,23 2017 | Breast | Ex = aerobic and resistance exercise vs Con = usual care |
% female = 100% Ex = 207 51.7 ± 8.8 years Con = 130 53.9 ± 8.3 years |
Aerobic-based and resistance-based: 180+ minutes, moderate-intensity exercise, to be accumulated on at least 4 days per week. Commencing at 6 weeks postsurgery. | Postsurgery Home exercise 8 months |
96 months | Mortality, DFS |
| Jones et al,25 2014 | Mix | Ex = aerobic training vs Con = usual care |
% female = 26% Ex = 47 64 ± 10 years Con = 43 66 ± 11 years Patients with heart failure |
Supervised aerobic training (treadmill or stationary cycle ergometer) sessions per week lasting 20 to 45 minutes per session at 60% to 70% of heart rate reserve. | Ambulatory treatment Home exercise 12 weeks |
12 months | All-cause mortality |
| Licker et al,26 2017 | Mix | Ex = high-intensity interval training vs Con = usual care |
% female = 40% Ex = 74 64 ± 13 years Con = 77 64 ± 10 years |
After a 5-minute warm-up period at 50% at peak work rate (peakWR); two 10-minute series of 15-second sprint intervals (at 80% to 100% peakWR) interspersed by 15-second pauses and a 4-minute rest between the 2 series; cooled down with a 5-minute active recovery period at 30% peakWR. | Presurgery Ambulatory treatment (2-3 times/week) |
30 days | Postoperative 30-day mortality |
| Rief et al,19 2014 | Mix | Ex = resistance training vs Con = control |
% female = 45% Ex = 30 61.3 ± 10.1 years Con = 30 64.1 ± 10.9 years |
Resistance training: 30 minutes | During hospitalization 2 weeks |
12 weeks | Mortality, pain score |
| Rief et al,20 2016 | Mix | Ex = resistance training vs Con = passive physical therapy |
% female = 45% Ex = 30 61.3 ± 10.1 years Con = 30 64.1 ± 10.9 years |
Resistance training: 30 minutes | During hospitalization 2 weeks |
10 months (range = 2-35 months) | Mortality, PFS, bone survival |
| Wiskemann et al,21 2015 | Allogeneic hematopoietic stem cell transplant | Ex = endurance exercises and resistance
exercises vs Con = usual care |
% female = 33% Ex = 50 48.2 ± 14.5 years Con = 53 50.0 ± 12.4 years |
A combination of endurance exercises, 3 to 5 times weekly, and resistance exercises twice weekly, with each session lasting 20 to 40 minute. | After allogeneic stem cell transplantation Before and after hospital admission After discharge: 8 weeks |
2 years | NRM, TM |
Abbreviations: Ex, exercise group; Con, control group; VO2max, maximum rate of oxygen consumption; DFS, disease-free survival; DDFS, distant DFS; RFI, recurrence-free interval; MET, metabolic equivalent of task; PFS, progression-free survival; NRM, non-relapse mortality; TM, total mortality.
Interventions in the RCTs were roughly classified into 2 categories: a short-term intervention rehabilitation program (with a duration of 2 weeks) that was performed in a hospital setting19,20 and ambulatory treatment or home exercise (with a duration of 2-8 months) for long-term outpatients.21-26 Regarding the content of the interventions, aerobic exercise and/or resistance training was conducted in all RCTs.
Study Quality
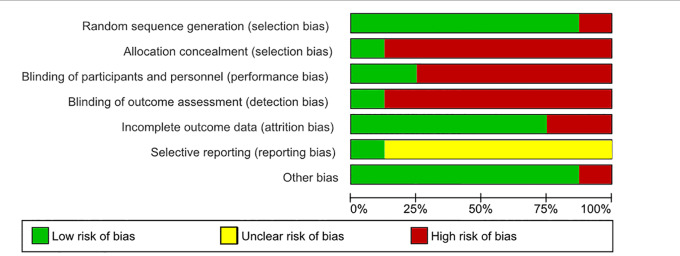
Risk of bias assessment is shown in Figures 2 and 3. Risk of bias for other bias domains varied across the included studies. Due to the nature of the interventions, it was expected that blinding of the participants and the personnel who delivered the interventions would be impossible. Consequently, the risk of performance bias in all studies was high. The quality of the studies rated by GRADE was moderate in each outcome (Table 2).
Figure 2.
Risk of bias graph based on the Cochrane Collaboration’s tool for assessing risk of bias.
Figure 3.
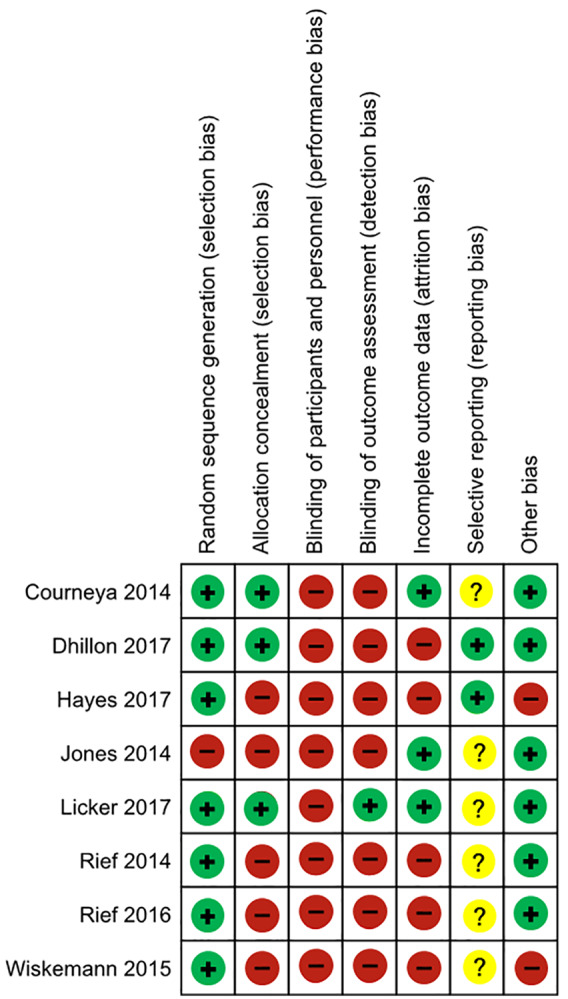
Summary of risk of bias based on the Cochrane Collaboration’s tool for assessing risk of bias.
Table 2.
GRADE Evaluation.
| Certainty Assessment |
No. of Patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Intervention | Control | Relative (95% CI) | Absolute (95% CI) | ||
| Mortality | ||||||||||||
| 8 | Randomized trials | Seriousa | Not serious | Not serious | Not serious | None | 93/656 (14.2%) | 125/579 (21.6%) | RR 0.76 (0.62-0.93) | 52 fewer per 1000 (from 82 fewer to 15 fewer) | ⨁⨁⨁◯ MODERATE |
9—critical |
| Recurrence | ||||||||||||
| 3 | Randomized trials | Seriousa | Not serious | Not serious | Not serious | None | 25/367 (6.8%) | 42/294 (14.3%) | RR 0.52 (0.29-0.92) | 69 fewer per 1000 (from 101 fewer to 11 fewer) | ⨁⨁⨁◯ MODERATE |
8—critical |
Abbreviations: GRADE, Grading of Recommendation Assessment, Development, and Evaluation; CI, confidence interval; RR, risk ratio.
All studies were judged to include a risk of bias.
Effect of Exercise on Risk of Mortality and Recurrence
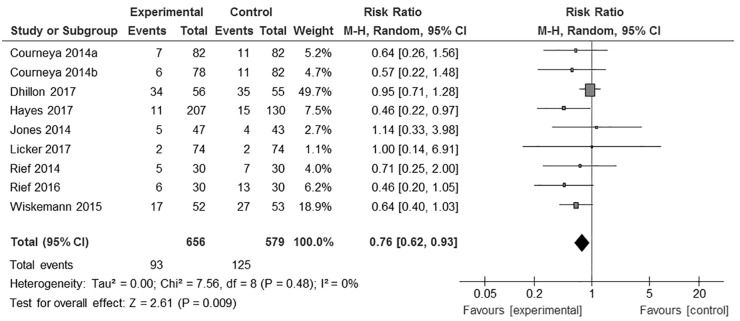
As a result, 9 exercise groups from 8 RCTs were included in a random-effects meta-analysis. This analysis comprised 656 patients in the exercise groups and 579 patients in the control groups. The efficacy of exercise in terms of risk of mortality and recurrence in patients with cancer was then estimated using a forest plot.
The meta-analysis of 9 exercise groups from 8 RCTs showed that physical exercise significantly reduced the risk of mortality in patients with cancer and in cancer survivors (RR = 0.76, 95% CI = 0.40-0.93, I2 = 0%, P = .009, n = 1235; Figure 4).
Figure 4.
Risk ratio for the effect of exercise on mortality in cancer patients and survivors.
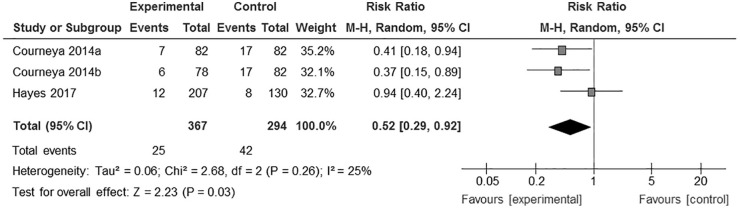
Cancer recurrence data were available in only 2 RCTs,23,24 one of which included 2 exercise groups. Thus, meta-analysis of 3 groups from 2 RCTs was performed, showing the effect of exercise on significant reduction of recurrence risk in cancer survivors (RR = 0.52, 95% CI = 0.29-0.92, I2 = 25%, P = .030, n = 661; Figure 5).
Figure 5.
Risk ratio for the effect of exercise on recurrence in cancer survivors.
Discussion
This systematic review and meta-analysis attempted to examine the evidence regarding the benefits of physical exercise on mortality and recurrence in patients with cancer. This review included breast, lung, hematological malignancy, and various cancer diagnoses. To the best of our knowledge, this is the first meta-analysis to determine the effects of exercise on mortality and recurrence in patients with cancer. Our quantitative analysis indicated that there is evidence to support the notion that physical exercise can improve the rates of mortality and recurrence in patients with cancer.
This meta-analysis also revealed that exercise interventions positively affect mortality rates in patients with cancer. The existing literature has shown the benefits of physical exercise training in patients with heart failure, including the reduction of cardiovascular mortality rates.27 Physical exercise has also been shown to improve exercise tolerance and QOL in patients with chronic obstructive pulmonary disease (COPD), as well as reduce hospital admissions and mortality rates.28 Another study showed that physical exercise interventions improve exercise capacity in patients with chronic kidney disease (CKD) and may reduce the risk of mortality.29 Patients with cancer may receive the same reduced mortality benefits from physical exercise as do patients with heart failure, COPD, and CKD. A previous report showed that physical activity was associated with significantly decreased hazards of cancer-specific and all-cause mortality in breast cancer survivors.30 In addition, the same study also illustrated that physical exercise can reduce cardiovascular mortality among breast cancer survivors.30 Furthermore, the intensity of leisure-time physical activity should be at least moderate, so that the beneficial effects of reducing overall cancer mortality can be achieved.31 Promoting physical activity of any intensity and duration is an important approach in reducing mortality risk in the general population.32 A dose-response association has also been observed between total leisure-time physical activity and risk of cancer-specific mortality. More vigorous physical activities have added benefits in the reduction of mortality rate in contrast to moderate physical activity.32 Increasing physical activity after or during an exercise intervention may have an effect on the reduction of mortality rates in patients with cancer. The American College of Sports Medicine reported that physical exercise (among others) is associated with numerous health benefits, including lower risk of cardiovascular disease, type 2 diabetes mellitus, some forms of cancer, and age-adjusted all-cause mortality.33 Exercise also contributes to the increase of muscle strength, muscle mass, and aerobic exercise stamina in patients with cancer.
This meta-analysis also showed that exercise has a positive effect on recurrence in patients with cancer. More particularly, an exercise intervention may help reduce the risk of recurrence of breast,34,35 colorectal,36 and prostate37 cancers. Moderate exercise seems to exert a protective effect on the immune system of the general population.38 Dhabhar39 suggests that the immunopotentiation from moderate exercise is due to the bidirectional effect of stress hormones on immunity, where subtle elevations are beneficial, whereas significant and sustained elevations (as seen with prolonged and/or intensive exertion) are detrimental to the host. The function of the immune system during or after anticancer therapy is important to cancer outcomes such as complications and/or the risk of recurrence.40 Strengthening the immune system may help patients with breast cancer at increased risk of disease recurrence.41 The knowledge of fundamental immunology suggests a suitable appreciation of the role of physical exercise in reducing the risk of cancer recurrence.40 Furthermore, it is reported that exercise can induce apoptosis of cancer cell and inhibit the growth of cancer in animal and in vitro experiments, although the mechanism of these effects of exercise is not clear.42,43
For the findings we obtained in this study, there is no particular limitation on the type of cancer to target, and the patient population may or may not have been treated for cancer. In addition, resistance training and aerobic exercise are listed as exercise interventions, so patients with high performance status are assumed to have been targeted (the intervention for patients with low performance status needs to be considered).
This review had several limitations that should be considered. The number of studies was too small for in-depth analysis with stratification according to cancer type, treatment time frame, and type of physical exercise. Particularly, this review included various cancer diagnoses including breast, lung, hematological malignancy, and other cancers. Physical symptoms and functions were distinguished by cancer type. This meta-analysis included 8 RCTs that examined the following physical exercise types: aerobic exercise, resistance training, or a combination of the two. Differences in the types of physical exercise may have had an impact on mortality and recurrence rates in patients with cancer. In addition, false-positive results could not be excluded because trial sequential analysis could not be performed. Finally, this review was limited to studies published in English; relevant studies published in other languages may offer other findings.
Conclusion
This systematic review and meta-analysis demonstrated that physical exercise has a positive effect on mortality and recurrence rates in patients with cancer. However, the existing data may be insufficient for determining this potential beneficial effect. In conclusion, there is need for better understanding of the different types of cancer, different treatment time frames, and the implementation of an exercise intervention, as well as the best objective or subjective measure for RCT studies.
Footnotes
Author‘s Note: Jiro Nakano is also affiliated with Kansai Medical University, Osaka, Japan.
Author Contributions: SM and JN made substantial contributions to the conception and design. YH and JN were accountable for collection and assembly of data. SM, YH, TF, TT, and JN performed the literature search and data analysis. SM, YH, TF, TT, JBF, and JN were major contributors in drafting and writing the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partly supported by a Grant-in-Aid for Niigata University of Health and Welfare, as well as the MD Anderson Cancer Center Support Grant CA 016672.
ORCID iDs: Shinichiro Morishita  https://orcid.org/0000-0002-4841-948X
https://orcid.org/0000-0002-4841-948X
Takuya Fukushima  https://orcid.org/0000-0001-7075-9264
https://orcid.org/0000-0001-7075-9264
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 2. Stark L, Tofthagen C, Visovsky C, McMillan SC. The symptom experience of patients with cancer. J Hosp Palliat Nurs. 2012;14:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. [DOI] [PubMed] [Google Scholar]
- 4. Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, Hojman P. Muscle dysfunction in cancer patients. Ann Oncol. 2014;25:947-958. [DOI] [PubMed] [Google Scholar]
- 5. Charalambous A, Kouta C. Cancer related fatigue and quality of life in patients with advanced prostate cancer undergoing chemotherapy. Biomed Res Int. 2016;2016:3989286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klassen O, Schmidt ME, Ulrich CM, et al. Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle. 2017;8:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gerritsen JK, Vincent AJ. Exercise improves quality of life in patients with cancer: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2016;50:796-803. [DOI] [PubMed] [Google Scholar]
- 8. Segal R, Zwaal C, Green E, et al. Exercise for people with cancer: a clinical practice guideline. Curr Oncol. 2017;24:40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patel JG, Bhise AR. Effect of aerobic exercise on cancer-related fatigue. Indian J Palliat Care. 2017;23:355-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakano J, Hashizume K, Fukushima T, et al. Effects of aerobic and resistance exercises on physical symptoms in cancer patients: a meta-analysis. Integr Cancer Ther. 2018;17:1048-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sweegers MG, Altenburg TM, Brug J, et al. Effects and moderators of exercise on muscle strength, muscle function and aerobic fitness in patients with cancer: a meta-analysis of individual patient data. Br J Sports Med. 2019;53:812. [DOI] [PubMed] [Google Scholar]
- 12. Segal R, Zwaal C, Green E, et al. Exercise for people with cancer: a systematic review. Curr Oncol. 2017;24:e290-e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morishita S, Tsubaki A, Fu JB. Does physical activity improve survival and mortality among patients with different types of cancer? Future Oncol. 2017;13:1053-1055. [DOI] [PubMed] [Google Scholar]
- 14. Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev. 2017;39:71-92. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Review Manager (RevMan) [computer program]. Copenhagen, Denmark: Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 19. Rief H, Omlor G, Akbar M, et al. Feasibility of isometric spinal muscle training in patients with bone metastases under radiation therapy—first results of a randomized pilot trial. BMC Cancer. 2014;14:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rief H, Bruckner T, Schlampp I, et al. Resistance training concomitant to radiotherapy of spinal bone metastases—survival and prognostic factors of a randomized trial. Radiat Oncol. 2016;11:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wiskemann J, Kleindienst N, Kuehl R, Dreger P, Schwerdtfeger R, Bohus M. Effects of physical exercise on survival after allogeneic stem cell transplantation. Int J Cancer. 2015;137:2749-2756. [DOI] [PubMed] [Google Scholar]
- 22. Dhillon HM, Bell ML, van der Ploeg HP, et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: a randomized controlled trial. Ann Oncol. 2017;28:1889-1897. [DOI] [PubMed] [Google Scholar]
- 23. Hayes SC, Steele ML, Spence RR, et al. Exercise following breast cancer: exploratory survival analyses of two randomised, controlled trials. Breast Cancer Res Treat. 2018;167:505-514. [DOI] [PubMed] [Google Scholar]
- 24. Courneya KS, Segal RJ, McKenzie DC, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. 2014;46:1744-1751. [DOI] [PubMed] [Google Scholar]
- 25. Jones LW, Douglas PS, Khouri MG, et al. Safety and efficacy of aerobic training in patients with cancer who have heart failure: an analysis of the HF-ACTION randomized trial. J Clin Oncol. 2014;32:2496-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Licker M, Karenovics W, Diaper J, et al. Short-term preoperative high-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol. 2017;12:323-333. [DOI] [PubMed] [Google Scholar]
- 27. Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67:1-12. [DOI] [PubMed] [Google Scholar]
- 28. Schaadt L, Christensen R, Kristensen LE, Henriksen M. Increased mortality in patients with severe COPD associated with high-intensity exercise: a preliminary cohort study. Int J Chron Obstruct Pulmon Dis. 2016;11:2329-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greenwood SA, Castle E, Lindup H, et al. Mortality and morbidity following exercise-based renal rehabilitation in patients with chronic kidney disease: the effect of programme completion and change in exercise capacity. Nephrol Dial Transplant. 2019;34:618-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spei ME, Samoli E, Bravi F, La Vecchia C, Bamia C, Benetou V. Physical activity in breast cancer survivors: a systematic review and meta-analysis on overall and breast cancer survival. Breast. 2019;44:144-152. [DOI] [PubMed] [Google Scholar]
- 31. Laukkanen JA, Rauramaa R, Mäkikallio TH, Toriola AT, Kurl S. Intensity of leisure-time physical activity and cancer mortality in men. Br J Sports Med. 2011;45:125-129. [DOI] [PubMed] [Google Scholar]
- 32. Zhao M, Veeranki SP, Li S, Steffen LM, Xi B. Beneficial associations of low and large doses of leisure time physical activity with all-cause, cardiovascular disease and cancer mortality: a national cohort study of 88,140 US adults. Br J Sports Med. 2019;53:1405-1411. [DOI] [PubMed] [Google Scholar]
- 33. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. [DOI] [PubMed] [Google Scholar]
- 34. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479-2486. [DOI] [PubMed] [Google Scholar]
- 35. Sternfeld B, Weltzien E, Quesenberry CP, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18:87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535-3541. [DOI] [PubMed] [Google Scholar]
- 37. Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brolinson PG, Elliott D. Exercise and the immune system. Clin Sports Med. 2007;26:311-319. [DOI] [PubMed] [Google Scholar]
- 39. Dhabhar FS. Stress-induced augmentation of immune function—the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16:785-798. [DOI] [PubMed] [Google Scholar]
- 40. Fairey AS, Courneya KS, Field CJ, Mackey JR. Physical exercise and immune system function in cancer survivors: a comprehensive review and future directions. Cancer. 2002;94:539-551. [DOI] [PubMed] [Google Scholar]
- 41. Standish LJ, Sweet ES, Novack J, et al. Breast cancer and the immune system. J Soc Integr Oncol. 2008;6:158-168. [PMC free article] [PubMed] [Google Scholar]
- 42. Dethlefsen C, Hansen LS, Lillelund C, et al. Exercise-induced catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res. 2017;77:4894-4904. [DOI] [PubMed] [Google Scholar]
- 43. Malicka I, Siewierska K, Kobierzycki C, et al. Impact of physical training on sex hormones and their receptors during. Anticancer Res. 2017;37:3581-3589. [DOI] [PubMed] [Google Scholar]