Abstract
Introduction: Viscum album L extracts (VA) are frequently used in integrative oncology. Aim of this study was to evaluate the impact of add-on VA applications on various patient-reported outcome measures. Methods: A longitudinal real-world study was conducted, using data from the Network Oncology clinical registry. Primary, nonmetastasized breast cancer patients treated with oncological standard therapy partly combined with VA applications were included. Internal Coherence Cancer-related Fatigue, and EORTC QLQ-C30 questionnaires were assessed at baseline and 6, 12, and 24 months later. Results: A total of 319 patients received standard oncological therapy and 40% of them additionally VA applications. After 6 and 12 months for patients treated with chemotherapy (Ctx) only a significant decline of the thermo-coherence, and worsening of fatigue was observed. For patients receiving VA applications but no Ctx, significant beneficial effects on thermo-coherence, fatigue, and seven EORTC QLQ-C30 scales were observed 24 months later. Adjusted multivariable long-term subgroup (n = 106) regression analysis revealed that Ctx, immuno-, and endocrine therapies had a worsening of 17, 17, and 6 point changes, respectively, for EORTC QLQ-C30 fatigue (P = .0004), while VA applications showed an improvement of 12 point change. A similar impact of improvement (add-on VA) and worsening (standard oncological treatment regimens) on EORTC QLQ-C30 insomnia (P = .009) and physical functioning (P = .005) were observed. Conclusions: In the present real-world study, add-on VA applications had a supportive effect on cancer-related fatigue, insomnia, physical functioning, and thermo-coherence. Thus, VA applications might be suited to alleviate symptom burden during anticancer therapy in breast cancer patients.
Keywords: breast cancer, mistletoe, Viscum album, health-related quality of life, cancer-related fatigue, internal coherence, integrative oncology
Introduction
Breast cancer is still the most common cancer in women worldwide, and due to the growing effectiveness of oncological therapies, the number of cancer survivors is increasing.1 However, many breast cancer survivors experience long-term adverse effects, resulting from previous anticancer treatments that deteriorate health-related quality of life (HRQL).2,3 In particular, cancer-related fatigue is a burdensome symptom for cancer patients.4,5 Fatigue affects about 50% of patients during breast cancer treatment and has significant effects on several aspects of HRQL and mood.6,7 Systemic oncologic therapies, in particular chemotherapy (Ctx), radiation, immunotherapy, and also endocrine treatments for breast cancer patients, have various side effects that have been shown to impair diverse aspects of HRQL.3 Numerous questionnaires have been developed to objectify and standardize patient-reported outcome (PRO) measures.8 One well-established multicultural instrument in oncology is the European Organization for Research and Treatment of Cancer Health-Related Quality of Life Core Questionnaire (EORTC QLQ-C30), which incorporates measurements of global health, functioning, and symptom scales including fatigue levels.9 Especially fatigue has a strong impact on HRQL, and other consequences of anticancer therapy can be the increase of menopausal symptoms including thermal discomfort and hot flushes.10 In the present study, fatigue was, in addition, assessed by the German Cancer Fatigue Scale (CFS-D),11,12 which enables researchers to distinguish between affective, physical, and cognitive proportions of fatigue. The sense of coherence is a resource that enables people to manage tension in a health-promoting manner and has a substantial impact on HRQL.13 A clinically useful measure for inner coherence and resilience is the Internal Coherence Scale (ICS) questionnaire, showing robust reliability and validity.14
The effectiveness of supportive complementary treatments such as mistletoe is of particular interest. Extracts of mistletoe, Viscum album L (VA), are frequently used in integrative medicine to enhance HRQL and to reduce adverse side effects.15,16 VA applications are usually well tolerated and have only few and mild side effects.17-19 Various studies have articulated the influence of mistletoe therapy on PROs in cancer patients, especially in breast cancer patients during Ctx.20-24
An important aspect of well-being is associated with feeling warm or cold, which might represent a relevant aspect of HRQL. Thermal dysregulation, such as “feeling cold” or hot flushes and congestive sweating, is often seen in patients with breast cancer, and this can explicitly affect HRQL.25 However, as thermal sensation or comfort is difficult to assess, the ICS questionnaire is a relevant tool since it considers a score for thermal comfort.14 Previously, it was described that the ICS questionnaire showed a good sensitivity to oncological patients, particularly regarding Ctx and VA therapy,26 and recently, we reported that VA therapy is associated with a positive impact on the thermo-coherence of breast cancer patients.27,28
The present longitudinal real-world study explored the impact of oncological treatment regimens as Ctx, immune, endocrine, and VA therapies on PROs in nonmetastasized breast cancer patients. Our objective was to evaluate how add-on VA applications affect HRQL of breast cancer patients, in particular their cancer-related fatigue and internal coherence during the course of anticancer treatment up to 24 months.
Methods
Study Design and Patients
We conducted a noncontrolled, nonrandomized real-world study by analyzing patient registry data (Network Oncology [NO]). The NO is a conjoint clinical register of hospitals, practitioners, and outpatient centers for the evaluation of integrative oncological therapy concepts in oncology health services research.29 Oncological patients from whom written informed consent had been obtained are included in the NO. Demographic data as well as information on diagnosis, histology, and treatment regimens are documented, and surveys of questionnaires on quality of life at different time points are conducted. For the present study, patients with a histologically proven primary diagnosis of nonmetastasized breast cancer were included.
Endpoints
The key element of this study was to evaluate the longitudinal effects of standard oncological treatment regimens and VA applications on PROs of nonmetastasized breast cancer patients. The primary outcomes of the present study were to analyze short- and medium-term effects of Ctx and VA therapy on the internal coherence and fatigue in breast cancer patients. In long-term analyses, the course of the effects of treatment regimens up to 24 months for the early breast cancer patients were evaluated.
Data Collection
For the present evaluation, as similarly reported previously,27,28,30 primary nonmetastasized breast cancer patients of the clinical database NO, who were seen at the certified Breast Cancer Center Gemeinschaftskrankenhaus Havelhöhe (GKH), Berlin, Germany, between June 2012 and October 2018, were screened. As the GKH actively supports shared decision-making, the patients have the chance to participate in the tumor board, and a joint recommendation for the therapy of the patient is given. According to guidelines and physician experiences, oncological therapies in conjunction with integrative interventions as well as VA therapy are offered to the patients.31 The breast cancer patients visited the surveillance and study center at 4 time points. The first visit took place after the surgery (T0), further visits were made after 6 months (T1), 12 months (T2), and 24 months (T3). During these visits, they received and pseudonymously answered the questionnaires.
All data reported here are based on retrievable data from the NO registry at cutoff date of October 15, 2018. Patients from whom assessable data sets at least for 2 measured time points were available were enrolled in the present study. Female primary nonmetastasized breast cancer patients >18 years were included. PROs were evaluated by analyzing the questionnaires for all 4 time points. The number of patients fulfilling all inclusion criteria and answering the questionnaires for at least 2 of the 4 time points determined the sample size. In addition, demographic and medical data (diagnosis, histology, pretreatment, and treatment) of the enrolled patients were retrieved from the clinical database NO. Furthermore, application of VA extracts in the context of an integrative oncological setting with start and end dates and application type used was retrieved. To identify influencing factors and to address potential sources of bias, adjusted multivariable linear regression analyses were performed.
HRQL Analysis and Group Allocation
For short-term analysis (6 months), the T1 surveys were compared with T0 surveys. For medium-term analysis (12 months), T2 surveys were evaluated, and for long-term analysis (24 months), the T3 PROs were assessed. The patients were treated with oncological standard therapy, according to the advice of the multidisciplinary tumor board case conferences, and were informed by physicians to make use of VA therapy. For group analyses, all included patients were assigned into treatment groups according to the therapies received. Classification to these groups was performed retrospectively. Patients who received neither Ctx nor VA applications within the first 6 months were allocated to the “control” group. Patients who received VA applications for at least 4 weeks, but no Ctx, were allocated to the “VA” group. Patients who received Ctx only were allocated to the “Ctx,” and patients receiving Ctx and VA were allocated to the “Ctx + VA” groups, respectively. Applied VA preparations included AbnobaViscum, Helixor, Iscador, and Iscucin VA extracts. VA therapy was applied subcutaneously according to the summary of product characteristics. Off-label intravenous applications were performed in individual cases. Eligible patients for long-term analysis were those from whom at least assessable data for T0 and T3 were available.
Ethics Approval and Consent to Participate
The study complies with the principles laid down in the Declaration of Helsinki. This NO study has been approved by the ethics committee of the Medical Association Berlin (Berlin—Ethik-Kommission der Ärztekammer Berlin). The Reference Number is Eth-27/10. This study had been retrospectively registered at the World Health Organization–approved register, German Register for Clinical Trials (Deutsches Register Klinischer Studien [DRKS]), trial registration number DRKS00013335 (http://www.drks.de/drks_web/setLocale_EN.do). Written informed consent has been obtained from all patients prior to study enrollment.
Patient-Reported Outcomes
For exploratory evaluation of longitudinal effects of Ctx or VA therapy on PROs, the ICS,14 the EORTC QLQ-C30,9 and the CFS-D11,12 were used and analyzed. The questionnaires were assessed for 4 time points, after surgery at baseline (T0), 6 months (T1), 12 months (T2), and 24 months (T3) later.
The ICS questionnaire is a short, highly reliable, and valid 10-item questionnaire based on a 5-point Likert-type scale (scale range: 10 [low ICS] to 50 [high ICS]). The ICS contains 2 subscales: 1 with 8 items Inner Coherence and Resilience and a second subscale Thermo Coherence with 2 items, which has been described earlier.14
The EORTC QLQ-C30 is structured into different subscales (1 global health, 5 functional, and 9 symptom scales).9 Equations were made as described in the EORTC QLQ-C30 manual. The EORTC scores range from 0 to 100. Higher scores represent a better self-reported level in the functional dimensions but a higher degree of symptom burden.
The CFS was originally developed in Japan,12 and after transcultural adaption, it was validated in German (CFS-D)11 and was used here. The CFS-D consists of 15-item questionnaire on 3 subscales (physical, cognitive, and affective fatigue) based on a 5-point Likert-type scale with a possible range of 0 (no fatigue) to 60 (maximum fatigue).11
Statistical Analysis
Continuous variables were described as median with interquartile range; categorical variables were summarized as frequencies and percentages. Student’s t test and Pearson’s χ2 test, respectively, were applied to detect longitudinal differences between the time points (for the groups separately), and the obtained P values were Holm-Bonferroni corrected. P values <.05 were considered to be significant. Data distributions were inspected graphically using box plots and diagrams. All statistical analyses were performed using Microsoft Excel and the software R (R Version 3.1.2 [2014]).32 For Pearson’s χ2 calculation, the basic R package was used; for Cohen’s d analyses, in addition, the “compute.es” package was used.
For long-term analyses, to quantify the strength of the relationship, the changes between the T0 and T3 scales and items were calculated and compared. To identify influencing factors and to address potential sources of bias, adjusted multivariable linear regression analyses were performed and potential confounders were addressed. Predicting variables (with regard to T0) were age (in years), body mass index (BMI; in classes: BMI <25, overweight [25 ≤ BMI < 30], obese [BMI ≥ 30]), hormonal stage (pre-/peri-/postmenopausal), and Union for International Cancer Control stage (UICC stage 0 or I/stage II or III). Furthermore, a comprehensive therapy sum parameter as a continuous variable was generated, which covers all different treatment regimens (Ctx, immune, endocrine, and VA therapies). For Ctx, the summand −3; for immune therapy, the summand −3; for endocrine therapy, the summand −1; for intravenous VA applications, the summand +2; and when only subcutaneous VA applications were applied, the summand +1 were assigned. Using recorded data of the NO registry, for all patients, their individual therapy sum parameter values were calculated. For example, a patient receiving only endocrine treatment received as therapy parameter value −1, patients receiving neither Ctx nor endocrine treatments received as therapy parameter value 0, as well as patients receiving only subcutaneous VA and endocrine applications (+1 −1 = 0), and another patient receiving Ctx, immune, endocrine, and intravenous VA therapies (−3 −3 −1 +2 = −5) received as therapy parameter value −5.
Results
Patients’ Characteristics
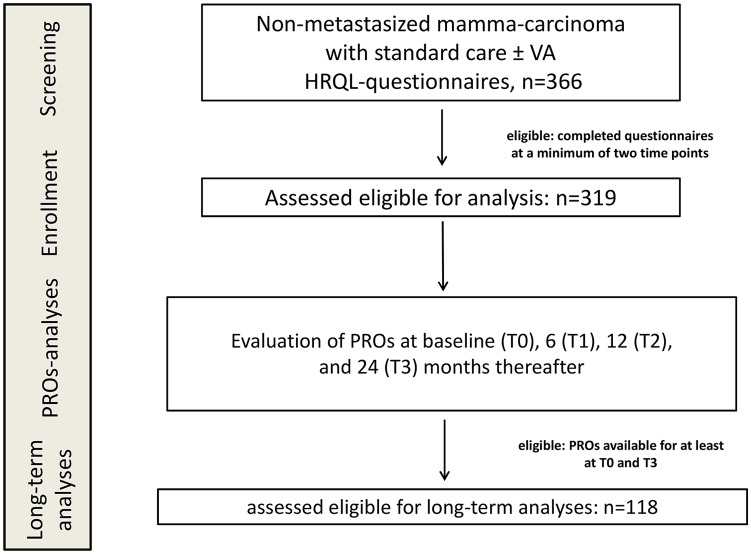
In total, 366 nonmetastasized breast cancer patients treated between 2012 and 2018 at the certified German Breast Cancer Center GKH answered the questionnaires at different time points. Eligibility for analysis was characterized by the availability of assessable data sets at a minimum of 2 time points. PROs were collected for 319 eligible patients (see also study flow chart, Figure 1), and complete data were retrieved from the NO registry. For longitudinal analyses of treatment regimens, all 319 patients were allocated to 4 different treatment groups, according to the therapies they received in the first 6 months. A total of 159 patients receiving neither Ctx nor VA were allocated to the control group, 73 patients receiving VA applications without Ctx were allocated to the VA group, 31 patients receiving Ctx without VA were allocated to the Ctx group, and 56 patients received Ctx and VA and were allocated to the Ctx + VA group. In Table 1, the main characteristics of analyzed patients are given for the entire study cohort and the groups separately. At T0, the median age of the entire study cohort was 59 years, with an interquartile range of 50 to 69 years. The median BMI was 25, 6 (1.8%) patients were underweight (BMI < 18.5), 169 (53%) normal (18.5 ≤ BMI < 25), 97 (30.4%) overweight (25 ≤ BMI < 30), and 44 (13.8) were obese (BMI ≥ 30). All different UICC tumor stages were represented. Most patients (59%) had early stage (UICC stage 0 or I) cancers (Table 1). The hormonal status of the majority (66.8%) was postmenopausal. Minor differences concerning age and BMI were obtained between the groups. The patients in the control group tended to be older and 74.8% were postmenopausal, while the patients in the Ctx group were younger, 51.6% were premenopausal, and 38.7% overweight (Table 1). The majority of patients were diagnosed as estrogen receptor positive (85%), progesterone receptor positive (81%), and Her2 negative (79%). Twenty-one (6.6%) patients were diagnosed triple negative. The interventions that were applied to the patients are listed in Tables 1 and 2. Most patients received radiations (78.7%) and endocrine therapy (65.5%). According to advised oncologic therapy, endocrine treatment was administered with the intention to treat for 5 years. A total of 209 patients (65.5%) received endocrine therapy. Tamoxifen was the most frequent therapy, applied to 142 patients (44.5%; for details see Table 2). Eighty-seven patients (27%) received Ctx, and 29 patients (9%) received, in addition, immunotherapy. Sixty-five patients (20.4%) received epirubicin, 63 (19.7%) paclitaxel, 70 (21.9%) cyclophosphamide, and 28 patients (8.8%) trastuzumab (for details see Table 2). A total of 129 patients (40.4%) received VA therapy of different producers (for details see Table 2). Abnoba extracts were the mistletoe remedies most often prescribed (n = 71), followed by Helixor remedies (n = 48), Iscador applications (n = 46), and Iscucin preparations (n = 16). A total of 119 patients (37%) received subcutaneous injections, and 40 patients (12.5%) received (off-label) intravenous VA treatments, usually accompanied by subcutaneous VA applications.
Figure 1.
Flow chart of the study population. HRQL, health-related quality of life; PROs, patient-related outcome measures; VA, Viscum Album L extracts.
Table 1.
Characteristics of Primary Breast Cancer Patientsa.
| Total | Control | VA | Ctx | Ctx + VA | |
|---|---|---|---|---|---|
| Number of patients, n (%) | 319 (100) | 159 (100) | 73 (100) | 31 (100) | 56 (100) |
| Age, years, median (IQR) | 59 (50-69) | 63 (53-71) | 56 (50-64) | 49 (43-56) | 57 (50-67) |
| BMI, median (IQR) | 25 (22-28) | 25 (22-28) | 23 (22-26) | 25 (23-28) | 24 (22-29) |
| Underweight (BMI <18.5) | 6 (1.9) | 1 (0.6) | 2 (2.7) | 2 (6.3) | 1 (1.8) |
| Normal (18.5 ⩽ BMI < 25) | 169 (53.0) | 79 (49.7) | 44 (60.3) | 13 (41.9) | 33 (58.9) |
| Overweight (25 ⩽ BMI < 30) | 97 (30.4) | 52 (32.7) | 20 (27.4) | 12 (38.7) | 13 (23.2) |
| Obese (BMI ⩾30) | 44 (13.8) | 24 (15.1) | 7 (9.6) | 4 (12.9) | 9 (16.1) |
| NA | 3 (0.9) | 3 (0.9) | 0 | 0 | 0 |
| UICC stages, n (%) | |||||
| 0 | 29 (9.1) | 17 (10.7) | 12 (16.4) | 0 | 0 |
| I | 155 (49.6) | 84 (52.8) | 40 (54.8) | 10 (32.3) | 21 (37.5) |
| II | 108 (33.9) | 53 (33.3) | 17 (23.3) | 13 (41.9) | 25 (44.6) |
| III | 25 (7.8) | 4 (2.5) | 3 (4.1) | 8 (25.8) | 10 (17.9) |
| NA | 2 (0.7) | 1 (0.6) | 1 (1.4) | 0 | 0 |
| ICD-10, n (%) | |||||
| C50.9 (Mammakarzinom) | 288 (90.3) | 140 (88.1) | 61 (83.6) | 31 (100) | 56 (100) |
| D05.1 (DCIS) | 31 (9.7) | 19 (11.9) | 12 (16.4) | 0 | 0 |
| Hormonal status, n (%) | |||||
| Premenopausal | 86 (27.0) | 28 (17.6) | 25 (34.2) | 16 (51.6) | 17 (30.4) |
| Perimenopausal | 10 (3.1) | 5 (3.1) | 0 | 3 (9.7) | 2 (3.6) |
| Postmenopausal | 213 (66.8) | 119 (74.8) | 46 (63.0) | 11 (35.5) | 37 (66.1) |
| NA | 10 (3.1) | 7 (4.4) | 2 (2.7) | 1 (3.2) | 0 |
| Estrogen receptor, n (%) | |||||
| Positive | 271 (85.0) | 149 (93.7) | 68 (93.2) | 19 (61.3) | 35 (62.5) |
| Negative | 43 (13.5) | 8 (5.0) | 3 (4.1) | 12 (38.7) | 20 (35.7) |
| NA | 5 (1.6) | 2 (1.3) | 2 (2.7) | 0 | 1 (1.8) |
| Progesterone receptor, n (%) | |||||
| Positive | 259 (81.2) | 146 (91.8) | 60 (82.2) | 19 (61.3) | 34 (60.7) |
| Negative | 54 (16.9) | 10 (6.3) | 11 (15.1) | 12 (38.7) | 21 (37.5) |
| NA | 6 (1.9) | 3 (0.9) | 2 (2.7) | 0 | 1 (1.8) |
| Her 2, n (%) | |||||
| Positive | 44 (13.8) | 8 (5.0) | 4 (5.5) | 10 (32.3) | 22 (39.3) |
| Negative | 251 (78.7) | 137 (86.2) | 61 (83.6) | 21 (67.7) | 32 (57.1) |
| NA | 24 (7.5) | 14 (8.8) | 8 (11.0) | 0 | 2 (3.6) |
| Triple-negative status, n (%) | |||||
| Yes | 21 (6.6) | 3 (0.9) | 2 (2.7) | 7 (22.6) | 9 (16.1) |
| No | 291 (91.2) | 153 (96.2) | 69 (94.5) | 24 (77.4) | 45 (80.4) |
| NA | 7 (2.2) | 3 (0.9) | 2 (2.7) | 0 | 2 (3.6) |
| Interventions, n (%) | |||||
| Radiation | 251 (78.7) | 127 (79.9) | 52 (71.2) | 27 (87.0) | 45 (80.4) |
| Endocrine therapy | 209 (65.5) | 110 (69.2) | 51 (69.9) | 14 (45.2) | 34 (60.7) |
| Chemotherapy | 87 (27.3) | 0 | 0 | 31 (100) | 56 (100) |
| Immunotherapy | 29 (9.1) | 0 | 1 (1.4) | 7 (22.6) | 21 (37.5) |
| Mistletoe therapy | 129 (40.4) | 0 | 73 (100) | 0 | 56 (100) |
Abbreviations: VA, Viscum album L therapy; Ctx, chemotherapy; IQR, interquartile range; BMI, body mass index; UICC, Union for International Cancer Control; ICD-10, International Classification of Diseases, 10th Revision.
TNM staging according to the UICC.
Table 2.
Interventionsa.
| Number of patients, n (%) | 319 (100) |
| Endocrine therapy, n (%) | 209 (65.5) |
| Tamoxifen | 142 (44.5) |
| Letrozole | 46 (14.4) |
| Anastrozole | 27 (8.5) |
| Exemestane | 15 (4.7) |
| Other | 6 (1.9) |
| Chemotherapy, n (%) | 87 (27.3) |
| Epirubicin | 65 (20.4) |
| Paclitaxel | 63 (19.7) |
| Cyclophosphamide | 70 (21.9) |
| Biphosphonate | 5 (1.6) |
| Other | 14 (4.4) |
| Immunotherapy, n (%) | 29 (9.1) |
| Trastuzumab | 28 (8.8) |
| Pertuzumab | 3 (0.9) |
| Other | 4 (1.3) |
| Mistletoe therapy, n (%) | 129 (40.4) |
| Subcutaneous | 119 (37.3) |
| Intravenous | 40 (12.5) |
| Abnobaviscum | 71 (22.3) |
| Helixor | 48 (15.0) |
| Iscador | 46 (14.4) |
| Iscucin | 16 (5.0) |
| Other | 3 (0.9) |
Characteristics of applied interventions (n = 319). Numbers in columns do not add to 319, as the patients have received various combinations of preparations and applications, respectively.
A total of 118 patients were eligible for long-term analyses (Figure 1). For long-term analyses, the changes of the PROs between the T0 and T3 scales and items were calculated and compared. A total of 201 patients were not eligible for long-term analysis, of which 66 patients were still under follow-up at cutoff time, while 116 patients did not complete the T3 questionnaires for unknown reasons or dropped out for the 24 months surveillance. Nineteen patients did not complete the T0 questionnaires for unknown reasons.
ICS Evaluation
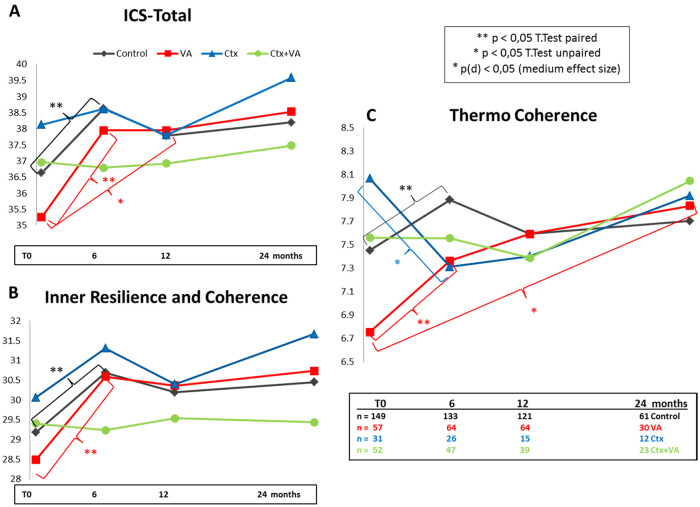
Completed ICS questionnaires were evaluated and analyzed for the entire study cohort. Assessable total ICS values from 289 patients for T0, from 268 for T1, 236 for T2, and 125 patients for T3 were obtained (Table 3). Obtained total ICS values were within the range as published previously,14,27,28 and no significant changes between the time points were observed for the entire study cohort (Table 3). For longitudinal treatment group analyses, the total and subscales “inner resilience and coherence” and “thermo coherence” (TC) were calculated, and mean values were determined for all scales, groups, and time points. In Figure 2, for all 4 different treatment groups in diagrams, the courses of the mean values for the total ICS (Figure 2A), the inner resilience and coherence (Figure 2B), and the thermo-coherence (Figure 2C) are shown. At T0, as similarly reported previously,27 differences between the groups were obtained for the ICS values. In particular, low ICS values were observed for the VA group (red in Figure 2). T test calculation (2-tailed, unpaired) revealed a significant (P = .005*) difference for the thermo-coherence between the Ctx and VA groups. Short-term ICS analysis with Student’s t test (2-tailed, paired) calculations (Holm-Bonferroni corrected) revealed a significant short-term increase of the total ICS and ICS subscores for the control and VA groups (P < .05; black and red in Figure 2A-C). In contrast, for the Ctx group (blue in Figure 2), the TC declined substantially (from TCCtxT0: 8.06 ± 1.68 to TCCtxT1: 7.231 ± 1.64). Chi-squared test (χ2 = 4.765) and Cohen’s d analyses revealed a significant medium effect, d (95% confidence interval [CI]) = 0.60 (0.04-1.16), with P(d) = .02 for this short-term decrease (blue in Figure 2C). Furthermore, t test calculation (2-tailed, unpaired) revealed only for the VA group a persistent significant (P = .028) long-term increase (from TCVAT0: 6.75 ± 2.18 in relation to TCVAT3: 7.83 ± 2.00) of the thermo-coherence (red in Figure 2C).
Table 3.
Total ICS and CFS-D Scores of the Entire Study Cohort.
| T0 | T1 | T2 | T3 | |
|---|---|---|---|---|
| ICS | ||||
| n | 289 | 268 | 236 | 125 |
| Mean ± SD | 36.57 ± 7.01 | 38.14 ± 6.64 | 37.69 ± 6.38 | 38.28 ± 5.98 |
| Median (IQR) | 38 (32-41) | 39 (34-43) | 38 (33-42) | 39 (34-42) |
| Min-max | 15-50 | 18-50 | 18-50 | 21-50 |
| CFS-D | ||||
| n | 282 | 270 | 235 | 127 |
| Mean ± SD | 20.75 ± 9.83 | 21.47 ± 10.93 | 22.08 ± 10.68 | 20.87 ± 10.23 |
| Median (IQR) | 20 (14-27) | 22 (13-29) | 22 (13.5-30) | 20 (13-28.5) |
| Min-max | 0-46 | 0-58 | 0-48 | 21-50 |
Abbreviations: ICS, Internal Coherence Scale; CFS-D, German Cancer Fatigue Scale; n, number of patients; SD, standard deviation; IQR, interquartile range.
Figure 2.
Internal Coherence Scale (ICS) for the treatment groups. For the entire study cohort, the ICS questionnaires at baseline (T0) and 6, 12, and 24 months, thereafter, were analyzed. The mean values of (A) the total ICS, (B) inner resilience and coherence, and (C) thermo-coherence scores for the control (black), Viscum album L therapy (VA; red), chemotherapy (Ctx; blue), and Ctx + VA (green) groups are shown.
Evaluation of Fatigue
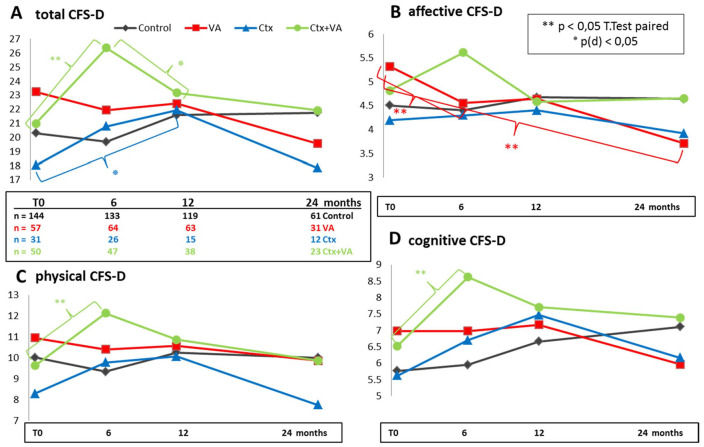
The CFS-D questionnaires were evaluated, and assessable values were obtained from 282 patients for T0, from 270 for T1, 235 for T2, and from 127 patients for T3 (Table 3). Obtained CFS-D values were within the range of formerly published results in breast cancer patients.11 For the entire study cohort at T1 and T2 in relation to T0, slightly elevated fatigue levels were observed, which decreased in the further course until T3 (Table 3). In Figure 3, for all 4 different treatment groups, the courses of the mean values for the total fatigue score (Figure 3A) and the subscales affective (Figure 3B), physical (Figure 3C), and cognitive (Figure 3D) fatigue are shown in diagrams. Significant short- and medium-term effects, respectively, were obtained for Ctx-treated groups (blue and green in Figure 3). T test calculation (2-sided, paired, Holm-Bonferroni corrected) revealed that in relation to T0, the Ctx + VA group (green in Figure 3) reported a significantly higher burden of fatigue at T1 (a mean impairment of 5 score points for total fatigue with P = .0006), while the Ctx-only group (blue in Figure 3) reported a significant medium-term increase of fatigue. Calculation of Pearson’s χ2 test (χ2 = 6.019) and Cohen’s d analysis revealed a medium effect size d (95% CI) = 0.77 (0.12-1.41) with P(d) = .02 for this medium-term increment of fatigue from T0 to T2 (blue in Figure 3A). For the Ctx + VA group, the initial short-term increment of fatigue reverses in the further course of 6 months (from T1 until T2); in particular, a mean reduction of 1.1 score points affective fatigue was observed (green in Figure 3B). Calculation of Pearson’s χ2 test (χ2 = 6.810) and Cohen’s d analysis revealed a medium effect size d (95% CI) = 0.59 (0.13-1.05) with P(d) = .01 for the decrease of total fatigue between T1 and T2 of the Ctx + VA group (green in Figure 3A). However, the VA group self-reported a significant amelioration of affective fatigue burden (red in Figure 3B). T test (2-sided, paired, Holm-Bonferroni corrected) calculation revealed for the VA group a mean reduction of 0.8 score points with P = .02 for short-term (T1 compared with T0, n = 48), and continually, 1.5 score points with P = .025 for long-term (T3 compared with T0, n = 27) affective fatigue (red in Figure 3B).
Figure 3.
Cancer Fatigue Scale (CFS-D) for the treatment groups. For the entire study cohort, the CFS-D at baseline (T0) and 6, 12, and 24 months, thereafter, were analyzed. The mean values of (A) the total CFS-D, (B) affective CFS-D, (C) physical CFS-D, (D) cognitive CFS-D scores for the control (black), Viscum album L therapy (VA; red), chemotherapy (Ctx; blue), and Ctx + VA (green) groups are shown.
EORTC QLQ-C30 Evaluation and Long-Term Analyses
EORTC QLQ-C30 questionnaires were evaluated, and assessable values were obtained from 284 patients for T0, from 270 for T1, 236 for T2, and from 127 patients for T3. The mean values for global health and functional scales for the entire study cohort are listed in Table 4. All EORTC QLQ-C30 scores at T0 were within the range of formerly published EORTC QLQ-C30 reference values for breast cancer patients33 and similar to published previously results30 (data not shown). Substantial significant short-term improvements for global health, role, emotional, and social functioning were observed (Table 4). On average, for the entire cohort, increments of 6 to 10 score points between T0 and T1 values were found, and t test calculations (2-sided, paired) revealed that these improvements were significant (P < .05). Also, significant medium- and long-term improvements for the entire cohort were observed (Table 4). The most pronounced longitudinal long-term increase for the entire study cohort was observed for role functioning (mean increment of 17 score points, with P < .001; Table 4). Regarding groups, in particular for the VA group, substantial and significant long-term improvements for 7 EORTC QLQ-C30 scales were observed (Table 5). The mean values, standard deviations, and t test P values for 10 EORTC QLQ-C30 scales for the VA group at T0 and T3 are listed in Table 5. In particular, the long-term analyses revealed the strongest improvements for global health (mean increment of 18 score points, with P = .0004), for role functioning (mean increment of 22 score points, with P = .001), and for social functioning (mean increment of 16 score points, with P = .004).
Table 4.
EORTC QLQ-C30 Global Health and Functional Scores of the Entire Study Cohorta.
| T0 | T1 | T2 | T3 | |
|---|---|---|---|---|
| Global health | ||||
| n | 278 | 268 | 235 | 125 |
| Mean ± SD | 58.18 ± 19.76 | 64.61 ± 21.12* | 65.71 ± 19.78** | 68.27 ± 19.60** |
| Physical functioning | ||||
| n | 284 | 270 | 236 | 127 |
| Mean ± SD | 74.69 ± 19.94 | 76.25 ± 21.13 | 76.55 ± 20.26 | 78.58 ± 19.56 |
| Role functioning | ||||
| n | 282 | 267 | 235 | 127 |
| Mean ± SD | 56.68 ± 33.27 | 66.85 ± 30.31** | 68.16 ± 28.91** | 74.02 ± 27.16** |
| Cognitive functioning | ||||
| n | 284 | 270 | 237 | 127 |
| Mean ± SD | 70.31 ± 25.03 | 72.04 ± 26.78 | 72.50 ± 25.53 | 73.49 ± 25.22 |
| Social functioning | ||||
| n | 280 | 269 | 236 | 126 |
| Mean ± SD | 61.61 ± 31.89 | 68.22 ± 30.16* | 69.21 ± 29.76* | 73.81 ± 28.67** |
| Emotional functioning | ||||
| n | 281 | 270 | 236 | 127 |
| Mean ± SD | 56.55 ± 26.65 | 61.39 ± 24.92* | 61.34 ± 25.74* | 62.80 ± 26.82* |
Abbreviations: EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Health-Related Quality of Life Core Questionnaire; SD, standard deviation.
T test calculations (2-sided, paired) for longitudinal changes when EORTC QLQ-C30 scales were compared with T0 scales; and significant P values are indicated: *P < .05; **P < .001.
Table 5.
Long-Term EORTC QLQ-C30 Changes of the VA Groupa.
| EORTC | Global Health | Functioning Scales |
Symptom Scales/Items |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Physical | Role | Emotional | Cognitive | Social | Fatigue | Insomnia | Appetite Loss | Nausea | ||
| T0 | ||||||||||
| n | 55 | 58 | 57 | 57 | 58 | 56 | 58 | 58 | 58 | 58 |
| Mean | 53.48 | 70.80 | 49.12 | 53.51 | 66.95 | 54.76 | 53.83 | 45.98 | 21.84 | 5.17 |
| SD | 20.45 | 21.29 | 35.65 | 26.35 | 25.61 | 30.81 | 27.28 | 34.92 | 28.74 | 11.25 |
| T3 | ||||||||||
| n | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Mean | 71.67 | 80.89 | 80.56 | 67.78 | 80.00 | 77.22 | 35.93 | 27.78 | 5.56 | 2.22 |
| SD | 19.08 | 15.18 | 23.21 | 25.43 | 22.93 | 25.63 | 20.82 | 27.33 | 15.11 | 7.11 |
| T test, n | 26 | 27 | 26 | 27 | 27 | 27 | 27 | 27 | 27 | 27 |
| P | .0004* | .0818 | .0011* | .0163* | .0367* | .0004* | .0139* | .0863 | .0038* | .0571 |
Abbreviations: EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Health-Related Quality of Life Core Questionnaire; VA, Viscum album L therapy; SD, standard deviation.
T test (2-sided, paired) for longitudinal EORTC QLQ-C30 changes from T0 compared with 24 months later (T3) were conducted; and P values determined. *P < 0.05.
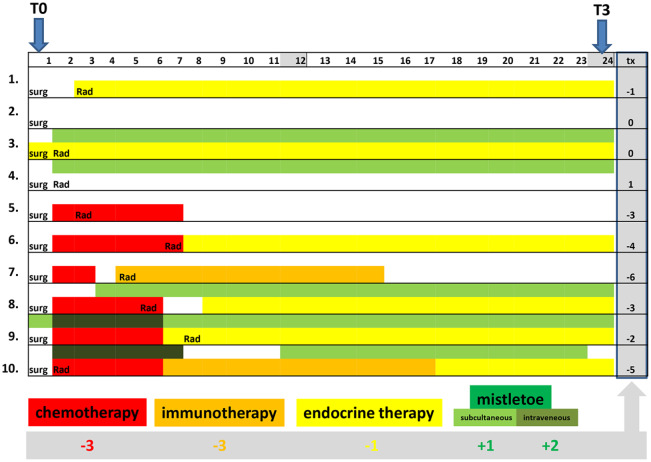
In order to take into account unequal distribution of demographic characteristics and to prevent selection bias, the EORTC QLQ-C30 score changes from T0 to the 24-month follow-up (T3) were calculated, and for these long-term analyses, multivariable regression analyses were carried out as outlined in the methods. A total of 118 patients were eligible for long-term analyses. The long-term group and the entire cohort were comparable in terms of their demographic parameters, hormonal status, and the interventions received. Thirty-four patients (29%) of this subgroup received Ctx, and 49 patients (41.5%) received VA treatment. Except for age as being a continuous variable, all other explanatory variables (BMI, cancer stage, and hormonal status) were of a categorical nature. Twelve patients did not complete all EORTC QLQ-C30 items for T0 or T3, respectively, and for 4 patients, the hormonal stage was not known and these were not included in the multivariate regression analyses. On multivariable analysis, Ctx was strikingly and significantly associated with a worsening of almost all measured PROs. As an example, in Table 6, the multivariable analyses for the longitudinal changes of the EORTC QLQ-C30 fatigue symptoms are shown. The application of Ctx was significantly associated with a worsening of fatigue (estimate β = 15 point change; P = .016; Table 6A). Endocrine therapy showed only minor, nonsignificant impairment of EORTC QLQ-C30 fatigue changes (β = 8.5 point change; P = .175; Table 6B). All 12 patients receiving immunotherapy also received Ctx; thus, for multivariate analyses, these linked variables were combined by assigning 1 to the patients receiving Ctx only and 2 to those patients receiving both Ctx and immunotherapy (Table 6C). Using this combined variable, multivariate analysis showed that Ctx and immunotherapy were both significantly associated with a worsening of the effects on fatigue of approximately 13 point changes each (P = .0022 in Table 6C). However, VA therapy showed only slight, nonsignificant improvements for the changes in fatigue (β = −0.5 point change; Table 6D). When a combined VA variable was used by assigning 1 to patients receiving only subcutaneous VA applications and 2 to patients additionally receiving intravenous VA applications, a nonsignificant improvement of 1.7 point change for subcutaneous VA and 3.4 point change for intravenous VA on fatigue was observed (Table 6E). Finally, considering Ctx and immunotherapy, the multivariate analysis in Table 6F shows a nonsignificant improving effect of 2.8 point change for subcutaneous VA and 5.6 point change for intravenous VA on fatigue. Since therapy regimens Ctx and VA applications were also linked, a comprehensive therapy sum parameter, which takes into account all interventions received (Ctx, immuno, endocrine, and VA therapies), as one continuous variable, was generated as illustrated in Figure 4 and described in the methods. In Figure 4, the interventions received over the course of 24 months for 10 patients of the long-term cohort are illustrated, and the calculation of the respective therapy sum parameter values is described. Using this comprehensive therapy parameter, significant associations for EORTC QLQ-C30 fatigue (estimate β = −5.874; P = .0004), insomnia (β = −5.693; P = .0086), and physical functioning (β = 2.882; P = .0046) were observed (Table 7). Similar associations were observed for global health, cognitive, and emotional functioning (data not shown). Improvements of insomnia or fatigue are expressed by negative estimate β values, while positive estimate β values on functioning scales indicate associations with improvements. Taking into account the assigned summands (Figure 4), the results for Ctx, immuno, and endocrine therapies were worsening of fatigue (P = .0004) with 17, 17, and 6 point changes, respectively, whereas an improvement of 6 point changes can be deduced for subcutaneous VA applications and 12 point changes for intravenous VA. A similar impact of worsening (standard oncological treatment regimens) and improvement (add-on VA) for insomnia (P = .009) and physical functioning (P = .005) were observed (Table 7). Furthermore, we observed that a premenopausal hormonal status was significantly associated with a substantial improvement of self-reported insomnia (β = −41.535; P = .001; Table 7).
Table 6.
| A |
B |
||||||
|---|---|---|---|---|---|---|---|
| Demographic Variables | Estimate β | SE | P | Demographic Variables | Estimate β | SE | P |
| Age | 0.052 | 0.346 | .88 | Age | 0.086 | 0.346 | .803 |
| BMI <25 | Reference | BMI <25 | Reference | ||||
| Overweight | 5.550 | 6.257 | .377 | Overweight | 4.470 | 6.281 | .478 |
| Obese | 5.089 | 8.935 | .57 | Obese | 5.765 | 8.912 | .519 |
| Postmenopausal | Reference | Postmenopausal | Reference | ||||
| Perimenopausal | −15.306 | 15.992 | .341 | Perimenopausal | −11.689 | 16.145 | .471 |
| Premenopausal | −9.931 | 9.511 | .299 | Premenopausal | −9.612 | 9.475 | .313 |
| UICC stage 0 or I | Reference | UICC stage 0 or I | Reference | ||||
| UICC stage II or III | −1.828 | 5.966 | .76 | UICC stage II or III | −2.355 | 5.954 | .693 |
| Interventions | Interventions | ||||||
| Ctx | 15.217 | 6.212 | .016* | Ctx | 18.563 | 6.654 | .0063* |
| Endocrine therapy | 8.508 | 6.229 | .175 | ||||
| C |
D |
||||||
| Demographic Variables | Estimate β | SE | P | Demographic Variables | Estimate β | SE | P |
| Age | 0.003 | 0.339 | .994 | Age | 0.052 | 0.348 | .882 |
| BMI <25 | Reference | BMI <25 | Reference | ||||
| Overweight | 5.905 | 6.151 | .339 | Overweight | 5.430 | 6.416 | .399 |
| Obese | 5.855 | 8.792 | .507 | Obese | 5.018 | 9.010 | .579 |
| Postmenopausal | Reference | Postmenopausal | Reference | ||||
| Perimenopausal | −14.134 | 15.685 | .37 | Perimenopausal | −15.545 | 16.272 | .342 |
| Premenopausal | −10.846 | 9.358 | .249 | Premenopausal | −9.942 | 9.557 | .301 |
| UICC stage 0 or I | Reference | UICC stage 0 or I | Reference | ||||
| UICC stage II or III | −2.427 | 5.853 | .679 | UICC stage II or III | −1.837 | 5.995 | .76 |
| Interventions | Interventions | ||||||
| Ctx + immune | 12.936 | 4.121 | .0022* | Ctx | 15.379 | 6.481 | .0195* |
| Only Ctx: 1; Ctx + immune: 2 | VA | −0.563 | 6.034 | .926 | |||
| E |
F |
||||||
| Demographic Variables | Estimate β | SE | P | Demographic Variables | Estimate β | SE | P |
| Age | 0.055 | 0.348 | .875 | Age | 0.0003 | 0.34 | .999 |
| BMI <25 | Reference | BMI <25 | Reference | ||||
| Overweight | 5.152 | 6.370 | .421 | Overweight | 5.235 | 6.254 | .405 |
| Obese | 4.870 | 8.990 | .589 | Obese | 5.539 | 8.830 | .532 |
| Postmenopausal | Reference | Postmenopausal | Reference | ||||
| Perimenopausal | −16.277 | 16.262 | .319 | Perimenopausal | −15.555 | 15.880 | .33 |
| Premenopausal | −9.760 | 9.561 | .31 | Premenopausal | −10.640 | 9.389 | .26 |
| UICC stage 0 or I | Reference | UICC stage 0 or I | Reference | ||||
| UICC stage II or III | −1.823 | 5.990 | .761 | UICC stage II or III | −2.396 | 5.870 | .684 |
| Interventions | Interventions | ||||||
| Ctx | 16.359 | 6.931 | .020* | Ctx + immuno | 14.180 | 4.559 | .0024* |
| VA sc/iv | −1.679 | 4.436 | .706 | VA sc/iv | −2.795 | 4.325 | .52 |
| Only sc VA: 1; iv VA: 2 | |||||||
Abbreviation: EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Health-Related Quality of Life Core Questionnaire; SE, standard error; BMI, body mass index; UICC, Union for International Cancer Control; Ctx, chemotherapy; VA, Viscum album L. therapy; sc, subcutaneous; iv, intravenous.
TNM staging according to the (UICC).
Negative estimate β values indicate an association with an improvement of fatigue, while positive estimate β values indicate an association with worsening of fatigue. *P < 0.05.
Figure 4.
The 24-month treatment regimen course summarized with a comprehensive therapy parameter. For 10 patients of the long-term cohort here is outlined roughly, which interventions they received after surgery (surg) between T0 and T3. The individual time frames of radiation (Rad), endocrine therapy (yellow), chemotherapy (Ctx; red), immunotherapy (orange), and mistletoe (subcutaneous VA (light green), and intravenous VA (dark green) applications are indicated. For Ctx, the summand −3, for immunotherapy −3, for endocrine therapy −1, for subcutaneous VA applications +1, and intravenous VA applications +2 were assigned (for details see Methods section Statistical analysis); and for all patients, their individual therapy sum parameter values (tx) were calculated.
Table 7.
Association Factors for Change of EORTC QLQ-C30 Scoresa.
| EORTC | Insomnia |
n = 106 |
Fatigue |
n = 105 |
Physical |
n = 106 |
|||
|---|---|---|---|---|---|---|---|---|---|
| Estimate β | SE | P | Estimate β | SE | P | Estimate β | SE | P | |
| Demographic variables | |||||||||
| Age | −0.878 | 0.439 | .048* | −0.002 | 0.334 | .994 | −0.232 | 0.206 | .262 |
| BMI <25 | Reference | Reference | Reference | ||||||
| Overweight | −6.367 | 8.003 | .428 | 3.394 | 6.035 | .575 | −5.266 | 3.745 | .163 |
| Obese | −5.312 | 11.488 | .645 | 5.845 | 8.656 | .501 | −8.603 | 5.355 | .111 |
| Postmenopausal | Reference | Reference | Reference | ||||||
| Perimenopausal | −17.278 | 20.523 | .402 | −14.838 | 15.454 | .339 | 13.092 | 9.603 | .176 |
| Premenopausal | −41.085 | 12.084 | .001* | −10.824 | 9.256 | .243 | −4.242 | 5.654 | .455 |
| UICC stage 0 or I | Reference | Reference | Reference | ||||||
| UICC stage II or III | −5.924 | 7.565 | .435 | −3.103 | 5.773 | .592 | 1.263 | 3.540 | .722 |
| Interventions | |||||||||
| Therapyb | −5.693 | 2.128 | .0086* | −5.874 | 1.605 | .0004* | 2.882 | 0.996 | .0046* |
Abbreviation: EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Health-Related Quality of Life Core Questionnaire; SE, standard error; BMI, body mass index; UICC, Union for International Cancer Control.
For insomnia or fatigue, negative estimate β values indicate an association with an improvement of symptoms. For physical functioning, positive β values indicate an association with an improvement.
A comprehensive therapy sum parameter as a continuous variable was generated. For all patients, their individual therapy sum parameter values were calculated as illustrated in Figure 4. *P < 0.05.
Discussion
In the present longitudinal study, the PROs of 319 primary nonmetastasized breast cancer patients treated with standard oncological treatment and VA applications were assessed over a period of 24 months. Significant short-term effects were observed, such as a substantial increase of the internal coherence, particularly the thermo-coherence, for patients treated with VA applications were detected, while a considerable decline of thermo-coherence and a worsening of fatigue was obtained for Ctx-treated patients. After 24 months, only for the VA group in absence of Ctx, significant beneficial long-term effects on fatigue, global health, and 5 further EORTC QLQ-C30 scales were observed. Adjusted multivariable linear regression analysis using a generated therapy sum parameter for all treatment regimens revealed that Ctx, immune, and endocrine therapies had a significant worsening impact, while add-on VA applications had supportive effects on HRQL.
Using the EORTC QLQ-C30 questionnaire, by Arndt et al,2 it was reported that in breast cancer patients 3 years after diagnosis deficits in EORTC QLQ-C30 scales were still apparent for several functioning and symptom scales including insomnia and fatigue. Cancer-related fatigue is pervasive and affects patients’ HRQL considerably.5 It was reported that fatigue levels were noted already after surgery (9%), increased during (49%) and at the end of Ctx (47%), were maintained after 1 year (31%), and still persisted (13%) 42 months after completing Ctx.7,34 Furthermore, persistent impairments, negatively influencing their daily life 3 years after Ctx, were described for a 453-member multicenter German breast cohort study.35 In this article, in line with those studies, we found significant associations of Ctx and immunotherapy with deficits for several PROs, in particular cancer-related fatigue, insomnia, and physical functioning scales 2 years after their cancer diagnosis.
The first questionnaires at T0 were collected after surgery, before the final decision on which anticancer treatment to apply. Although in the present study cohort only minor differences between groups were observed concerning baseline characteristics (Table 1), substantial differences for all PROs were observed at T0 (Figures 2 and 3). Interestingly, with regard to their breast cancer stages, the proportion of early-stage cancer is highest in the VA group (71%; Table 1), but on the other hand, it appears that the patients who chose the option of additional VA therapy had lower ICS values (Figure 2) and a higher fatigue burden (Figure 3) compared with the other groups at T0. This probably depends on the individual perceived severity of breast cancer, as various psychologically distressing symptoms may occur at the time of cancer diagnosis, and fatigue in particular is one of the most commonly reported symptoms.36 In particular, breast cancer patients show a high need for psychological attendance37 and are interested in the usage of complementary and integrative medicine.38,39 It has also been reported that women with higher distress levels use complementary medicine more often.40 Previously, associations of the ICS and self-regulation at baseline with the course of fatigue were evaluated, suggesting that adaptive capacities are appropriate outcome predictors for cancer-related fatigue.41 For healthy woman, the mean total ICS value has been described to be in the range of 40 and when diagnosed with breast cancer is lowered to 36.14 Appropriate ICS mean values were obtained at T0 for the entire study cohort here (Table 3 and Figure 2). Regarding fatigue, in relation to the mean values of the entire study cohort (Table 3) or control group (black in Figure 3), the VA group (red in Figure 3) had mean increased fatigue levels at T0. As has been reported, in 9% of breast cancer patients, fatigue symptoms are identified already after surgery before onset of oncological treatment.7,34 In our survey, the first questionnaires (T0) were filled in after surgery, and we observed marked differences for the ICS and CFS-D scores between the treatment groups (Figures 2 and 3). Notably, for the VA group, increased fatigue levels (red in Figure 3) and lowered ICS values (red in Figure 2) were observed at T0, while the Ctx-only group had throughout anticancer therapy remarkable high ICS scales (blue in Figure 2) and their fatigue scores, which worsened during Ctx treatment, recovered in the second year (blue in Figure 3). A relationship between the prevalence for developing fatigue and psychological parameters, such as depression and anxiety, has been reported.7,34 Hence, in the moment when confronted with the diagnosis of cancer, independent of therapy, the individual sense of coherence and the prevalence for developing fatigue might be correlated. In particular, such sensitive patients with low ICS have an urgent need for supportive therapy and care.
Aims of VA therapy are the improvement of HRQL and the reduction of side effects associated with conventional anticancer strategies.16,42,43 For breast cancer patients, beneficial associations between add-on VA applications and HRQL have been reported.15,16 For example, in a clinical investigation of 270 breast cancer patients, HRQL was stabilized and improved during Ctx and concurrent VA therapy.20 Similarly, in randomized controlled trials (RCTs) with breast cancer patients, an improvement of HRQL was observed in patients who received add-on VA during Ctx treatment, whereas no improvement was observed in the control group who received only Ctx.24 Positive effects on some EORTC QLQ-C30 scores were also observed.44 No RCT reported an unfavorable effect of VA on PROs, and all published RCTs regarding breast cancer patients observed considerable improvements for the mistletoe groups.16 Thus, evidences from RCTs support the view that VA applications offer benefits on HRQL during Ctx for breast cancer patients.17 Currently, in the clinical practice guidelines on the use of integrative therapies during breast cancer treatment, add-on VA applications received Grade C for improving quality of life, indicating that add-on VA can be considered for the use.45 In line with those findings, using the CFS-D and the EORTC QLQ-C30 questionnaires, here, we observed significant and clinical effective improvements for fatigue and several EORTC QLQ-C30 scales including global health status for VA-treated breast cancer patients in the absence of Ctx (Figures 2C and 3B; Table 5).
Significant beneficial effects of VA applications on PROs were observed in this study. Various VA preparations (Table 2) and different dosages and individual application protocols were considered in this real-world study. The patients receiving intravenous VA injections also generally received subcutaneous VA applications over a period of at least 6 months, while patients receiving only subcutaneous VA applications have sometimes received only low VA dosages. To enable a distinction between high and low VA dosages, received VA applications were classified into intravenous VA applications and only subcutaneous VA applications, respectively (Table 6E). From the multivariate analyses shown in Table 6A to F, it can be deduced how such qualitative and quantitative differences can be summarized by a single linear variable. This led to the generation of a comprehensive therapy sum parameter that can take all treatment regimens into account, in a dose-dependent fashion (Figure 4). These analyses revealed that Ctx, immune, and endocrine therapies have a worsening impact, while VA is associated with improving effects on fatigue, insomnia, and physical functioning (Table 7).
In context of fatigue also thermal regulation plays an important role, and breast cancer patients are frequently deficient in achieving thermal comfort,25 and sudden onset of treatment-induced menopausal symptoms including hot flushes and night sweats are common problems for breast cancer survivors.46 From large and well-controlled studies of breast cancer survivors, a linkage between inflammation and fatigue is suggested, and a basic model proposes that the tumor itself and also oncologic treatments activate pro-inflammatory cytokines.47 Cyclooxygenases (COX) are key enzymes for inflammatory reactions, and preclinical studies have shown that some VA compounds reduce selectively COX-2 levels.48 It was suspected that phytochemicals as VA preparations may exert an anti-inflammatory effect via this COX-2 pathway.49 Previously, the influence of VA therapy on breast cancer patients on the ICS and, in particular, the thermo-coherence was observed for short-term effects.27,28 From the prolonged evaluation of present study, it can be assumed that a persistent long-term effect on the thermo-coherence can be achieved by VA applications (Figure 2B).
Due to the study design, in this investigation, we cannot distinguish between direct drug effects on PROs and possible indirect effects from therapy expectations or intentions. Unwanted biases may have been introduced in the analysis, for example, the assignment of treatment with VA was performed in a nonrandomized, noncontrolled, and unblinded fashion. Further confounders may influence ICS and fatigue, especially patients may differ in their choices of options for receiving VA, since the patients’ preferences and therapy decisions directed allocation into groups. Not all initially participating patients filled in all questionnaires for different reasons, representing a major limitation. Furthermore, comorbidities or received additional routine medications of the patients were not considered for analyses. Strengths of this study are the prospective, longitudinal data collection, and that the physicians’ recommendations were independent of participation into this study. By conducting multivariate analyses, potential confounders such as BMI, UICC stages, and the menopausal status were taken into account.
Conclusions
In the present longitudinal real-world study, the impact of oncological therapies on PROs in breast cancer patients is described. The strongest stressful long-term impacts on PROs have Ctx and immunotherapy, worsening fatigue, lowering thermo-coherence, and affecting physical functioning. In contrast, VA applications coadministered with conventional treatments had improving effects on fatigue, insomnia, and physical functioning. Thus, add-on VA applications might be suited to alleviate symptom burden during anticancer strategies in breast cancer patients.
Acknowledgments
We would like to thank all medical documentation officers at the Gemeinschaftskrankenhaus Havelhöhe and the Forschungsinstitut Havelhöhe involved in the present work.
Footnotes
Author’s Note: Matthias Kröz is also affiliated with Research Department, Hospital Arlesheim, Arlesheim, Switzerland.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FS reports grants from Helixor Heilmittel GmbH (travel costs and honoraria for speaking), grants from Abnoba GmbH, and grants from Iscador AG, outside the submitted work. HM is a member of the board of directors of Weleda AG and a member of the Network Arbeitsgemeinschaft der Wissenschaftlichen Fachgesellschaften (AWMF e.V.) guideline committee for integrative oncology (Guideline for Complementary Medicine in the Treatment of Oncological Patients). HM has an endowed professorship at the Charité Universitätsmedizin Berlin, which is financed by the Software AG Foundation, outside the submitted work. MK received a lecturer honorarium from Helixor Heilmittel GmbH. The other authors have declared that no competing interests exist. No payment was received for any other aspects of the submitted work. There are no patents, products in development, or marketed products to declare. There are no other relationships/conditions/circumstances that present a potential conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Network Oncology was partially funded by Iscador AG, Arlesheim, Switzerland; Abnoba GmbH, Pforzheim, Germany; and Helixor Heilmittel GmbH, Rosenfels, Germany. By contract, researchers were independent from the funder.
ORCID iD: Shiao Li Oei  https://orcid.org/0000-0002-5772-550X
https://orcid.org/0000-0002-5772-550X
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 2. Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Persistence of restrictions in quality of life from the first to the third year after diagnosis in women with breast cancer. J Clin Oncol. 2005;23:4945-4953. [DOI] [PubMed] [Google Scholar]
- 3. Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol. 2000;11:971-975. [DOI] [PubMed] [Google Scholar]
- 5. Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(suppl 1):22-34. [DOI] [PubMed] [Google Scholar]
- 6. Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fabi A, Falcicchio C, Giannarelli D, Maggi G, Cognetti F, Pugliese P. The course of cancer related fatigue up to ten years in early breast cancer patients: what impact in clinical practice? Breast. 2017;34:44-52. [DOI] [PubMed] [Google Scholar]
- 8. Shimozuma K, Okamoto T, Katsumata N, et al. Systematic overview of quality of life studies for breast cancer. Breast Cancer. 2002;9:196-202. [DOI] [PubMed] [Google Scholar]
- 9. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 10. Mortimer JE, Flatt SW, Parker BA, et al. ; WHEL Study Group. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat. 2008;108:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kroz M, Zerm R, Reif M, et al. Validation of the German version of the Cancer Fatigue Scale (CFS-D). Eur J Cancer Care (Engl). 2008;17:33-41. [DOI] [PubMed] [Google Scholar]
- 12. Okuyama T, Akechi T, Kugaya A, et al. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000;19:5-14. [DOI] [PubMed] [Google Scholar]
- 13. Eriksson M, Lindström B. Antonovsky’s sense of coherence scale and its relation with quality of life: a systematic review. J Epidemiol Community Health. 2007;61:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kroz M, Bussing A, von Laue HB, et al. Reliability and validity of a new scale on internal coherence (ICS) of cancer patients. Health Qual Life Outcomes. 2009;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kienle GS, Glockmann A, Schink M, Kiene H. Viscum album L extracts in breast and gynaecological cancers: a systematic review of clinical and preclinical research. J Exp Clin Cancer Res. 2009;28:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kienle GS, Kiene H. Review article: influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr Cancer Ther. 2010;9:142-157. [DOI] [PubMed] [Google Scholar]
- 17. Horneber MA, Bueschel G, Huber R, Linde K, Rostock M. Mistletoe therapy in oncology. Cochrane Database Syst Rev. 2008;(2):CD003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steele ML, Axtner J, Happe A, Kröz M, Matthes H, Schad F. Adverse drug reactions and expected effects to therapy with subcutaneous mistletoe extracts (Viscum album L) in cancer patients. Evid Based Complement Alternat Med. 2014;2014:724258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steele ML, Axtner J, Happe A, Kröz M, Matthes H, Schad F. Use and safety of intratumoral application of European mistletoe (Viscum album L) preparations in oncology. Integr Cancer Ther. 2015;14:140-148. [DOI] [PubMed] [Google Scholar]
- 20. Eisenbraun J, Scheer R, Kröz M, Schad F, Huber R. Quality of life in breast cancer patients during chemotherapy and concurrent therapy with a mistletoe extract. Phytomedicine. 2011;18:151-157. [DOI] [PubMed] [Google Scholar]
- 21. Piao BK, Wang YX, Xie GR, et al. Impact of complementary mistletoe extract treatment on quality of life in breast, ovarian and non-small cell lung cancer patients. A prospective randomized controlled clinical trial. Anticancer Res. 2004;24:303-309. [PubMed] [Google Scholar]
- 22. Semiglasov VF, Stepula VV, Dudov A, Lehmacher W, Mengs U. The standardised mistletoe extract PS76A2 improves QoL in patients with breast cancer receiving adjuvant CMF chemotherapy: a randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2004;24(2C):1293-1302. [PubMed] [Google Scholar]
- 23. Semiglazov VF, Stepula VV, Dudov A, Schnitker J, Mengs U. Quality of life is improved in breast cancer patients by standardised mistletoe extract PS76A2 during chemotherapy and follow-up: a randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2006;26(2B):1519-1529. [PubMed] [Google Scholar]
- 24. Tröger W, Zdrale Z, Tišma N, Matijašević M. Additional therapy with a mistletoe product during adjuvant chemotherapy of breast cancer patients improves quality of life: an open randomized clinical pilot trial. Evid Based Complement Alternat Med. 2014;2014:430518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kokolus KM, Hong CC, Repasky EA. Feeling too hot or cold after breast cancer: is it just a nuisance or a potentially important prognostic factor? Int J Hyperthermia. 2010;26:662-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kröz M, Reif M, Zerm R, et al. Mistletoe and chemotherapy responsiveness of different scales of oncological patients undergoing chemotherapy. In: Scheer R, Alban S, Becker H, et al. , eds. Die Mistel in der Tumortherapie. Vol 3 Essen, Germany: KVC Verlag; 2013:417-437. [Google Scholar]
- 27. Oei SL, Thronicke A, Kroz M, Herbstreit C, Schad F. The internal coherence of breast cancer patients is associated with the decision-making for chemotherapy and Viscum album L treatment. Evid Based Complement Alternat Med. 2018;2018:1065271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oei SL, Thronicke A, Kröz M, Herbstreit C, Schad F. Supportive effect of Viscum album L extracts on the sense of coherence in non-metastasized breast cancer patients. Eur J Integr Med. 2019;27:97-104. [Google Scholar]
- 29. Schad F, Axtner J, Happe A, et al. Network Oncology (NO)—a clinical cancer register for health services research and the evaluation of integrative therapeutic interventions in anthroposophic medicine. Forsch Komplementmed. 2013;20:353-360. [DOI] [PubMed] [Google Scholar]
- 30. Thronicke A, Kröz M, Merkle A, Matthes H, Herbstreit C, Schad F. Psychosocial, cognitive, and physical impact of elaborate consultations and life review in female patients with non-metastasized breast cancer. Complement Med Res. 2018;25:92-101. [DOI] [PubMed] [Google Scholar]
- 31. Schad F, Thronicke A, Merkle A, et al. Implementation of an Integrative oncological concept in the daily care of a German certified breast cancer center. Complement Med Res. 2018;25:85-91. [DOI] [PubMed] [Google Scholar]
- 32. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 33. Scott NW, Fayers PM, Aaronson NK, et al. EORTC-QLQ-C30 reference values. https://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf. Published July 2008. Accessed March 21, 2020.
- 34. Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116:5740-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hurtz HJ, Tesch H, Göhler T, et al. Persistent impairments 3 years after (neo)adjuvant chemotherapy for breast cancer: results from the MaTox project. Breast Cancer Res Treat. 2017;165:721-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al-Azri M, Al-Awisi H, Al-Moundhri M. Coping with a diagnosis of breast cancer-literature review and implications for developing countries. Breast J. 2009;15:615-622. [DOI] [PubMed] [Google Scholar]
- 37. Thewes B, Butow P, Girgis A, Pendlebury S. The psychosocial needs of breast cancer survivors; a qualitative study of the shared and unique needs of younger versus older survivors. Psychooncology. 2004;13:177-189. [DOI] [PubMed] [Google Scholar]
- 38. Fremd C, Hack CC, Schneeweiss A, et al. Use of complementary and integrative medicine among German breast cancer patients: predictors and implications for patient care within the PRAEGNANT study network. Arch Gynecol Obstet. 2017;295:1239-1245. [DOI] [PubMed] [Google Scholar]
- 39. Gross AM, Liu Q, Bauer-Wu S. Prevalence and predictors of complementary therapy use in advanced-stage breast cancer patients. J Oncol Pract. 2007;3:292-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kristoffersen AE, Fønnebo V, Norheim AJ. Do cancer patients with a poor prognosis use complementary and alternative medicine more often than others? J Altern Complement Med. 2009;15:35-40. [DOI] [PubMed] [Google Scholar]
- 41. Kröz M, Reif M, Zerm R, et al. Do we have predictors of therapy responsiveness for a multimodal therapy concept and aerobic training in breast cancer survivors with chronic cancer-related fatigue? Eur J Cancer Care. 2015;24:707-717. [DOI] [PubMed] [Google Scholar]
- 42. Bussing A, Raak C, Ostermann T. Quality of life and related dimensions in cancer patients treated with mistletoe extract (iscador): a meta-analysis. Evid Based Complement Alternat Med. 2012;2012:219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friedel WE, Matthes H, Bock PR, Zänker KS. Systematic evaluation of the clinical effects of supportive mistletoe treatment within chemo- and/or radiotherapy protocols and long-term mistletoe application in nonmetastatic colorectal carcinoma: multicenter, controlled, observational cohort study. J Soc Integr Oncol. 2009;7:137-145. [PubMed] [Google Scholar]
- 44. Pelzer F, Tröger W, Nat DR. Complementary treatment with mistletoe extracts during chemotherapy: safety, neutropenia, fever, and quality of life assessed in a randomized study. J Altern Complement Med. 2018;24:954-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67:194-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501-514. [DOI] [PubMed] [Google Scholar]
- 47. Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(suppl):S48-S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hegde P, Maddur MS, Friboulet A, Bayry J, Kaveri SV. Viscum album exerts anti-inflammatory effect by selectively inhibiting cytokine-induced expression of cyclooxygenase-2. PLoS One. 2011;6:e26312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bock PR, Hanisch J, Matthes H, Zänker KS. Targeting inflammation in cancer-related-fatigue: a rationale for mistletoe therapy as supportive care in colorectal cancer patients. Inflamm Allergy Drug Targets. 2014;13:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]