Abstract
Objective
To clarify the efficacy of functional magnetic stimulation (FMS) in improving hemiplegic upper extremity function in patients with sub-acute stroke.
Methods
In this randomized controlled trial, 40 sub-acute stroke patients with hemiplegia were recruited from inpatient wards in the Department of Rehabilitation and randomly assigned to two groups. In the FMS group, magnetic stimulation was applied to extensor muscle groups of the affected upper extremity. In the low-frequency repetitive transcranial magnetic stimulation (LF-rTMS) group, stimulation was applied to the contralesional primary motor cortex. All patients received occupational therapy. Hand and upper extremity motor function was evaluated using the Fugl–Meyer Assessment for upper extremity (FMA-UE), and the Barthel Index (BI) evaluated daily living abilities.
Results
The FMA-UE and BI scores were significantly increased in both groups following stimulation. Furthermore, a significant between-group difference was observed in both FMA-UE and BI scores after 2 weeks of therapy. In the FMS group, 6 of 19 patients regained wrist and finger extension abilities, but only 2 patients regained equivalent motor skills in the LF-rTMS group
Conclusions
FMS improves paretic upper extremity function and leads to better recovery of motor activity than LF-rTMS. FMS may be a novel modality to improve motor function.
Keywords: Functional magnetic stimulation, repetitive transcranial magnetic stimulation, hemiplegia, upper extremity, randomized controlled trial, Fugl–Meyer Assessment, Barthel Index
Introduction
Stroke survivors often have varying degrees of motor function impairment, especially in the upper extremities, which can lead to long-term disability and poor quality of life.1 Several methods have been proposed for the rehabilitation of hemiplegic upper extremities;2–4 however, it remains the most common deficit following a stroke.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation technique in which magnetic stimulation is delivered by a coil over the scalp, to generate an electric field within the brain.5 Low-frequency rTMS (LF-rTMS, ≤1 Hz) suppresses cortical excitability, while high-frequency rTMS (HF-rTMS, ≥5 Hz) enhances cortical excitability.6 Both types of rTMS are reportedly effective for improving upper extremity function after stroke,7,8 although a meta-analysis revealed that LF-rTMS may be more effective than HF-rTMS for regaining motor function.9 However, contraindications such as seizures and metal implants limit the application of rTMS, especially in stroke patients.10 Thus, studies have recently begun to develop novel modalities of magnetic stimulation. It has been reported that, when delivered peripherally, magnetic stimulation can generate electrical stimulation of selected nerves or muscles; this technique is usually known as functional magnetic stimulation (FMS).11 Although it is similar to functional electrical stimulation, FMS has a deeper reach and generates less pain.11 Reports have demonstrated the beneficial role of FMS in improving swallowing and respiratory functions after stroke,12,13 but to our knowledge, no study has yet investigated its role in motor dysfunction. Therefore, this study aimed to explore the efficacy of FMS on upper extremity function in stroke patients.
Methods
Participants
We enrolled first-time stroke patients with upper extremity dysfunction who were admitted to the Department of Rehabilitation Medicine of The First Hospital of Jilin University between 4 July 2017 and 29 December 2017. All patients met the following inclusion criteria: 1) sub-acute stroke patients (cerebral infarction) with upper extremity hemiparesis; 2) 18 to 65 years old; and 3) able to follow the therapist’s instructions. The exclusion criteria were as follows: 1) cerebral hemorrhage; 2) a history of epilepsy or recent administration of anti-epileptic drugs; 3) medically unstable, such as a severe cardiopulmonary situation, severe liver and kidney dysfunction, or malignant tumor; 4) severe cognitive dysfunction or aphasia with mental illness; 5) severe visual and auditory problems; 6) electronic and/or magnetic implants; and 7) spasticity of the affected upper extremity, with Modified Ashworth Scale > 2. Basic patient information was collected, including sex, height, weight, handedness, and stroke characteristics (side and location).
This project is registered with chictr.org.cn (ChiCTR1800019757). The study protocol was reviewed and approved by the local ethics review committee of the First Hospital of Jilin University (protocol no. 2107-343). All of the patients signed written informed consent for this study. The therapy procedure did not harm the patients and they could stop at any time if they felt uncomfortable.
Experimental model
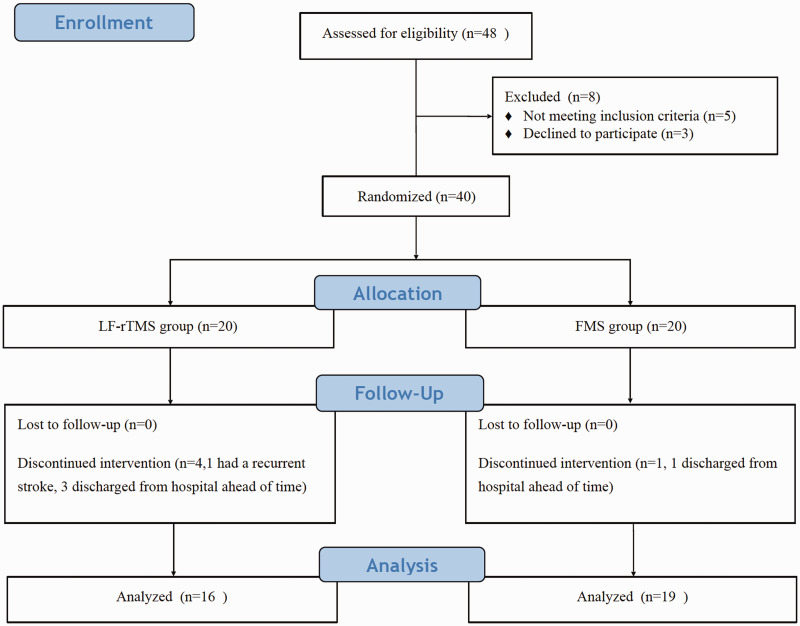
Patients were randomly assigned to either the FMS or the LF-rTMS group. Randomization was performed using a random number table by a researcher blinded to patient details. The researcher who enrolled the patients and the occupational therapist and doctor who evaluated the patients were unaware of the randomization results. The trial flow diagram is shown in Figure 1. Clinical features and demographic data were collected. During hospitalization, patients received occupational therapy after FMS or LF-rTMS; each patient received one session per day, with a total of 10 sessions over 2 weeks.
Figure 1.
Flow diagram of the study design. LF-rTMS, low-frequency repetitive transcranial magnetic stimulation; FMS, functional magnetic stimulation.
Application of FMS and LF-rTMS
For the FMS group, stimulation was delivered via a parabolic coil (MMC-140 coil, Magpro R30 stimulator, Magventure, Farum, Denmark), at a frequency of 30 Hz, and with an intensity of approximately 20% to 40% of its maximal output without generating local pain. The extensor muscles of the upper extremity and shoulder muscles were sequentially stimulated while patients simultaneously contracted; this was repeated five times for each site. Occupational therapy was conducted immediately after FMS. For the LF-rTMS group, stimulation was delivered via a slightly bent butterfly coil (MCF-B70 Butterfly Coil, Magventure) on the contralesional M1 area, at 45° to the sagittal direction, at an intensity of 90% of the resting motor threshold (RMT), with 1 Hz frequency and 1,500 pulses in total. The RMT was evaluated before therapy using a previously reported method.14 Briefly, the electromyography signal was monitored using disposable Ag/AgCl electrodes. The active electrode was attached to the skin overlying the contralesional abductor pollicis brevis, while the reference electrode was placed over the adjacent joint. The RMT was determined as the minimum TMS intensity that generated at least five motor evoked potentials of ≥50 µV peak-to-peak amplitude per 10 consecutive stimuli.
After magnetic stimulation, all patients immediately received occupational therapy, which included muscle stretching, muscle strengthening exercises, and passive and active upper extremity movements. These movements included shoulder flexion, extension, and rotation; elbow extension and flexion; wrist extension, flexion, supination, and pronation; and thumb flexion. A repetitive task-oriented training approach was taken, using movements such as reaching and weight bearing, repetitive hand motor skills, and activities related to daily living (ADL). All therapies were performed by two occupational therapists who were trained to perform the same occupational procedures.
To evaluate improvements in upper extremity function and ADL performance, respectively, the Fugl–Meyer Assessment for upper extremity (FMA-UE)15 and the Barthel Index (BI)16 were performed pre- and post-therapy. An experienced physician performed all evaluations in a blinded manner.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Data are presented as the mean (standard deviation [SD]) if they showed a normal distribution. The χ2 test and independent t-test were used to compare baseline characteristics. Paired t-tests were used for pre- and post-therapy data comparisons within groups. To investigate changes between groups, an independent samples t-test was used. A P-value of less than 0.05 was considered to be statistically significant.
Results
The patients’ clinical characteristics are displayed in Table 1. There were no significant differences in clinical and demographical characteristics between the two groups. The patients who completed this study were all right-handed, and lesion areas included the primary motor cortex, premotor cortex, supplementary motor cortex, basal ganglia, and brainstem. All patients underwent anticoagulant therapy and supportive care before admission to the Department of Rehabilitation. Of the 40 enrolled patients, one patient in the FMS group and four patients in the LF-rTMS group were excluded because they failed to complete the magnetic stimulation sessions (Figure 1). No adverse effects of therapy were reported.
Table 1.
Demographic features of the groups.
| FMS (n = 19) | LF-rTMS (n = 16) | P | |
|---|---|---|---|
| Sex (male:female) | 14:5 | 13:3 | 0.700 |
| Age (years), mean (SD) | 52.56 (11.65) | 48.68 (14.11) | 0.387 |
| Height (m), mean (SD) | 1.74 (0.07) | 1.73 (0.07) | 0.69 |
| Weight (kg), mean (SD) | 72.38 (6.95) | 71.05 (9.68) | 0.64 |
| BMI (kg/m2), mean (SD) | 23.88 (1.61) | 23.66 (2.43) | 0.76 |
| Side of stroke (left:right) | 9:10 | 9:7 | 0.738 |
| FMA-UE, mean (SD) | 16.21 (14.54) | 15.75 (12.73) | 0.922 |
| BI, mean (SD) | 38.42 (22.43) | 34.06 (19.08) | 0.544 |
FMS, functional magnetic stimulation; LF-rTMS, low-frequency repetitive transcranial magnetic stimulation; SD, standard deviation; BMI, body mass index; FMA-UE, Fugl–Meyer Assessment for upper extremity; BI, Barthel Index.
There were no significant differences in FMA-UE or BI scores between the two groups before therapy. After 10 sessions of therapy, there were significant improvements in both FMA-UE and BI scores in both groups compared with the baseline values (P < 0.05, Table 2). Furthermore, post-therapy FMA-UE scores were significantly higher in the FMS group than in the LF-rTMS group (P < 0.05, Table 2). Similarly, the post-therapy BI scores were significantly higher in the FMS group than in the LF-rTMS group (P < 0.05, Table 2). In the FMS group, 6 of 19 patients regained some motor activity in the upper extremity, such as wrist extension and finger extension. In the LF-rTMS group, only 2 of 16 patients regained the equivalent motor skills.
Table 2.
Evaluation of upper limb function after therapy.
| FMS group |
LF-rTMS group |
Between groups |
|||||
|---|---|---|---|---|---|---|---|
| Pre-therapy, mean (SD) | Post-therapy, mean (SD) | P | Pre-therapy, mean (SD) | Post-therapy, mean (SD) | P | P | |
| FMA-UE | 16.21 (14.54) | 31.42 (13.49) | 0.002 | 15.75 (12.73) | 26.25 (12.90) | 0.027 | 0.003 |
| BI | 38.42 (22.43) | 58.16 (19.66) | 0.007 | 34.06 (19.08) | 48.75 (19.62) | 0.040 | 0.011 |
FMS, functional magnetic stimulation; LF-rTMS, low-frequency repetitive transcranial magnetic stimulation; SD, standard deviation; FMA-UE, Fugl-Meyer Assessment for upper extremity; BI, Barthel Index.
Discussion
The results of this study suggest that both FMS and LF-rTMS increase function in the paralyzed upper extremity and improve ADL performance in sub-acute stroke patients. LF-rTMS has previously been reported to have a beneficial effect on upper limb function in stroke patients.17,18 Here, we aimed to investigate a new modality of non-invasive magnetic stimulation, so we used LF-rTMS as a control rather than using sham stimulation; thus, all patients received treatment during the ‘golden period’ of therapy.
Compared with the LF-rTMS group, patients in the FMS group gained better motor function and performance, as measured by FMA-UE scores, and greater improvements in ADL, as evaluated by BI scores. The underlying mechanisms for these improvements remain unclear. It has been reported that peripheral stimuli preferentially activate the proprioceptive afferents that carry information from mechanoreceptors, as well as muscle spindles and deep connective tissue sensors; these in turn may enhance M1 excitability.19 Kumru et al.20 demonstrated that peripheral magnetic stimulation of the extensor carpi radialis, followed by 1 Hz rTMS on the M1 area, increased corticospinal excitability and decreased intracortical inhibition. Furthermore, peripheral magnetic stimulation has also been revealed to increase sensory function in paretic limbs, which may also have a positive impact on motor recovery.21,22 Therefore, magnetic stimulation over muscles or nerves may help motor recovery by facilitating plastic changes in the M1 area, as well as by providing sensory input. In the present study, six patients in the FMS group reported that they were able to extend their wrist and fingers following treatment, which they had been unable to do before magnetic stimulation; in contrast, only two patients reported this improvement in the LF-rTMS group. This result suggests that FMS may be more beneficial for regaining motor performance in post-stroke patients.
FMS has been extensively used to treat urodynamic stress incontinence, constipation, and pain.23–25 One previous study reported that FMS improved dysphagia in stroke patients.12 In addition, by conditioning the inspiratory and expiratory muscles, FMS has also been reported to improve voluntary respiratory functions.13 However, no studies have previously reported the impact of FMS on motor function in stroke. In the current study, FMS was effective in improving paretic upper extremity function. As well as the possible mechanisms mentioned in the previous paragraph, active muscle contractions may enhance the efficiency of proprioceptive afferent input transmission to the cortex.26 We thus speculate that FMS during active muscle contractions may enhance synaptic connectivity in the proprioceptive projections, which leads to better motor performance in stroke patients. In addition, neuromodulation over direct nerve stimulation may also improve these effects. However, further studies are needed to demonstrate the underlying mechanisms.
The present study has some limitations. Further studies should include a larger sample size, and should also have longer follow-up periods to observe how long the beneficial effects may last. Moreover, the lower extremities were not included in the current study, and should be considered in future studies. However, even with these limitations, we were able to observe the promising effect of FMS on motor function.
rTMS has many contraindications, including epilepsy and the presence of metal implants, which are very common in patients after stroke and traumatic brain injury. These contraindications greatly narrow the application of this non-invasive magnetic stimulation treatment. FMS, however, is expected to have wider applications for brain-injured patients. Future studies also need to investigate the optimal frequency and stimulating times to achieve the best therapeutic effects.
Acknowledgements
The authors thank Matthias Kienle for technical support and help throughout this study.
Author contributions
Xiaowei Chen, Xuncan Liu, and Zhenlan Li contributed to the design and monitoring of the project. Xiaowei Chen wrote the manuscript. Xuncan Liu enrolled and managed the patients and decided on the analytical strategy. Yinxing Cui performed all evaluations in both groups. Guoxing Xu was responsible for the FMS and LF-rTMS delivery. Lu Liu carried out the occupational therapy. Xueru Zhang and Kun Jiang collected all data and performed the statistical analyses. All authors discussed the results and contributed to the final manuscript.
Clinical message
FMS can improve paretic upper extremity function and allow patients to regain hand function more efficiently than LF-rTMS.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the Changchun Science and Technology Bureau (grant no. 17YJ016).
ORCID iD
Zhenlan Li https://orcid.org/0000-0002-3177-1073
References
- 1.Raghavan P. Upper limb motor impairment after stroke. Phys Med Rehabil Clin N Am 2015; 26: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatem SM, Saussez G, Della Faille M, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci 2016; 10: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paik YR, Lee JH, Lee DH, et al. Effect of mirror therapy and electrical stimulation on upper extremity function in stroke with hemiplegic patient: a pilot study. J Phys Ther Sci 2017; 29: 2085–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guang H, Ji L, Shi Y, et al. Dynamic modeling and interactive performance of PARM: a parallel upper-limb rehabilitation robot using impedance control for patients after stroke. J Healthc Eng 2018; 2018: 8647591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology 2007; 68: 484–488. [DOI] [PubMed] [Google Scholar]
- 6.Dayan E, Censor N, Buch ER, et al. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci 2013; 16: 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludemann-Podubecka J, Bosl K, Theilig S, et al. The effectiveness of 1 Hz rTMS over the primary motor area of the unaffected hemisphere to improve hand function after stroke depends on hemispheric dominance. Brain Stimul 2015; 8: 823–830. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Yim J. Effects of high-frequency repetitive transcranial magnetic stimulation combined with task-oriented mirror therapy training on hand rehabilitation of acute strokepPatients. Med Sci Monit 2018; 24: 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu WY, Cheng CH, Liao KK, et al. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke 2012; 43: 1849–1857. [DOI] [PubMed] [Google Scholar]
- 10.Lomarev MP, Kim DY, Richardson SP, et al. Safety study of high-frequency transcranial magnetic stimulation in patients with chronic stroke. Clin Neurophysiol 2007; 118: 2072–2075. [DOI] [PubMed] [Google Scholar]
- 11.Szecsi J, Schiller M, Straube A, et al. A comparison of functional electrical and magnetic stimulation for propelled cycling of paretic patients. Arch Phys Med Rehabil 2009; 90: 564–570. [DOI] [PubMed] [Google Scholar]
- 12.Momosaki R, Abo M, Watanabe S, et al. Functional magnetic stimulation using a parabolic coil for dysphagia after stroke. Neuromodulation 2014; 17: 637–641; discussion 41. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Plow E, Ranganthan V, et al. Functional magnetic stimulation of inspiratory and expiratory muscles in subjects with tetraplegia. PM R 2016; 8: 651–659. [DOI] [PubMed] [Google Scholar]
- 14.Bashir S, Edwards D, Pascual-Leone A. Neuronavigation increases the physiologic and behavioral effects of low-frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr 2011; 24: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31. [PubMed] [Google Scholar]
- 16.Wade DT, Skilbeck CE, Hewer RL. Predicting Barthel ADL score at 6 months after an acute stroke. Arch Phys Med Rehabil 1983; 64: 24–28. [PubMed] [Google Scholar]
- 17.Askin A, Tosun A, Demirdal US. Effects of low-frequency repetitive transcranial magnetic stimulation on upper extremity motor recovery and functional outcomes in chronic stroke patients: a randomized controlled trial. Somatosens Mot Res 2017; 34: 102–107. [DOI] [PubMed] [Google Scholar]
- 18.Kondo T, Yamada N, Momosaki R, et al. Comparison of the effect of low-frequency repetitive transcranial magnetic stimulation with that of theta burst stimulation on upper limb motor function in poststroke patients. Biomed Res Int 2017; 2017: 4269435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litvak V, Zeller D, Oostenveld R, et al. LTP-like changes induced by paired associative stimulation of the primary somatosensory cortex in humans: source analysis and associated changes in behaviour. Eur J Neurosci 2007; 25: 2862–2874. [DOI] [PubMed] [Google Scholar]
- 20.Kumru H, Albu S, Rothwell J, et al. Modulation of motor cortex excitability by paired peripheral and transcranial magnetic stimulation. Clin Neurophysiol 2017; 128: 2043–2047. [DOI] [PubMed] [Google Scholar]
- 21.Krewer C, Hartl S, Muller F, et al. Effects of repetitive peripheral magnetic stimulation on upper-limb spasticity and impairment in patients with spastic hemiparesis: a randomized, double-blind, sham-controlled study. Arch Phys Med Rehabil 2014; 95: 1039–1047. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Liu F, Yan Z, et al. Therapeutic effects of sensory input training on motor function rehabilitation after stroke. Medicine (Baltimore) 2018; 97: e13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim YH, Song JM, Choi EH, et al. Effects of repetitive peripheral magnetic stimulation on patients with acute low back pain: a pilot study. Ann Rehabil Med 2018; 42: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanishi T, Suzuki T, Sato R, et al. Effects of magnetic stimulation on urodynamic stress incontinence refractory to pelvic floor muscle training in a randomized sham-controlled study. Low Urin Tract Symptoms 2019; 11: 61–65. [DOI] [PubMed] [Google Scholar]
- 25.Yun YC, Yoon YS, Kim ES, et al. Transabdominal functional magnetic stimulation for the treatment of constipation in brain-injured patients: a randomized controlled trial. Ann Rehabil Med 2019; 43: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunesch E, Knecht S, Schnitzler A, et al. Somatosensory evoked potentials elicited by intraneural microstimulation of afferent nerve fibers. J Clin Neurophysiol 1995; 12: 476–487. [DOI] [PubMed] [Google Scholar]