Abstract
Objective:
Due to physiological and metabolic immaturity, prematurely born infants are at increased risk because of maternal separation in many neonatal intensive care units (NICUs). The stress induced from maternal–infant separation can lead to well-documented short-term physiologic instability and potentially lifelong neurological, sociological, or psychological sequelae. Based on previous studies of kangaroo mother care (KMC) that demonstrated improvement in physiologic parameters, we examined the impact of KMC on physiologic measures of stress (abdominal temperature, heart rate, oxygen saturation, perfusion index, near-infrared spectrometry), oxidative stress, and energy utilization/conservation in preterm infants.
Methods:
In this randomized, stratified study of premature neonates, we compared the effects on urinary concentrations of biomarkers of energy utilization and oxidative stress of 1 hr of KMC versus incubator care on Day 3 of life in intervention-group babies (n = 26) and control-group babies (n = 25), respectively. On Day 4, both groups received 1 hr of KMC. Urinary samples were collected 3 hr before and 3 hr after intervention/incubator care on both days. Energy utilization was assessed by measures of adenosine triphosphate (ATP) degradation (i.e., hypoxanthine, xanthine, and uric acid). Oxidative stress was assessed using urinary allantoin. Mixed-models analysis was used to assess differences in purine/allantoin.
Results:
Mean allantoin levels over Days 3 and 4 were significantly lower in the KMC group than in the control group (p = .026).
Conclusions:
Results provide preliminary evidence that KMC reduces neonatal oxidative stress processes and that urinary allantoin could serve as an effective noninvasive marker for future studies.
Keywords: kangaroo mother care, biochemical marker, hypoxanthine, xanthine, uric acid, allantoin
Premature infants admitted to the neonatal intensive care unit (NICU) are born with physiologic and metabolic immaturity (Blass, 2015). These infants are at increased risk, at least in part, because they are separated from their mothers in most NICUs and experiencing the negative consequences of such early maternal separation (Craig et al., 2016; Sutton & Darmstadt, 2013). Further, these infants are vulnerable to a variety of internal and external events that increase energy losses (Blass, 2015; Tan et al., 2018). Findings from a review of kangaroo mother care (KMC), in which a parent holds an infant skin-to-skin on their bare chest for extended periods of time, suggest that KMC reduces morbidity and mortality among premature infants and is associated with improved neonatal physiologic stability and decreased stress levels, as evidenced by lower infant cortisol levels (Chi Luong et al., 2016; Johnson, 2013; Pados, 2019).
The mechanisms by which KMC seems to be associated with energy preservation include stabilizing physiologic parameters and maximizing energy-conserving physiologic and behavioral effectors mediated by both peripheral and central processes (Blass, 2015). However, the specific physiologic processes that mediate this energy conservation have not been adequately investigated. In the present study, we attempted to link biochemical markers to the theorized physiologic effects of KMC on stress responses. We quantified energy utilization in infants by examining urinary purines as biochemical markers of adenosine triphosphate (ATP) degradation (i.e., hypoxanthine [Hx], xanthine [Xa], uric acid [UA]) and oxidative stress (i.e., allantoin) (Angeles et al., 2015; Plank et al., 2011). We used a prospective, stratified, randomized controlled design to test the hypothesis that exposure to KMC would significantly alter physiologic measures of stress and biochemical markers of ATP utilization and oxidative stress among preterm neonates.
The morbidities and associated stress that preterm infants experience can be partially explained by an oxygen supply that cannot meet the body’s demands. This imbalance results in a switch from aerobic to anaerobic metabolism in which ATP is broken down to generate energy (Plank et al., 2011). The degradation of ATP results in elevated levels of adenosine monophosphate and free adenosine, which are subsequently catabolized into inosine monophosphate and inosine, respectively. Both inosine monophosphate and inosine are then converted into Hx, then to Xa, and finally to UA. UA has the ability to act as an antioxidant by scavenging reactive oxygen species, which converts the compound mainly to allantoin, a marker of oxidative stress in humans (Kand’ár et al., 2006). Interventions to reduce anaerobic metabolism and oxidative stress, such as KMC, may partially blunt the occurrence and severity of morbidities in preterm infants (Mörelius et al., 2016). Thus, we chose biomarkers involved in the degradation of ATP and oxidative stress to examine the effects of KMC on infants’ stress response.
In one study of 82 late-preterm infants in which researchers collected data on Days of life 3 and 6 (Holden et al., 2014), the measure of choice of ATP utilization was urinary Hx. Investigators assigned infants to one of the four groups based on a number of clinical characteristics: poor nippling only (n = 8), hyperbilirubinemia plus poor nippling (n = 21), early respiratory disease and poor nippling (n = 26), and only respiratory disease (n = 27). The infants diagnosed with respiratory disease alone had significantly higher urinary Hx concentrations than the other infants, which suggests that specific clinical conditions can change ATP metabolism and increase purine breakdown. Findings from that study demonstrate that Hx is stable in urine and can be used to evaluate ATP metabolism in preterm infants. It is well-documented that preterm infants have lower energy stores and higher energy demands compared to full-term infants. If purine biochemical markers are reliable and stable measures of ATP utilization, then purines should be reliable indicators of energy conservation in preterm neonates.
In terms of the theoretical rationale for the use of KMC, Mefford (2004) developed a new middle-range theory of health promotion for preterm infants that gives impetus to the study of energy conservation. This theory outlines principles that can be used to guide nursing interventions that address the unique challenges that preterm infants face during their adaptation to extrauterine life. Accordingly, nursing interventions should be centered on keeping the mother–infant dyad together as much as possible as a single unit that is complemented by participation from extended family members.
Some of the expected direct effects of KMC are stabilization of breathing, improved oxygen saturation and heart rate, and improved breastfeeding in infants along with increased milk production in the mother (Bergman & Bergman, 2013). Although observational studies have documented benefits to both mother and infant (Bergman et al., 2004; Chi Luong et al., 2016; Feldman et al., 2014; Moore et al., 2012; Nyqvist et al., 2010), the neurobiological mechanisms that underlie the effects of KMC remain unclear. One hypothesis for the efficacy of KMC is that the direct skin-to-skin contact connects the sensory nerve pathways of the mother and infant. The physiological effects associated with skin-to-skin contact, described above, suggest that energy is synthesized and conserved in order to achieve physiologic stability. In one study that used proxy indicators of energy expenditure (i.e., heart rate, activity level, and behavioral state) to examine energy conservation in preterm infants who received KMC (Ludington, 1990), all of the indicators showed improvements. However, whether these indicators translate into physiologic measures of energy conservation remains unknown. Many causes of morbidities in preterm infants are linked to increased metabolic needs. The present study is the first to evaluate the effects of KMC on physiologic markers of energy conservation (i.e., Hx, Xa, UA) and oxidative stress (i.e., allantoin).
Method
Patients and Settings
We collected data in an 84-bed, Level IV NICU at Loma Linda University Children’s Hospital, a large regional children’s hospital in Southern California. The NICU cares for both inborn and outborn infants, with an admission rate of over 1,200 neonates per year. The institutional review boards at the Loma Linda University and the University of San Diego approved this study.
Mothers with premature infants who were born at the facility and admitted to the NICU for care were eligible to participate in the present study. Eligible mothers had infants who were born prematurely between 24 and 36 weeks’ gestation, were less than 1 day of age, and were deemed medically stable as determined by a Score for Neonatal Acute Physiology Perinatal Extension (SNAPPE-II) of <9 (Harsha & Archana, 2015; Richardson et al., 2001). Mothers were excluded if their infants required surgery; had intraventricular hemorrhage (IVH) Grade 3 or 4; were receiving medications such as morphine sulfate, fentanyl, midazolam, muscle relaxants, phenobarbital, or phenytoin; or had renal injuries (plasma creatinine > 1 mg/dl), severe cyanotic heart disease, severe respiratory distress, known abdominal wall or intestinal anomalies or injuries, chromosomal anomalies, or facial anomalies.
Sample Size
During planning, we calculated the power necessary to detect differences in purine levels among premature infants with a moderate effect size of 0.35. The calculations assumed an outcome with a Type I error rate (α) of 0.05, a Type II error rate (β) of 0.20 (power of 0.80), and a two-tailed repeated measures analysis of variance (ANOVA) statistical test. The power analysis determined that a sample size of 50 mother–infant dyads, randomized with 25 each in the control and intervention groups based on birth weight, would meet these requirements.
Study Recruitment
We approached mothers of preterm infants who met the inclusion criteria and had none of the exclusion criteria for informed consent soon after the birth. Once mothers provided their consent and their infants were deemed medically stable, we randomized the dyads into the intervention or control group using permuted blocks randomly ordered and stratified by birth weight into small (≤1,000 g) or large (>1,000 g) categories. A researcher who was not part of the team placed assignments to the intervention or control condition in opaque, numbered, and sealed envelopes. After the baby’s mother consented to participate in the study, the hospital identification sticker was placed on the envelope which would then be opened by the researcher.
Intervention Protocol
The primary investigator (PI) organized the KMC intervention for all of the participants. The PI followed existing unit policy guidelines for transferring the infant from the incubator to the mother’s chest, positioning the infant, assessing tolerance, and recording information specific to the research project. The infants in the intervention group were given 1 hr of KMC on Day 3 of life. The infants in the control group had only incubator care on Day 3. Both groups had 1 hr of KMC on Day 4 of life. Dependent physiologic variables of heart rate, respiratory rate, oxygen saturation, abdominal near infrared spectroscopy (NIRS), perfusion index, fraction of inspired oxygen (FiO2), ventilator setting, and abdominal temperature were monitored both via the bedside monitors and via manual recordings by the PI. These data were collected on Days 3 and 4 in both the intervention and control groups for a period of 1 hr beginning from baseline at the intervention start and noted every 15 min until the intervention ended (i.e., T1 = baseline, T2 = baseline + 15 min, T3 = baseline + 30 min, T4 = baseline + 45 min, T5 = baseline + 60 min). We collected the control data for Day 3 in an analogous manner while the babies were in the incubator.
Outcome Measures
We collected data on birth weight, gender, gestational age at birth, gestational age at time of sampling, severity of illness, medications, heart rate, oxygen saturation, perfusion index, abdominal temperature, and NIRS measurement of abdominal tissue saturation during the hour of KMC intervention or incubator care. We quantified current illness severity using the SNAPPE-II tool, a risk-adjustment instrument with established validity and reliability (Harsha & Archana, 2015; Richardson et al., 2001).
NIRS
We measured splanchnic tissue oxygen saturation using the Fore-Sight II NIRS tissue oximeter monitor. The NIRS probe (Casmed, Fore-Sight II, Branford, CT) was placed on the lower left abdominal quadrant immediately before KMC with mother or during incubator care and was discontinued after the intervention or, in the case of the control group on Day 3, after 1 hr of monitoring (Akotia et al., 2016).
Abdominal temperature
We measured abdominal temperatures using an incubator skin temperature probe, which was placed on the abdomen 3 hr before the study began (Knobel-Dail et al., 2017).
Neonate urine collection
We instructed bedside nurses caring for the infants on the urine collection procedure at the start of Day 3 of life. Nurses collected urine in 3-hr aliquots before and after KMC by placing cotton balls over the opening of the infant’s urethra, as described by Holden et al. (2014). They removed the urine-soaked cotton balls at the diaper change and placed them in a special, labeled, iced container located bedside, where they were stored at 0°C. We placed urine-soaked cotton balls in a syringe, 6–12 hr after collection and used pressure to extract the urine. Urine was aliquoted into Eppendorf tubes and centrifuged for 30 min at 18,000 g, 4°C. The supernatant was transferred to syringes and filtered through a Millex syringe-driven filter (low-protein-binding Durapore PVD filter, 0.45 µm, 13 mm, Millipore Corp.). The filtrate was transferred to new Eppendorf tubes and stored at −80°C until analysis at 4–6 months after collection.
Neonatal purine quantification
We measured urinary Hx, Xa, UA, and creatinine concentrations using the adapted high-performance liquid chromatography (HPLC) method described by Holden et al. (2014). Urine samples were thawed and sonicated before 200 μl was transferred to an Eppendorf tube containing 1 × 10−7 mol of 2-aminopurine (internal standard). Samples were analyzed on a UHPLC system (Dionex Ultimate 3000 RS System) by injecting 35 μl onto a SUPELCOSIL LC-18-S 15 cm × 4.6 mm, 5 µm column (SGE, Austin, TX) with the following isocratic conditions: 10 mM potassium dihydrogen phosphate buffer, pH 4.7, flow rate 1.0 ml/min. Creatinine, Hx, Xa, UA, and 2-aminopurine were quantitated by obtaining peak areas at the appropriate retention times (∼3.5, 8, 9, 5, and 13.5 min, respectively) and wavelengths (230, 248, 267, 288, and 305 nm, respectively). The area ratios of each compound to 2-aminopurine were determined and converted into concentrations using standard curves. Samples were analyzed in triplicate, and we included values with coefficients of variation of <10% in the final analysis. The limits of detection were creatinine = 3.2 μM, Hx = 1.58 μM, Xa = 1.32 μM, and UA = 5.0 μM.
Neonatal allantoin quantification
Allantoin measurements in urine samples were performed at OpAns, LLC, using an adaptation of the methods published by Tolun et al. (2010). Dr. Millington’s lab kindly provided the allantoin standard, labeled allantoin internal standard, blank synthetic urine, and pooled patient quality control (QC) sample to OpAns. Specifically, the adaptations included the liquid chromatography with tandem mass spectrometry (LC/MS/MS) system, using an Agilent 1260 binary HPLC pump with a thermostated column compartment, a CTC HTS PAL autosampler, and an Agilent 6410A triple quadrupole mass spectrometer. Additionally, a Waters Atlantis HILIC (3 µm, 2.1 × 50mm) HPLC column was used. The calibration range was 2–500 μM with prepared QCs at 3, 30, and 300 μM. The pooled patient QC at 153 μM provided by Dr. Millington was also included. The accuracy range for all calibration, QC, and patient QC sample injections was 92–109%, 94–106%, and 92–104%, respectively.
Statistical Analysis
We performed all statistical analyses using the Statistical Package for the Social Sciences (SPSS) software (Version 25.0, IBM Corp.). Categorical variables are presented with the number and percentage. We compared quantitative variables between the two groups using t tests when assumptions of parametric tests were met or the Mann–Whitney U test when extreme outliers were present. To assess the association of categorical variables between the two groups, we used a χ2 test. When assumptions of χ2 were not met, we used Fisher’s exact test. In order to analyze the associations between KMC and biochemical markers of ATP degradation and oxidative stress in the urine of preterm infants while accounting for missing data, we used a mixed-model ANOVA with an unstructured covariance matrix. We adjusted for multiple comparisons using the Bonferroni method to assess changes and account for missing data in the outcome measures.
Results
Study Cohort
Participant enrollment occurred from August 2017 to January 2018. We recruited 61 mother–infant dyads, 5 of the mothers declined consent, and 5 later withdrew because they became ill or changed their mind. We randomized 51 dyads to either the KMC intervention group or the modified standard of care (control) group, stratified by weight of ≤1,000 g or >1,000 g (see Figure 1).
Figure 1.
Enrollment flowchart.
Sample Demographics
A total of 25 infants were in the control group and 26 in the KMC intervention group. Randomization successfully created two treatment groups (intervention and control) with no significant differences in maternal age, gravidity, parity, gestational age at birth, corrected gestational age, or gestational age at intervention (Table 1).
Table 1.
Characteristics of Study Participants by Study Group.
| Subject Characteristics | KMC | Control | p Value |
|---|---|---|---|
| Maternal age (years)a | 30.1 ± 4.9 | 28.1 ± 6.9 | .245 |
| Graviditya | 3.7 ± 2.4 | 3.2 ± 2.7 | .454 |
| Paritya | 2.4 ± 1.8 | 1.7 ± 1.4 | .125 |
| Gestation at birth (weeks)a | 32.0 ± 2.6 | 31.4 ± 2.1 | .405 |
| Gestation corrected (weeks)a | 31.8 ± 2.5 | 31.3 ± 2.1 | .430 |
| Gestation at sampling (weeks)a | 31.8 ± 2.5 | 31.4 ± 2.1 | .559 |
| Birth weighta (g) | 1827 ± 492 | 1642 ± 545 | .210 |
| 1-min Apgara | 6.5 ± 2.2 | 6.6 ± 2.5 | .647 |
| 5-min Apgara | 8.2 ± 1.0 | 7.8 ± 1.4 | .260 |
| SNAPPE II scores on day of samplingb | 0.0 (0–49) | 0.0 (0–49) | .675 |
| Infant gender, malec | 10 (38.5) | 16 (64.0) | .068 |
| Ethnicityc | .027* | ||
| White non-Hispanic | 15 (57.7) | 5 (20.0) | |
| Black non-Hispanic | 6 (23.1) | 9 (36.0) | |
| Hispanic/Mexican | 5 (19.2) | 8 (32.0) | |
| Others | 0 (0) | 3 (12.0) | |
| Mode of birthc | .681 | ||
| Vaginal | 11 (44.0) | 7 (30.4) | |
| Planned CS | 13 (52.0) | 15 (65.2) | |
| Emergency CS | 1 (4.0) | 1 (4.3) | |
| Mode of oxygen delivery | .341 | ||
| Spontaneous RA | 17 (65.4) | 11 (45.8) | |
| Nasal cannula/HFNC | 5 (19.2) | 4 (16.7) | |
| NCPAP | 4 (15.4) | 6 (25.0) | |
| NIPPV | 0 (0) | 1 (4.2) | |
| SIMV | 0 (0) | 2 (8.3) |
Note. CS = cesarean; HFNC = high-flow nasal cannula; KMC = kangaroo mother care; NCPAP = nasal continuous positive airway pressure; NIPPV = nasal intermittent positive pressure ventilation; RA = room air; SIMV = synchronized intermittent mechanical ventilation.
a Values represent mean ± SD. bValues represent median (min–max). cValues represent n (%).
* Significant at an α of .05.
Similarly, birth weight and procedure weight between groups were homogenous. SNAPPE-II gathered as a measure of illness severity in the first 12–24 hr and at the commencement of the study yielded parallel results in both groups. APGAR (i.e., Appearance, Pulse, Grimace, Activity, and Respiration) scores at 1 and 5 min in both groups were similar. In addition, birth mode was similar between the two groups, with cesarean delivery highest in each. Oxygen delivery mode for both groups was similar, with most infants maintained on room air or very minimal respiratory support (Table 1).
However, we did find a statistically significant difference in the ethnic distribution between the two groups (p = .027), which occurred by random chance. Specifically, there were 15 White mothers in the intervention group and 5 in the control group. Conversely, the control group had more Black non-Hispanic and Hispanic mothers than the intervention group (nine Black, non-Hispanic and eight Hispanic mothers in the control group and six Black non-Hispanic and five Hispanic mothers in the intervention group).
KMC and Physiologic Measures of Stress
The mean heart rate, respiratory rate, oxygen saturation, and abdominal NIRS measures were homogenous across the groups. The FIO2 median scores for both intervention and control groups ranged from 21% to 40%, with no statistically significant difference between the two groups. Most participants were maintained on room air or 21% FIO2. While we found no statistically significant differences in the means of abdominal temperature (Figure 2a) or perfusion index (Figure 2b) between the groups, we did find statistically significant increases over time in the sample as a whole (p = .004 and p = .031, respectively). Changes over time in the abdominal perfusion index suggest divergent trends between the treatment and control groups, implying that a larger cohort might demonstrate significant KMC benefits.
Figure 2.
Changes over time in infant abdominal temperature (a) and perfusion index scores (b) in the kangaroo mother care (KMC; n = 26) and control (n = 25) groups. Values are plotted as means ± SD.
Association of KMC With Urinary Biochemical Markers
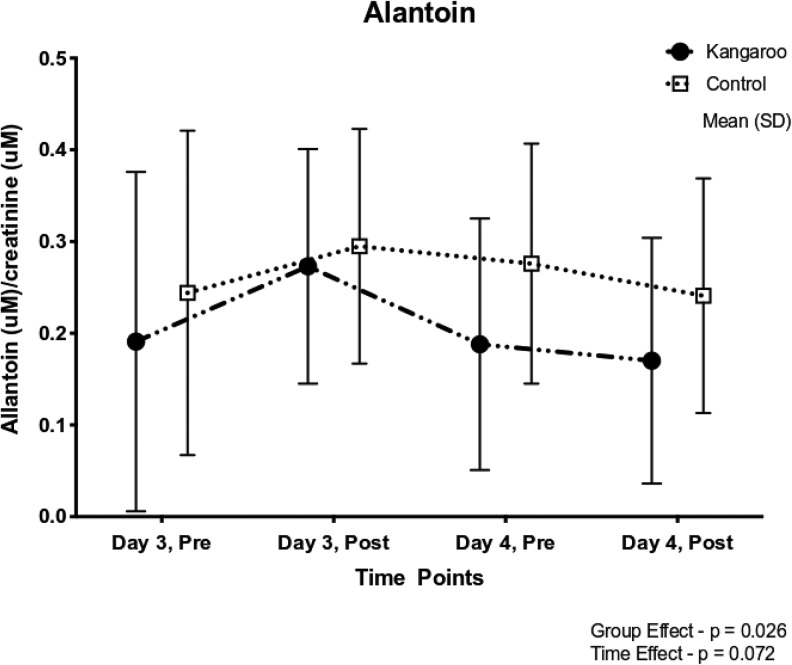
Mean urinary allantoin levels in the KMC intervention group were significantly lower than those of the control group (p = .026; see Figure 3). However, this result was largely due to lower allantoin levels measured on Day 4 for babies who initially received KMC on Day 3. These findings suggest that neonates in the KMC intervention group had decreased levels of stress-related biochemical markers 1 day after an initial session of KMC, signaling a general reduction in the inflammatory tone as a result of prior KMC experience, compared to infants in the control group who received only incubator care on Day 3.
Figure 3.
Changes over time in urinary allantoin levels between the kangaroo mother care (KMC, n = 26) and control (n = 25) groups. Values are plotted as means ± SD.
Median levels of Hx (Table 2) did not differ significantly between the intervention and control groups or over time. Similarly, we found no significant differences in Xa or UA levels between the two groups. However, Xa levels increased modestly over time (p = .042), while UA levels decreased with time (p = .025).
Table 2.
Change in the Levels of Uric Acid (UA), Xanthine (Xa), Hypoxanthine (Hx), and Allantoin (Al) for the Intervention and Control Groups.
| Metabolite and Time | Kangaroo Care | Control | Group Effect | Time Effect |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p Value | p Value | |
| Uric acid (UA(uM)/Cr(uM)) | .562 | .025* | ||
| 1 (Ref.) | .422 ± .298 | .398 ± .298 | ||
| 2 | .463 ± .300 | .433 ± .300 | ||
| 3 | .418 ± .298 | .364 ± .298 | ||
| 4 | .332 ± .298 | .284 ± .305 | ||
| Xanthine (Xa(uM)/Cr(uM)) | .193 | .042* | ||
| 1 (Ref.) | .014 ± .011 | .013 ± .011 | ||
| 2 | .014 ± .011 | .013 ± .011 | ||
| 3 | .021 ± .011 | .016 ± .011 | ||
| 4 | .016 ± .011 | .014 ± .012 | ||
| Hypoxanthine (Hx(uM)/Cr(uM)) | .586 | .645 | ||
| 1 (Ref.) | .017 ± .038 | .010 ± .037 | ||
| 2 | .015 ± .037 | .009 ± .039 | ||
| 3 | .029 ± .038 | .013 ± .040 | ||
| 4 | .014 ± .039 | .015 ± .041 | ||
| Allantoin (Al(uM)/Cr(uM)) | .026* | .072 | ||
| 1 (Ref.) | .191 ± .185 | .244 ± .177 | ||
| 2 | .273 ± .128 | .295 ± .128 | ||
| 3 | .188 ± .137 | .276 ± .131 | ||
| 4 | .170 ± .134 | .241 ± .128 |
Note. Cr = creatinine.
* Significant at an α of .05.
Discussion
In the present stratified, randomized study of KMC for neonates of 24–36 weeks’ gestational age, we examined urinary purines as biochemical markers of energy utilization (i.e., Hx, Xa, UA), a urinary marker of oxidation stress (i.e., allantoin), infant abdominal temperature, and near-infrared perfusion of the abdomen. We found significantly lower allantoin levels in the treatment group (p = .026) compared to the control group on the fourth day of life, which was the day after infants in the treatment group received a single 1-hr session of KMC and those in the control group received incubator care. This finding suggests that at least one benefit of KMC treatment is time dependent. In addition, this finding supports the hypothesis that the KMC intervention may lower oxidative stress in preterm infants.
Abdominal temperatures improved over time in both groups, and abdominal perfusion increased modestly (but not statistically significantly) in KMC treated babies over the same period. Previous work suggested that KMC can help regulate an infant’s body temperature via thermal regulators in the mother’s breast, heating or cooling the baby as needed, thus optimizing the infant’s temperature (Bergman et al., 2004; Chi Luong et al., 2016). Our findings do not directly demonstrate this positive impact of KMC on temperature. However, this inconsistency may be related to the limitations of the current small study size, doing KMC for only 1 hr per session, having only 1-hr difference in total KMC duration between the groups, and/or the appropriateness of incubator and environmental settings. Prolonged or repeated treatments may have additional beneficial effects that the present study design could not demonstrate.
Evidence exists that infants treated with KMC have better food digestion and metabolism, generally leading them to thrive better and gain weight more quickly, compared to infants given incubator care only (Boundy et al., 2016). The biological mechanisms for this finding remain unclear. Strategic nursing and technical measures are employed to ensure infant normothermia (Knobel-Dail et al., 2017). Future studies should further investigate the impact of the KMC intervention on infant intestinal perfusion and oxygenation to clarify any impact on infant digestion.
The present study has some limitations. Although most cohort characteristics were similar between the two groups, the distribution of race/ethnicity was not. The intervention group was, by random chance, enriched with White mothers. Research has documented that preterm Black non-Hispanic infants, depending on gestational age, differ with respect to rates of morbidity and mortality from White infants (Anderson et al., 2018; Wallace et al., 2017). It is uncertain how this difference between groups might have affected our study outcomes. We would expect a larger sample size to lead to a more balanced racial composition of the groups.
Additionally, it appears that our estimated effect size was too generous for our cohort. Moreover, the study implementation of the KMC intervention (compared to incubator care) was limited to 1 day for each participant, and data collection was limited to just 2 days for each participant, with the intervention group receiving a total of 2 hr of KMC during the data collection period and the control group receiving only 1 hr total of KMC during the same period. It is possible that a larger difference in the dose of KMC between the groups would have resulted in more significantly different outcomes for the two groups. A longer study protocol might improve the likelihood of seeing larger and clinically meaningful differences between groups.
In addition, the span of gestational ages of enrolled infants was very wide, which could have had an impact on the statistical significance of the results. In future investigations, a closer focus on the stable very low birthweight group might provide more evidence that starting KMC early has benefits that outweigh the risks.
Conclusions
In the present study, we found significantly lower levels of urinary allantoin, a biochemical marker of oxidative stress, in preterm infants treated with 1 hr of KMC on the previous day compared with preterm infants in the control group, who had been treated with incubator care on the previous day. This finding suggests that infants treated with KMC exhibit lower inflammatory tone over time. Our results support the practice of early KMC intervention in the NICU with the ultimate goal of reducing stress and promoting the health and well-being of preterm infants.
Footnotes
Author Contribution: Dorothy Forde, Douglas D. Deming, and Mary K. Barger contributed to conception and design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Khaled Bahjri contributed to design and analysis and interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Raylene M. Phillips contributed to conception and design, acquisition, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Eileen K. Fry-Bowers contributed to design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. John C. Tan contributed to analysis, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Danilyn M. Angeles contributed to conception and design and acquisition, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Danilo S. Boskovic contributed to conception and design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by an award from the American Association of Colleges of Nursing (AACN) 2016 and 2017, a dissertation scholarship from the Western Institute of Nursing Research (WIN) in 2017, National Institutes of Health grant no. R01 NR011209-05, and National Institute of Nursing Research Biobehavioral Research Training in Symptom Science Grant (T32NR016920).
ORCID iDs: Dorothy Forde  https://orcid.org/0000-0001-8016-2955
https://orcid.org/0000-0001-8016-2955
Danilo S. Boskovic  https://orcid.org/0000-0001-6919-3726
https://orcid.org/0000-0001-6919-3726
References
- Akotia D. H., Durham J. T., Arnell K. M., Petruzzelli D. L., Katheria A. C. (2016). Relationship between near-infrared spectroscopy and transabdominal ultrasonography: Noninvasive monitoring of intestinal function in neonates. Medical Science Monitor, 22, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. G., Rogers E. E., Baer R. J., Oltman S. P., Paynter R., Partridge J. C., Rand L., Jelliffe-Pawlowski L. L., Steurer M. A. (2018). Racial and ethnic disparities in preterm infant mortality and severe morbidity: A population-based study. Neonatology, 113, 44–54. 10.1159/000480536 [DOI] [PubMed] [Google Scholar]
- Angeles D. M., Asmerom Y., Boskovic D. S., Slater L., Bacot-Carter S., Bahjri K., Mukasa J., Holden M., Fayard E. (2015). Oral sucrose for heel lance enhances adenosine triphosphate use in preterm neonates with respiratory distress. SAGE Open Medicine, 3 10.1177/2050312115611431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J., Bergman N. (2013). Whose choice? Advocating birthing practices according to baby’s biological needs. Journal of Perinatal Education, 22, 8–13. 10.1891/1058-1243.22.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman N. J., Linley L. L., Fawcus S. R. (2004). Randomized controlled trial of skin-to-skin contact from birth versus conventional incubator for physiological stabilization in 1200- to 2199-gram newborns. Acta Paediatrica (Oslo, Norway: 1992), 93, 779–785. [DOI] [PubMed] [Google Scholar]
- Blass E. (2015). Energy conservation in infants. Behavioural Processes, 117, 35–41. 10.1016/j.beproc.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Boundy E. O., Dastjerdi R., Spiegelman D., Fawzi W. W., Missmer S. A., Lieberman E., Kajeepeta S., Wall S., Chan G. J. (2016). Kangaroo mother care and neonatal outcomes: A meta-analysis. Pediatrics, 137, e20152238 10.1542/peds.2015-2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Luong K., Long Nguyen T., Huynh Thi D. H., Carrara H. P. O., Bergman N. J. (2016). Newly born low birthweight infants stabilise better in skin-to-skin contact than when separated from their mothers: A randomised controlled trial. Acta Paediatrica, 105, 381–390. 10.1111/apa.13164 [DOI] [PubMed] [Google Scholar]
- Craig B. M., Hartman J. D., Owens M. A., Brown D. S. (2016). Prevalence and losses in quality-adjusted life years of child health conditions: A burden of disease analysis. Maternal and Child Health Journal, 20, 862–869. 10.1007/s10995-015-1874-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R., Rosenthal Z., Eidelman A. I. (2014). Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biological Psychiatry, 75, 56–64. 10.1016/j.biopsych.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Harsha S. S., Archana B. R. (2015). SNAPPE-II (Score for Neonatal Acute Physiology with Perinatal Extension-II) in predicting mortality and morbidity in NICU. Journal of Clinical and Diagnostic Research, 9, SC10–SC12. 10.7860/JCDR/2015/14848.6677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden M. S., Hopper A., Slater L., Asmerom Y., Esiaba I., Boskovic D. S., Angeles D. M. (2014). Urinary hypoxanthine as a measure of increased ATP utilization in late preterm infants. Infant, Child & Adolescent Nutrition, 6, 240–249. 10.1177/1941406414526618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. (2013). Maternal-infant bonding: A review of literature. International Journal of Childbirth Education, 28, 17–22. [Google Scholar]
- Kand’ár R., Záková P., Muzáková V. (2006). Monitoring of antioxidant properties of uric acid in humans for a consideration measuring of levels of allantoin in plasma by liquid chromatography. Clinica Chimica Acta, International Journal of Clinical Chemistry, 365, 249–256. 10.1016/j.cca.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Knobel-Dail R. B., Tanaka D. T., Holditch-Davis D., White J. (2017). Perfusion index in very low birth weight premature infants during their first 2 weeks of life. Biological Research for Nursing, 19, 41–52. 10.1177/1099800416656914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludington S. M. (1990). Energy conservation during skin-to-skin contact between premature infants and their mothers. Heart & Lung: The Journal of Critical Care, 19, 445–451. [PubMed] [Google Scholar]
- Mefford L. C. (2004). A theory of health promotion for preterm infants based on levine’s conservation model of nursing. Nursing Science Quarterly, 17, 260–266. 10.1177/0894318404266327 [DOI] [PubMed] [Google Scholar]
- Moore E. R., Anderson G. C., Bergman N., Dowswell T. (2012). Early skin-to-skin contact for mothers and their healthy newborn infants Cochrane Database of Systematic Reviews. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003519.pub3/abstract [DOI] [PMC free article] [PubMed]
- Mörelius E., Örtenstrand A., Theodorsson E., Frostell A. (2016). OC09—Early maternal contact has an impact on preterm infants’ brain systems that manage stress. Nursing Children and Young People, 28, 62–63. 10.7748/ncyp.28.4.62.s40 [DOI] [PubMed] [Google Scholar]
- Nyqvist K. H., Anderson G. C., Bergman N., Cattaneo A., Charpak N., Davanzo R., Ewald U., Ibe O., Ludington-Hoe S., Mendoza S., Pallás-Allonso C., Ruiz Peláez J. G., Sizun J., Widström A.-M. (2010). Towards universal kangaroo mother care: Recommendations and report from the first European conference and seventh international workshop on kangaroo mother care. Acta Paediatrica (Oslo, Norway: 1992), 99, 820–826. 10.1111/j.1651-2227.2010.01787.x [DOI] [PubMed] [Google Scholar]
- Pados B. F. (2019). Physiology of stress and use of skin-to-skin care as a stress-reducing intervention in the NICU. Nursing for Women’s Health, 23, 59–70. 10.1016/j.nwh.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Plank M. S., Calderon T. C., Asmerom Y., Boskovic D. S., Angeles D. M. (2011). Biochemical measurement of neonatal hypoxia. Journal of Visualized Experiments, e2948 10.3791/2948 [DOI] [PMC free article] [PubMed]
- Richardson D. K., Corcoran J. D., Escobar G. J., Lee S. K. (2001). SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. Journal of Pediatrics, 138, 92–100. [DOI] [PubMed] [Google Scholar]
- Sutton P. S., Darmstadt G. L. (2013). Preterm birth and neurodevelopment: A review of outcomes and recommendations for early identification and cost-effective interventions. Journal of Tropical Pediatrics, 59, 258–265. 10.1093/tropej/fmt012 [DOI] [PubMed] [Google Scholar]
- Tan J. B. C., Boskovic D. S., Angeles D. M. (2018). The energy costs of prematurity and the neonatal intensive care unit (NICU) experience. Antioxidants (Basel, Switzerland), 7 10.3390/antiox7030037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A. A., Zhang H., Il’yasova D., Sztáray J., Young S. P., Millington D. S. (2010). Allantoin in human urine quantified by ultra-performance liquid chromatography-tandem mass spectrometry. Analytical Biochemistry, 402, 191–193. 10.1016/j.ab.2010.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. E., Mendola P., Kim S. S., Epps N., Chen Z., Smarr M., Hinkle S. N., Zhu Y., Grantz K. L. (2017). Racial/ethnic differences in preterm perinatal outcomes. American Journal of Obstetrics and Gynecology, 216, 306.e1–306.e12. 10.1016/j.ajog.2016.11.1026 [DOI] [PMC free article] [PubMed] [Google Scholar]