Abstract
Background:
The postpartum period can be a vulnerable time during which many women are prone to mood disturbances. Since telomere length (TL) is known to be associated with dysphoric moods, inflammation, and stress in many populations, this study’s objective was to assess the relationships among TL, dysphoric moods, stress, and inflammation during the postpartum period.
Method:
This cross-sectional pilot study is a secondary analysis of data collected in a larger parent study of anti-thyroid peroxidase (TPO) enzyme antibody positive versus negative women. The parent study followed selected mothers every month for 6 postpartum months. From this parent study, a random sample of preserved peripheral blood mononuclear cells from 97 participants collected at 2–4 months postpartum were measured for TL. Data were available on the production of interleukin-6 (IL-6), an inflammatory cytokine, in stimulated ex vivo cultures for 59 of these women. Dysphoric moods and stress were measured. Pearson correlations and linear regressions were performed, controlling for postpartum thyroiditis status and age.
Results:
There were no statistically significant relationships between TL and demographic factors, stress, depression, or TPO status. There were significant negative correlations between TL and anxiety and a trend for a relationship between TL and IL-6 levels. IL-6 levels were significantly, positively associated with negative moods.
Conclusions:
Higher anxiety scores and inflammation were associated with shorter TL. Inflammation was related to anxiety and other dysphoric moods and was marginally associated with shorter TLs.
Keywords: telomeres, postpartum, anxiety, inflammation
Prior studies, including ours, have suggested that the postpartum period extends physiologically and psychologically through the first 6 months to 1 year after birth (Groer, Jevitt, & Ji, 2015; Groer et al., 2014). This period is an understudied but potentially critical time of life (Bauer, Damschroder, Hagedorn, Smith, & Kilbourne, 2015) during which mothers adopt new roles and responsibilities, physically recover from pregnancy and labor, and lactate for varying amounts of time. Women are at risk for a variety of postpartum mood disorders, from mild dysphoria to severe depression and psychosis (O’Hara & McCabe, 2013; Stewart & Vigod, 2016). Postpartum depression can persist for many months (Miller, 2002). Mothers also cope with many demands and stressors that can sometimes feel overwhelming (Jevitt, Groer, Crist, Gonzalez, & Wagner, 2012). Thus, for some women, the postpartum period is psychologically difficult and may also be a biologically vulnerable time.
Our group has studied dysphoric moods, stress, thyroid disease, and immune function in women through the first postpartum year (Groer et al., 2015; Groer et al., 2014; Groer, Jevitt, Sahebzamani, Beckstead, & Keefe, 2013; Groer & Vaughan, 2013; Groer et al., 2011; Jevitt et al., 2012; Thibeau et al., 2016). We completed a longitudinal study of postpartum women who were either negative or positive during pregnancy testing for the anti-thyroid peroxidase (TPO) enzyme antibody. TPO is a common antibody that occurs in up to 11% of childbearing women and increases their risk for later development of postpartum thyroiditis (PPT: Meena, Chopra, Jain, & Aggarwal, 2016). We found that dysphoric moods were more common in TPO positive women with PPT than in TPO positive women who did not experience PPT. In qualitative work, we found that some women, regardless of TPO or PPT status, were extremely affected by postpartum moods and stress (Jevitt et al., 2012). Of course, mood disorders and stress are not confined to women with thyroid disease. In our earliest studies of the postpartum period, in a fairly large sample, we discovered that there was a wide range of stress scores and dysphoric moods (Groer, 2005).
Dysphoric moods and stress are associated with poor health outcomes and negatively impact longevity (Russo et al., 2007; Slavich, 2016). Moreover, shortened telomere length (TL), a potential biomarker of senescence, has been shown to be associated with adverse events in a person’s life such as stress, depression, childhood trauma, socioeconomic status, or conditions that produce oxidative stress and inflammation as well as genetic factors and heritability (Bell et al., 2019; Chander, Yadav, Jain, Bhadada, & Dhawan, 2018; Korakas, Dimitriadis, Raptis, & Lambadiari, 2018; Li, Hao, Fan, & Zhang, 2018; Pearce et al., 2018; Peng, Xiao, Hu, & Zhang, 2018). Telomeres are repetitive DNA hexamer tandem sequences of TTAGGG at the ends of chromosomes, which protect the chromosome from degrading (Wai, 2004). They are found in all chromosomes in all cells, and, as cells divide, there is loss of the DNA hexamer, which, in most differentiated human cells, cannot be repaired (Calado & Young, 2009). Activated lymphocytes are able to repair telomere damage through the enzyme telomerase, which repairs shortened ends of the chromosomes and can restore TL in some cells, such as germ or immune cells (de Punder, Heim, Wadhwa, & Entringer, 2019). Nevertheless, the shortening of telomeres on lymphocytes and other cells is a marker of biological aging and strongly correlates with life span (Effros & Pawelec, 1997; Harley, Futcher, & Greider, 1990; Kipling et al., 1999). Thus, TL may be utilized as an effective biomarker indicative of biological repercussions of psychosocial factors. Peripheral blood mononuclear cells (PBMCs) are a mix of lymphocytes and monocytes, and many studies have examined relationships among PBMC TL and various psychosocial and physiological variables.
In light of previous findings, we hypothesized that postpartum stress and dysphoric moods would be associated with inflammation and shortened TL in preserved PBMCs in our postpartum sample, even when PPT status was controlled. To date, there have been no published studies relating postpartum moods with TL.
Materials and Methods
Study Population
The institutional review board at the university approved the study. All participating women gave written informed consent. The parent study population comprised postpartum women who had been assessed during pregnancy for antibodies against TPO (N = 631), and eligible TPO negative and positive women were followed for 6 months in the postpartum period (N = 121). Exclusion criteria for the parent study, determined by examination of the medical record, included history of depression or other mental illness, HIV or hepatitis, and use of drugs that would alter immune function. For the current investigation, we randomly selected PBMC samples from 97 of the 121 women to measure for TL.
Data Collection
In the parent study, blood samples were taken at pregnancy (mean week of collection was 16 weeks’ gestation), 1-week postpartum, and then every month for 6 months. Investigators collected 15 ml of blood by venipuncture at morning home visits, and PBMCs and plasma were preserved. The majority of samples for the present study were from 2 months, but in order to increase the sample size, we selected about 20% of the samples from Months 3 and 4.
Measures
Participants completed a demographic questionnaire, the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1992), an investigator-developed thyroid symptom checklist (Groer & Jevitt, 2014), and the Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983) at each visit. The thyroid symptom checklist included Hyperthyroid and Hypothyroid subscales. The Hyperthyroidism subscale comprised nine 4-point Likert-type items (maximum severity score = 36) and the Hypothyroidism subscale had 10 4-point Likert-type items (maximum severity score = 40). The Cronbach’s α for the Hyperthyroid Symptom subscale was .70 and for the Hypothyroid subscale was .77. The long form of the POMS is a 65-item rating scale for measuring how often the respondent experienced a specific mood during the past week, including the day of measurement (McNair et al., 1992). The POMS consists of a total mood disturbance (TMD) score and six subscales: tension–anxiety, depression–dejection, anger–hostility, vigor–activity, fatigue–inertia, and confusion–bewilderment. The internal consistency reliabilities for the subscales range from .87 to .95. The validity of the scale (face validity, factorial validity, predictive validity, and construct validity) is reported to be excellent (McNair et al., 1992). The PSS is a widely used measure of perceptions of stress related to helplessness and control. Respondents rank 14 items on a 4-point Likert-type scale. The internal consistency reliability has been reported to be .84 to .86 in young adults. Congruent and criterion validity has been shown to be excellent, although predictive validity declines over time (Cohen et al., 1983).
Ex Vivo Cultures and Interleukin-6 (IL-6) Measurement
We used a whole-blood sample in an ex vivo culture on the day of collection, measuring cytokine synthesis over time in mitogen-stimulated cultures. Whole blood (1.2 ml) was suspended in Roswell Park Memorial Institute-1640 media (4.8 ml) with 10 μg/ml gentamycin, 5.0 μg/ml of phytohemagglutinin (Sigma-Aldrich, St.Louis, MO) and 5.0 μg/ml of lipopolysaccharide (ICN Biomedicals, OH) and placed in the wells of a 16-well plate. The plates were incubated at 37 °C with 5% CO2 for 48 hr. The contents were centrifuged at 400 g, and the supernatants were frozen at −80 °C. IL-6 was analyzed by enzyme-linked immunoassay (ELISA; eBioscience, San Diego, CA). The results of this assay matched to the time of TL measurement were available for 59 participants.
Telomere Assay
We selected preserved PBMCs from a random sample of 97 participants for telomere assay. PBMCs were isolated by density-gradient centrifugation, and cells were preserved in 10% DMSO in liquid nitrogen until analysis.
Genomic DNA was extracted from PBMCs with DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA), and DNA concentration and quality were evaluated by 260/280 UV nanodrop spectrophotometer; only samples with a 260/280 ratio > 1.7 were included in subsequent analyses. DNA was extracted from lymphocytes (Bio-Rad, Hercules, CA) for quantitative polymerase chain reaction (qPCR) with iCycler real-time PCR system. Samples were diluted to 7 ng/μl for the PCR reaction. Each well contained 4 μl of sterile water, 5 μl (35 ng) of genomic DNA, 0.5 μl of each primer, and 10 μl of iQ SYBR Green Supermix Bio-Rad (reaction buffer with dNTPs, iTaq DNA polymerase, 6 mM MgCl2, SYBR Green I, fluorescein, and stabilizers), and 7 ng/μl of DNA. The telomere primers were Telo-F (CGG TTT GTT TGG GTT TGG GTT TGG GTT TGG GTT TGG GTT) and Telo-R (GGC TTG CCT TAC CCT TAC CCT TAC CCT TAC CCT TAC CCT). The primers for the reference control gene, 36B4 single-copy gene, were 36B4-F (CAG CAA GTG GGA AGG TGT AAT CC) and 36B4-R (CCC ATT CTA TCA TCA ACG GGT ACA A). The concentration of all primers was 10 μM, and the PCR cycle conditions consisted of an initial denaturation step at 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s and at 60 °C for 60 s. Next, the reaction was run as follows: 1 min at 95 °C, 1 min at 55 °C, and 81 repeats of 10 s at 55 °C. Reactions were set up in triplicate in 96-well plates, each with five DNA quantity standards (serial dilutions of a reference DNA Hela cells giving final DNA quantities of between 28 and 1.75 ng/μl per reaction), one negative control and one internal control. Each sample was run 2 times on different plates. If the mean of values on one plate differed by >15% from the mean of the samples of the second plate, the sample was run a third time, and the mean of the two closest measurements was used. A standard curve was made by serial dilutions of DNA from Hela cells. The amount of each subject’s DNA sample was determined relative to the single reference DNA sample by a standard curve. Telomeres were measured as T/S ratios. This is a computation by which the sample differs in its ratio of telomere repeat copy number to a single-copy gene copy number. The telomere signal was normalized to the signal from the single-copy gene to generate the TL, which was indicative of relative TLs across all chromosomes in a cell. The coefficient of variation was 1.4%.
Statistical Analyses
The data distributions were analyzed first for normality and transformed, when necessary, before parametric techniques were used to analyze relationships. Pearson correlations were performed between TL and demographic characteristics, mood states, stress, and cytokines assayed at the time of TL measurement. After determining that only anxiety and IL-6 were related to TL, linear regression models of TL and anxiety and TL and IL-6, using the pairwise option and controlling for PPT status and age for anxiety and age only for IL-6, were performed. For descriptive purposes, as is often done in telomere studies, TL was sorted into tertiles (0.56–1.05 [shortest], 1.06–1.26 [medium length], and 1.3–2.1[longest]), and contingency table analysis was used for nominal variables of ethnicity, marital status, income, educational level, and breastfeeding status. A p value of .05 was accepted for statistical significance. Power analysis was not performed for this cross-sectional, exploratory, pilot study, as we were constrained by our use of a limited data set from the parent study.
Results
Telomeres
The TL (T/S ratio) was normally distributed. The mean (SD) TL ratio was 1.17 ± 0.32. The range was 0.31–2.10.
Demographics
The sample consisted of 59 TPO negative and 38 TPO positive postpartum females. At the time of telomere measurement, only two women reported smoking, and only five reported more than an hour of exercise per week. The mean age of the sample was 29.6 years (SD = 6.3, range 19.0–46.0), and the mean body mass index (BMI) was 28.8 kg/m2 (SD = 5.9, range 19.4–46.0). The majority (72%) were unemployed, and 72% were married, 15% divorced and 13% single. Annual income was over US$40,000 for 55%, and the majority had completed high school, with 54% college completion or postgraduate education preparation. The sample was 76% Caucasian (39% of whom were of Hispanic origin) and 15% African American. The remaining 9% were Asian or other racial categories. There were no statistically significant relationships between TL and age, BMI, income level, marital status, parity, number in household, or ethnicity. However, Hispanics had the lowest TLs of all ethnic groups (NS; η2 = .095) and highest depression scores (28%, but also NS).
Thyroid Status
Analysis of TL by TPO status revealed essentially equal TLs (TPO positive TL = 1.174, n = 39; TPO negative TL = 1.172, n = 59) . TL was not significantly different in the 11 women who were experiencing symptoms of PPT (fatigue, dry skin, lack of energy) at the time of TL measurement compared to the rest of the sample.
Mood and Stress
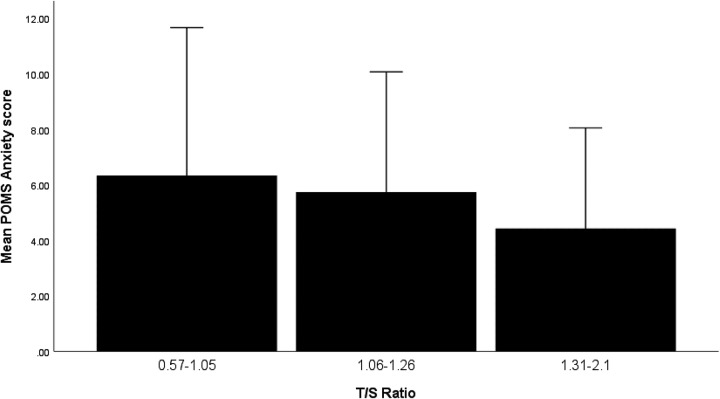
For descriptive purposes, a frequent method of illustrating telomere data in research publications is sorting TL into tertiles (0.36–1.05 [shortest], 1.06–1.26 [medium length], and 1.3–2.1[longest]). Anxiety by TL tertile is depicted in Figure 1. All dysphoric mood states followed this pattern. This method gives an indication of the directional trends of relationships, but we used the actual values of mood and anxiety scores and TL in correlations and regressions. This sample had generally low mood disturbance scores (see Table 1). For example, the TMD score, which sums the dysphoric moods and subtracts the vigor score, was 8.5.
Figure 1.
Anxiety by tertiles of telomere length. POMS = Profile of Mood States; T/S ratio = measure of telomere length.
Table 1.
Profile of Mood States (POMS) and Perceived Stress Scale Scores.
| Variables | n | Minimum | Maximum | M | SD |
|---|---|---|---|---|---|
| POMS anxiety | 95 | 0.00 | 26.00 | 5.54 | 4.54 |
| POMS depression | 95 | 0.00 | 36.00 | 4.00 | 6.480 |
| POMS anger | 95 | 0.00 | 40.00 | 4.79 | 6.40 |
| POMS vigor | 95 | 0.00 | 28.00 | 16.1 | 6.74 |
| POMS fatigue | 95 | 0.00 | 26.00 | 5.31 | 4.94 |
| POMS confusion | 95 | 0.00 | 23.00 | 4.98 | 3.56 |
| Total mood disturbance | 95 | −24.00 | 123.00 | 8.53 | 25.8 |
| Perceived stress | 94 | 3 | 38 | 19.5 | 7.56 |
Perceived stress scores were not significantly related to TL but were positively correlated with all the negative mood states (anxiety: r = .52, p < .001; depression: r = .48, p < .001; anger: r = .47, p < .001; fatigue: r = .30, p < .01; TMD: r = .67, p < .001).
In regression models examining subscales of the TMD score as predictors of TL as a continuous variable, only anxiety was significantly related to TL. Subsequently, we fit a second regression model to examine predictions of TL by anxiety scores, controlling for PPT status (because of the possibility of a confounding effect on mood) and age (because TL is generally shorter with increasing age). The results of the regression indicated that the three predictors explained 8.2% of the variance in TL, R 2 = .082, F(3, 88) = 2.63, p < .055. We found that only anxiety predicted TL (β = −.24, p < .02).
IL-6
The release of cytokines into the supernatant of ex vivo whole-blood mitogen-stimulated cultures was only available for 59 women due to lab issues and inadequate volumes of blood available for the assay for every visit. We analyzed the levels of IL-6 from these cell cultures in the participants. IL-6 levels were correlated with dysphoric moods (anxiety: r = .32, p < .009; anger: r = −.4, p < .001; fatigue: r = .26, p < 04; TMD: r = .36, p < .03) .There were no significant differences in IL-6 release from ex vivo cultures between TPO negative and positive women. We fit a regression model to examine predictions of TL by IL-6, controlling for age. The results of the regression indicated that the two predictors explained 8.2% of the variance in TL, R 2 = .082, F(2, 59) = 2.62, p < .08. Age did not significantly predict TL (β = −.18, p < .16). IL-6 marginally predicted TL (β = −.29, p < .07).
Discussion
Many animal and human studies have shown relationships among stress, depression, inflammation, senescence and age-related disease, and shortened TL. However, the relationship between postpartum moods and TL has not been studied. In a study across pregnancy and the postpartum period, previous researchers found that TL remained stable and was affected, not by present conditions, but rather by childhood socioeconomic factors and low social support (Mitchell, Kowalsky, Epel, Lin, & Christian, 2018). We found in this preliminary work that TL was related to anxiety mood scores in postpartum women, controlling for PPT status and age. This finding is interesting, as our study population was generally healthy, even though some women had evidence of PPT with hypothyroid symptoms (Groer & Jevitt, 2014; Groer & Vaughan, 2013). Nevertheless, the sample was generally low on stress and mood scores. The mean perceived stress score of 19.5 in the present study is nearly identical with the mean score of women in a normative sample similar to the one in this study (Cohen & Williamson, 1988). A study of Australian mothers at the 2-month postpartum time point found a much higher TMD score of 16.5 (compared to the mean in the present study of 8.53) but with a comparable anxiety score to that in our study (Cooklin et al., 2018)
Most of the studies on telomeres and behavior or mood have focused on clinically significant diagnosed disorders rather than mood states. Mood states can be an indicator of underlying psychiatric pathology. In a meta-analysis of psychological stress, depression, and TL, authors found evidence for shortening in stress but lengthening in major depressive disorders (Deng, Cheung, Tsao, Wang, & Tiwari, 2016). Likewise, in a recent meta-analysis of TL and anxiety, authors found a small but significant relationship between shortened telomeres and anxiety (r = −.06; Malouff & Schutte, 2017). Our hypothesis regarding mood and TL in the present study was partially supported by the relationship we found between TL and anxiety.
We also found a trend for a relationship between TL and inflammation, when controlling for age. Specifically, our finding in the present study of a marginal relationship between IL-6, a proxy for inflammation, and shortening of TL suggests a possible association between inflammation and TL. Telomere shortening due to stress or psychological states, such as depression or anxiety, may be related to inflammatory and oxidative processes. Inflammation is known to be associated with dysphoric moods and stress (Felger, 2018). Excessive or prolonged inflammation leads to damage to the organism. Inflammation is associated with telomere shortening, and the oxidative stress associated with inflammation is a key factor implicated in diseases of aging (Zhang et al., 2016). Telomeres have a large number of guanines and thus are sensitive to oxidative damage, which may result in cell aging and death due to DNA damage (Saretzki, 2009). An interesting mechanistic hypothesis about the association of shortened telomeres and aging is that immune and inflammatory mechanisms involve a metabolic cost to the organism that reduces the efficacy of processes that maintain telomeres (Criscuolo et al., 2018). Also of interest are the findings that, not only do senescent cells have shortened telomeres, the transcription factor nuclear factor-κB is overexpressed in cellular aging, leading to overproduction of inflammatory cytokines such as tumor necrosis factor-α, IL-6, and gamma interferon in circulating macrophages (Zhang et al., 2016). Further exploration of postpartum inflammation and TL is warranted.
Limitations
Limitations of the study include generally low self-reported dysphoric mood and stress at the time of TL measurement in the study population. A history of depression was an exclusion criterion in the parent study, and this criterion may have impacted the prevalence of dysphoric mood states and stress in our sample because mothers with a history of depression are more likely to have higher levels of stress and anxiety in the postpartum period. While we did find that higher anxiety scores were significantly associated with TL, the effect sizes of all relationships with TL were small. Another key variable associated with shortened telomeres is childhood trauma, and future studies should include this important factor.
Conclusion
In summary, our cross-sectional study of 97 postpartum women 2–4 months postpartum identified a significant relationship between TL and anxiety and a marginal relationship between TL and IL-6. Trends in the mood scores were apparent when we divided the TL data into tertiles. We found no relationships with other dysphoric moods or stress. A larger study of women with more significant and better characterized anxiety and depression is needed to determine the significance of these findings. In the present study, effect sizes were small, and multiple factors that are certainly involved in telomere shortening were not included in the data available from the parent study for these women (e.g., adverse childhood events, traumas).
The postpartum period is a vulnerable and unique time in a woman’s life. The possibility of long-term effects of postpartum states, events and moods on later disease or even longevity requires further research in women with greater levels of mood disturbance, stress, and postpartum depression. This research has important implications for nursing, as providers can offer interventions that address and improve many negative postpartum issues, which not only affects the quality of life for women and their infants in the postpartum period, it may also affect later health and even longevity.
Footnotes
Author Contributions: Maureen Groer contributed to conception and design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Adetola Louis-Jacques contributed to conception and design, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Laura Szalacha contributed to design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Laura Redwine contributed to conception and design, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Roberta Dracxler contributed to conception and design, acquisition, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. David Keefe contributed to conception and design, acquisition, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institute of Nursing Research (R01 NR05000).
ORCID iD: Maureen Groer  https://orcid.org/0000-0002-5526-2927
https://orcid.org/0000-0002-5526-2927
References
- Bauer M. S., Damschroder L., Hagedorn H., Smith J., Kilbourne A. M. (2015). An introduction to implementation science for the non-specialist. BMC Psychology, 3 doi:10.1186/s40359-015-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. S., Spencer J. I., Yates R. L., Yee S. A., Jacobs B. M., DeLuca G. C. (2019). Invited review: From nose to gut—The role of the microbiome in neurological disease. Neuropathology and Applied Neurobiology, 45(3), 195–215. doi:10.1111/nan.12520 [DOI] [PubMed] [Google Scholar]
- Calado R. T., Young N. S. (2009). Telomere diseases. New England Journal of Medicine, 361, 2353–2365. doi:10.1056/NEJMra0903373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander A. M., Yadav H., Jain S., Bhadada S. K., Dhawan D. K. (2018). Cross-talk between gluten, intestinal microbiota and intestinal mucosa in celiac disease: Recent advances and basis of autoimmunity. Frontiers in Microbiology, 9, 2597 doi:10.3389/fmicb.2018.02597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Cohen S., Williamson G. (1988). Perceived stress in a probability sample of the U.S In Spacapam S., Oskamp S. (Eds.), Claremont symposium on applied social psychology. Newbury Park, CA: Sage. [Google Scholar]
- Cooklin A. R., Amir L. H., Nguyen C. D., Buck M. L., Cullinane M., Fisher J. R. W., Donath S. M. (2018). Physical health, breastfeeding problems and maternal mood in the early postpartum: A prospective cohort study. Archives of Women’s Mental Health, 21, 365–374. doi:10.1007/s00737-017-0805-y [DOI] [PubMed] [Google Scholar]
- Criscuolo F., Sorci G., Behaim-Delarbre M., Zahn S., Faivre B., Bertile F. (2018). Age-related response to an acute innate immune challenge in mice: Proteomics reveals a telomere maintenance-related cost. Proceedings of the Royal Society B: Biological Sciences, 285 doi:10.1098/rspb.2018.1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Cheung S. T., Tsao S. W., Wang X. M., Tiwari A. F. (2016). Telomerase activity and its association with psychological stress, mental disorders, lifestyle factors and interventions: A systematic review. Psychoneuroendocrinology, 64, 150–163. doi:10.1016/j.psyneuen.2015.11.017 [DOI] [PubMed] [Google Scholar]
- de Punder K., Heim C., Wadhwa P. D., Entringer S. (2019). Stress and immunosenescence: The role of telomerase. Psychoneuroendocrinology, 101, 87–100. doi:10.1016/j.psyneuen.2018.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R. B., Pawelec G. (1997). Replicative senescence of T cells: Does the Hayflick limit lead to immune exhaustion? Immunology Today, 18, 450–454. [DOI] [PubMed] [Google Scholar]
- Felger J. C. (2018). Imaging the role of inflammation in mood and anxiety-related disorders. Current Neuropharmacology, 16, 533–558. doi:10.2174/1570159x15666171123201142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer M., Jevitt C. (2014). Symptoms and signs associated with postpartum thyroiditis. Journal of Thyroid Research, 2014, 531969 doi:10.1155/2014/531969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer M. E., Jevitt C., Ji M. (2015). Immune changes and dysphoric moods across the postpartum. American Journal of Reproductive Immunology, 73, 193–198. doi:10.1111/aji.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer M. W. (2005). Differences between exclusive breast feeders, formula-feeders, and controls: A study of stress, mood, and endocrine variables. Biological Research for Nursing, 7, 106–117. doi:10.1177/1099800405280936 [DOI] [PubMed] [Google Scholar]
- Groer M. W., El-Badri N., Djeu J., Williams S. N., Kane B., Szekeres K. (2014). Suppression of natural killer cell cytotoxicity in postpartum women: Time course and potential mechanisms. Biological Research for Nursing, 16, 320–326. doi:10.1177/1099800413498927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer M. W., Jevitt C. M., Sahebzamani F., Beckstead J. W., Keefe D. L. (2013). Breastfeeding status and maternal cardiovascular variables across the postpartum. Journal of Womens Health (Larchmt), 22, 453–459. doi:10.1089/jwh.2012.3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer M. W., Vaughan J. H. (2013). Positive thyroid peroxidase antibody titer is associated with dysphoric moods during pregnancy and postpartum. Journal of Obstetrics, Gynecology and Neonatal Nursing, 42, E26–32. doi:10.1111/j.1552-6909.2012.01425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer M. W., Yolken R. H., Xiao J. C., Beckstead J. W., Fuchs D., Mohapatra S. S.…Postolache T. T. (2011). Prenatal depression and anxiety in toxoplasma gondii-positive women. American Journal of Obstetrics and Gynecology, 204, 433 e431–437. doi:10.1016/j.ajog.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. (1990). Telomeres shorten during ageing of human fibroblasts. Nature, 345, 458–460. doi:10.1038/345458a0 [DOI] [PubMed] [Google Scholar]
- Jevitt C. M., Groer M. W., Crist N. F., Gonzalez L., Wagner V. D. (2012). Postpartum stressors: A content analysis. Issues in Mental Health Nursing, 33, 309–318. doi:10.3109/01612840.2011.653658 [DOI] [PubMed] [Google Scholar]
- Kipling D., Wynford-Thomas D., Jones C. J., Akbar A., Aspinall R., Bacchetti S.…Faragher R. G. (1999). Telomere-dependent senescence. Nature Biotechnology, 17, 313–314. doi:10.1038/7827 [DOI] [PubMed] [Google Scholar]
- Korakas E., Dimitriadis G., Raptis A., Lambadiari V. (2018). Dietary composition and cardiovascular risk: A mediator or a bystander? Nutrients, 10 doi:10.3390/nu10121912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Hao Y., Fan F., Zhang B. (2018). The role of microbiome in insomnia, circadian disturbance and depression. Frontiers in Psychiatry, 9, 669 doi:10.3389/fpsyt.2018.00669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouff J. M., Schutte N. S. (2017). A meta-analysis of the relationship between anxiety and telomere length. Anxiety, Stress and Coping, 30, 264–272. doi:10.1080/10615806.2016.1261286 [DOI] [PubMed] [Google Scholar]
- McNair D. M., Lorr M., Droppleman L. F. (1992). Profile of Mood States manual. San Diego, CA: Educational and Industrial Testing Service. [Google Scholar]
- Meena M., Chopra S., Jain V., Aggarwal N. (2016). The effect of anti-thyroid peroxidase antibodies on pregnancy outcomes in euthyroid women. Journal of Clinical Diagnosis Research, 10, QC04–QC07. doi:10.7860/jcdr/2016/19009.8403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J. (2002). Postpartum depression. Journal of the American Medical Association, 287, 762–765. doi:10.1001/jama.287.6.762 11851544 [Google Scholar]
- Mitchell A. M., Kowalsky J. M., Epel E. S., Lin J., Christian L. M. (2018). Childhood adversity, social support, and telomere length among perinatal women. Psychoneuroendocrinology, 87, 43–52. doi:10.1016/j.psyneuen.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara M. W., McCabe J. E. (2013). Postpartum depression: Current status and future directions. Annual Reviews of Clinical Psychology, 9, 379–407. doi:10.1146/annurev-clinpsy-050212-185612 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Coia H. G., Karl J. P., Pantoja-Feliciano I. G., Zachos N. C., Racicot K. (2018). Intestinal in vitro and ex vivo models to study host-microbiome interactions and acute stressors. Frontiers in Physiology, 9, 1584 doi:10.3389/fphys.2018.01584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Xiao X., Hu M., Zhang X. (2018). Interaction between gut microbiome and cardiovascular disease. Life Sciences, 214, 153–157. doi:10.1016/j.lfs.2018.10.063 [DOI] [PubMed] [Google Scholar]
- Russo A., Cesari M., Onder G., Zamboni V., Barillaro C., Pahor M.…Landi F. (2007). Depression and physical function: Results from the aging and longevity study in the Sirente geographic area (iLSIRENTE study). Journal of Geriatric Psychiatry and Neurology, 20, 131–137. doi:10.1177/0891988707301865 [DOI] [PubMed] [Google Scholar]
- Saretzki G. (2009). Telomerase, mitochondria and oxidative stress. Experimental Gerontology, 44, 485–492. doi:10.1016/j.exger.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Slavich G. M. (2016). Life stress and health: A review of conceptual issues and recent findings. Teaching of Psychology, 43, 346–355. doi:10.1177/0098628316662768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D. E., Vigod S. (2016). Postpartum depression. New England Journal of Medicine, 375, 2177–2186. doi:10.1056/NEJMcp1607649 [DOI] [PubMed] [Google Scholar]
- Thibeau S., D’Apolito K., Minnick A. F., Dietrich M. S., Kane B., Cooley S., Groer M. (2016). Relationships of maternal stress with milk immune components in African American mothers of healthy term infants. Breastfeeding Medicine, 11, 6–14. doi:10.1089/bfm.2015.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai L. K. (2004). Telomeres, telomerase, and tumorigenesis—A review. Medscape General Medicine, 6, 19. [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Rane G., Dai X., Shanmugam M. K., Arfuso F., Samy R. P.…Sethi G. (2016). Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Research Reviews, 25, 55–69. doi:10.1016/j.arr.2015.11.006 [DOI] [PubMed] [Google Scholar]