Abstract
Background:
Nonpharmacologic stress reduction interventions provide an opportunity to modify chronic pain trajectories; however, the biological mechanisms underlying these interventions are poorly understood.
Objectives:
To examine clinical literature published in 2012–2018 with the goals of (1) identifying which biological mechanisms or biomarkers are currently being measured in nonpharmacologic stress reduction intervention studies for individuals with chronic pain and (2) evaluating the evidence to determine whether these stress reduction interventions lead to changes in (a) pain outcomes and/or (b) measured biomarkers.
Data sources:
Scientific articles in the electronic databases PubMed/Medline, Cumulative Index of Nursing and Allied Health Literature, PsychINFO, and SCOPUS following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Study selection:
Randomized controlled trials and quasi-experimental studies that recruited subjects with a chronic pain condition, examined a relationship between a nonpharmacologic stress reduction intervention and pain-related outcome(s), and included measurement of a biomarker.
Results:
The 13 articles that met inclusion criteria spanned four nonpharmacologic stress reduction categories: mindfulness-based stress reduction, physical exercise, manual therapies, and biofeedback. Methods for studying biomarkers included measuring biological samples, neurological function, and autonomic control. Although all studies investigated both biological measures and pain outcomes, only three demonstrated an association between the biomarker(s) and pain-related outcomes.
Conclusions:
The results of this review highlight the complex nature of stress–pain relationships and the lack of rigorous clinical research identifying specific stress-related biological factors that modulate pain outcomes. Stress reduction interventions remain a favorable method for symptom management in patients living with chronic pain, but consistency in study measures and design is needed for robust evaluation.
Keywords: chronic pain, nonpharmacologic intervention, stress reduction intervention, psychological stress, biomarkers
For many, the experience of chronic pain (CP) impacts daily well-being and may lead to long-term physical and psychological consequences (Fine, 2011; Global Burden of Diseases, Injuries, and Risk Factors Study 2017 Risk Factor Collaborators, 2018; Pitcher et al., 2018). Approximately one in five individuals are living with a CP condition worldwide, including 20.4% of the population in the United States (Dahlhamer et al., 2018; International Association for the Study of Pain [IASP], n.d.). CP is broadly defined as pain extending beyond 3 months or lasting longer than the expected time of healing (IASP, n.d.; Treede et al., 2015). It may be diagnosed as a primary disease, as seen with idiopathic chronic low back pain (CLBP), or secondary to another medical condition such as cancer or diabetes mellitus (Fine, 2011; IASP, n.d.; Treede et al., 2019; U.S. Department of Health and Human Services, 2016). The duration, severity, and impact of CP differ among individuals, emphasizing that pain is a personalized experience (Dahlhamer et al., 2018; Pitcher et al., 2018).
CP is often conceptualized using the biopsychosocial model, which specifically describes an intricate network of processes that contribute to the maintenance and the outcomes of CP (Gatchel, 2004; Gatchel et al., 2007). This model clearly outlines the relationships among biological processes (e.g., nociception, central sensitization), cognitive–affective systems (e.g., perceived stress, pain evaluation), and external factors (e.g., culture, relationships) elicited from the social world that are involved in CP (Gatchel, 2004; Gatchel et al., 2007). In particular, the role of stress in the development of CP is a crucial consideration because research has identified stressors that are intrinsic to pathophysiological pain processes and those that are extrinsically located in the social environment. Biological responses to stressors initiate neuroendocrine cascades within the hypothalamic–pituitary–adrenal (HPA) axis, eliciting both physiological and behavioral changes (Smith & Vale, 2006). Changes in the HPA axis, including dysregulation of neurotransmitters and endogenous hormones, may further influence CP, leading to alterations in cerebral plasticity and nociceptive tone (Eller-Smith et al., 2018; Vachon-Presseau et al., 2013).
Although acute stress initially creates an analgesic effect for the individual experiencing pain, it can also have a negative, hyperalgesic effect on pain modulated by the magnitude (i.e., high or low) of an individual’s stress response (Geva & Defrin, 2018; Vachon-Presseau, 2018). Moreover, repeated, long-term exposure to stressors and maladaptive emotional reactions to stressors (e.g., anxiety, fear, depression), can modify the pain response by contributing to neurobiological changes in pain processing pathways, a phenomenon termed stress-induced hyperalgesia (Jennings et al., 2014; Olango & Finn, 2014). Individual differences in genetic predisposition are also critical factors, as varying genotypes influence an individual’s susceptibility to stress and pain in addition to subsequent responses to and outcomes of a painful experience (Gatchel, 2004; Gatchel et al., 2007; Novais et al., 2016; Olango & Finn, 2014).
Nonpharmacologic interventions often target stress reduction as the mechanism of action to reduce pain and other symptoms. Multiple types of nonpharmacologic stress reduction techniques exist that generally fall within broader categories such as relaxation techniques, physical training programs, cognitive behavioral therapy, massage, and biofeedback. Although health care clinicians frequently encourage patients with CP to use these methods and the methods have good feasibility, the findings regarding their effectiveness in improving pain-related outcomes are conflicting (Eller-Smith et al., 2018; Fisher et al., 2018; Majeed et al., 2018; Petersen & la Cour, 2016; Skelly et al., 2018; Theadom et al., 2015). One reason for this lack of agreement may be a limited understanding of how these methods target the biological mechanisms within the stress-response system.
To advance the knowledge of interactions between these stress reduction techniques and the biological mechanisms underlying the stress response, we reviewed the current literature to identify scientific studies that specifically examined biological mechanisms, termed biomarkers, associated with specific nonpharmacologic stress reduction interventions for individuals with CP. Strimbu and Tavel (2010) define biomarkers as the “objective, quantifiable characteristics of normal biological processes” (p. 2). Empirical evidence supporting the effectiveness of targeted nonpharmacologic stress reduction interventions will help to formulate recommendations for optimal application across clinical settings. The primary questions driving this systematic review were the following: Which biological mechanisms are being measured in current research on nonpharmacologic stress reduction interventions for individuals with CP? and What evidence has been published showing that nonpharmacologic stress reduction interventions lead to a change in the measured biological process and/or pain outcomes?
Method
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guided this systematic review (Moher et al., 2009). We employed the electronic databases PubMed/Medline, the Cumulative Index of Nursing and Allied Health Literature, PsycINFO, and SCOPUS to identify relevant articles. The primary database search terms included stress reduction, non-pharmacologic*, integrative, intervention, chronic pain, pain disorder, biomarker, and biological mechanism. Searches in PubMed/Medline included the specific Medical Subject Headings phrase “chronic pain [majr].” We also reviewed the reference lists of qualifying articles for additional relevant publications to screen.
Studies met inclusion criteria if they (a) were published in English between January 2012 and December 2018 due to recent advancements and increased accessibility to biological measurement techniques (e.g., RNA-seq, genome-wide association studies), (b) implemented a randomized controlled trial (RCT) or quasi-experimental study design, (c) recruited subjects with a CP condition, (d) examined a relationship between a nonpharmacologic stress reduction intervention and a reported pain-related outcome(s), and (e) included one or more biological measurements. For the purposes of the present review, biological measurements included one or more of the following: Biological samples extracted from participants, use of imaging techniques to characterize pathophysiological processes, and/or parasympathetic measurements used to show changes in biological processes. We defined nonpharmacologic stress reduction interventions broadly as techniques used to diminish symptoms and promote well-being, including educational/cognitive, psychological, and/or physiological methods. We excluded studies if they were (a) an assessment of pain in animal models, (b) psychometric studies, (c) surveys, (d) case studies, or (e) dissertations.
The first and last authors (KBC and AS) independently screened abstracts and full-text articles according to the PRISMA checklist and inclusion/exclusion criteria (Moher et al., 2009). After identifying the final sample of articles, KBC extracted data from each study into an electronic table and confirmed results with the last author (AS). We completed a narrative analysis of each study to evaluate the effects of the stress reduction interventions on pain outcomes and biological measures.
We assessed quality and risk of bias using the Joanna Briggs Institute (JBI) critical appraisal checklists for RCTs (13 items) and quasi-experimental studies (9 items; Tufanaru et al., 2017). For these checklists, each item has the following possible responses: “Yes,” “No,” “Unclear,” or “Not applicable.” Yes is scored as 1 and all other answers as 0. We established score ranges for evaluating the methodological quality of the RCT reports as follows: low quality (1–4), moderate quality (5–7), and high quality (8–13; Moola et al., 2017). For the quasi-experimental studies, the comparable ranges were as follows: low quality (1–3), moderate quality (4–6), and high quality (7–9). KBC and AS assessed the methodological quality for each of the included studies, and they subsequently reviewed together. We made the decision by consensus a priori to include all articles which scored as moderate or high quality in the present review. We resolved any lack of consensus regarding quality scores by discussion.
Results
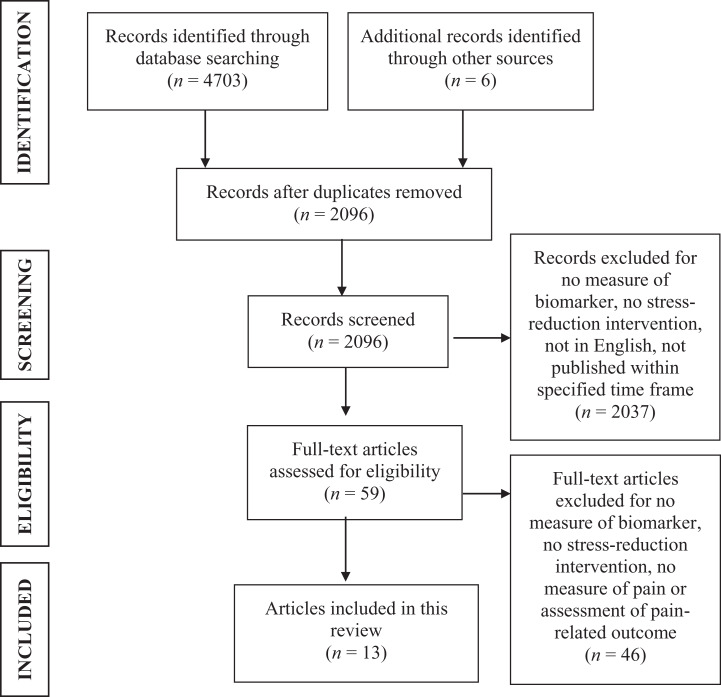
After removing duplicate articles, we screened a total of 2,096 article titles and abstracts and assessed 59 full-text articles for meeting selection criteria. A final sample of 12 studies (reported in 13 articles) met the criteria for the present review, with two journal articles reporting different study results from a single interventional trial (Cho et al., 2015; Lee et al., 2014). We evaluated all 12 studies as moderate-to-high quality and included them in this systematic review. Figure 1 displays the PRISMA flow diagram of the article-screening process. Descriptive information we obtained from each individual article, including study design, subject descriptions, stress reduction intervention, outcome measures, major results, and quality appraisals, is included in Table 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram of the systematic review article-screening procedure.
Table 1.
Study Characteristics of Selected Articles for Systematic Review by Intervention Type.
| Authors (Year) | Study Design | Final Sample | Stress Reduction Intervention | Pain-Related Measures | Biological Measure(s) | Major Results | QA |
|---|---|---|---|---|---|---|---|
| MBSR | |||||||
| Ardito et al. (2017) | QE | N = 28 (17 active participants; 11 wait-list controls). Chronic low-back pain. M age = 48.14 years | 8-week MBSR program including body scan meditation, sitting/walking meditation, yoga, relational mindfulness exercises, and home practice | Pain severity: NRS | Salivary cortisol levels | Pain: Significant postintervention difference between the
intervention and control groups’ pain distributions,
t(22) = 2.69, p = .01,
d = 1.03. Cortisol: Significant difference in postintervention evening cortisol levels, t(15) = 3.18, p = .006. |
7/9 |
| Braden et al. (2016) | QE, P | N = 23 (12 intervention group; 11 reading control group). Chronic low-back pain. M age T = 46 years | 4-week MBSR program including breath work, body scan, guided imagery, mindful practices, emotion observation, and home practice | Oswestry Low Back Pain Scale | Neural network activity assessed with fMRI during sadness-induction and control vision tasks to measure BOLD neural signals in emotional processing regions of the brain | Pain: Intervention group experienced significant improvement of
low-back pain postintervention, t(9) = 2.30,
p = .04, d =
0.28. MRI: Intervention group showed postintervention changes in BOLD signals during sadness-induction task in the left sgACC, t(11) = 20.37, p = .0009, d = 1.68; right sgACC, t(11) = 7.53; p = .019, d = 1.06; and left vlPFC, t(11) = 4.95, p = .032, d = 1.04; BOLD signal in left sgACC correlated with sad valence in intervention group, r(10) = .65, p = .021. |
9/9 |
| Crisp et al. (2016) | QE, P | N = 9. Women with chronic pelvic pain. M age = 31.4 years | 8-week MBSR training; daily-diary home practice; encouraging message sent via text/email/instant 3 days per week | BPI | Cytokine profiles from vaginal swabs, collected pre- and postintervention; levels of IL-1β and TNF-α assayed | Pain: No statistically significant changes. Cytokine profiles: No statistically significant changes. |
5/9 |
| Grossman et al. (2017) | RCT | N = 163 (130 experimental subjects, 33 healthy controls). Women with FMS. M age T = 54.1 years | Three-armed trial: (1) 8-week MBSR training including mindfulness and yoga; (2) RELAX active control group completed 8-week course of progressive relaxation and yoga with physical therapy; and (3) wait-list control group | PPS | Continuous respiratory, cardiac, and accelerometry signal monitoring; HRV | Pain: No statistically significant
changes. Physiological/HRV: No statistically significant changes. |
9/13 |
| Schmidt et al. (2015) | QE, P | N = 21. Chronic low-back pain > 1 year. M age = 47.8 years | 8-week MBSR intervention including mindfulness, yoga, and home practice | PPS; VAS | Patterns of thalamocortical dysrhythmia measured by EEG | Pain: Postintervention, participants rated improvements in
affective pain (p = .038, d =
0.5), average maximum (p = .003;
d = 1.15) and minimum (p =
.002, d = 0.64) pain severity from
baseline. EEG: No statistically significant changes. |
6/9 |
| Zgierska et al. (2016) | RCT, P | N = 35 (21 active participants; 14 wait-list controls). Chronic low-back pain with treatment of ≥30 mg/day morphine-equivalent dose of opioid for >3 months. M age = 51.8 years | 8-week mixed mindfulness meditation and CBT program including mindfulness practice, coping strategies, breath work, movement exercises, and home practice | BPI; pain intensity and unpleasantness with thermal stimuli | Serum levels of CRP and cytokines (IL-1β, TNF-α, IL-6, and INF-γ) | Pain: Intervention group reported decreased average pain at 8
(p = .045, 95% CI [0.01–1.7],
d = 0.69) and 26 weeks (95% CI [0.2–1.9],
d = 0.86); the intervention group had
reduced pain intensity at 8 (p = .024) and 26
weeks (p = .049) and reduced pain
unpleasantness at 8 (p = .003) and 26 weeks
(p = .028) compared to
controls. CRP: No statistically significant changes at 8 (95% CI [−0.3 to 2.2], d = 0.48) or 26 weeks (95% CI [−0.4 to 2.1], d = 0.34). IL-1β: No statistically significant changes at 8 (95% CI [0.8–0.2], d = 0.51) or 26 weeks (95% CI [−0.6 to 0.5], d = 0.05). IL-6: No statistically significant changes at 8 (95% CI [−0.4 to 2.7], d = 0.53) or 26 weeks (95% CI [−1.3 to 1.8], d = −0.47). TNF-α: No statistically significant changes at 8 (95% CI [−3.2 to 8.4], d = 0.34) or 26 weeks (95% CI [−2.5 to 9.2], d = 0.38). INF-γ: No statistically significant changes at 8 (95% CI [−6.6 to 4.4], d = 0.16) or 26 weeks (95% CI [−8 to 3.7], d = 0.33). |
10/13 |
| Physical exercise | |||||||
| Gerdle et al. (2016) | QE | N = 57 (29 FMS, 28 healthy controls). Women with FMS. M age T = 53.7 years | 15-week exercise intervention targeting muscle strength and health status, including twice weekly sessions with a physical therapist | VAS; pressure pain threshold with algometer | Microdialysis samples of vastus lateralis muscle measuring concentrations of lactate, pyruvate, glutamate, glucose, and glycerol | Pain: FMS participants had lower postintervention pain intensity
at all five data collection time points (p =
.01–.07). Microdialysis: Postintervention, FMS group had decreases in glucose (p = .039), glutamate (p = .006), and pyruvate (p = .007). Mean pain intensity: Significantly correlated with decreases in pyruvate and glucose concentrations in FMS group (R 2 = .13, p < .05). |
8/9 |
| Lee et al. (2014) a | QE | N = 25 women (14 intervention group; 11 control group). Premenopausal women with back pain >3 months. M age = 43.3 years | 12 weeks of 60-min group hatha yoga classes 3 times/week; each class included 12 poses of increasing difficulty | VAS | Serum levels of BDNF and serotonin | Pain: Intervention group had decreased postintervention VAS
scores (p < .001). BDNF: Increased postintervention levels in intervention group (p < .01). Serotonin: No statistically significant changes in intervention group. |
8/9 |
| Cho et al. (2015) a | Same as Lee et al. (2014) | Same as Lee et al. (2014) | Same as Lee et al. (2014) | RMDQ | Serum levels of cortisol, CRP, and TNF-α | Pain: Intervention group had decreased postintervention RMDQ
scores (p < .05). Cortisol: Intervention group experienced postintervention decrease (p < .05). CRP: No statistically significant changes. TNF-α: No statistically significant changes. |
8/9 |
| Scioli-Salter et al. (2016) | QE, P | N = 12 (5 with CP/PTSD; 7 TC). Musculoskeletal pain >3 months and PTSD. M age = 39 years | One session of a peak cardiopulmonary exercise test | Pain interference; cold pressor test for pain threshold and tolerance | Plasma levels of neurohormones, including NPY, ALLO, cortisol, and DHEA | Pain interference: Not reported. NPY: No significant changes across time; no correlation to pain measures noted when BMI used as covariate. ALLO: Statistically significant increases across time, F(3, 27) = 18.01, p < .001, partial η2 = 0.67; CP/PTSD participants had increased levels compared to the TC group over time, F(1, 8) = 7.12, p < .05, with BMI as a covariate; no correlation to pain measures noted with BMI as a covariate. Cortisol: No significant changes across time; pre- to 30-min postexercise associated with pain tolerance after exercise (r = −.69, p < .05) with BMI as a covariate. DHEA: No significant changes across time; pre- to 30-min postexercise associated with pain tolerance (r = −.58, p < .05) with BMI as a covariate. |
7/9 |
| Manual therapies | |||||||
| Törnhage et al. (2013) | RCT, P | N = 44 (29 in experimental group; 16 active controls). Parkinson’s disease >2 years with CP. M age T = 62.5 years | Intervention group completed 10 sessions tactile-massage intervention with music over 8 weeks; control group participated in 10 rest-to-music sessions over 8 weeks | UPDRS I–IV | Salivary cortisol levels | Pain: No statistically significant changes. Cortisol concentration: Immediately after first session, experimental group had decrease (p = .002); 30 min after first session, both the experimental (p = .0003) and the active control (p = .016) groups had decrease; after eighth session, both the experimental and the active control groups had posttreatment decrease immediately (p = .003; p = .028, respectively) and 30 min after (p = .0006; p = .028, respectively). Total cortisol secretion: Significantly decreased immediately after the eighth intervention in the experimental (p = .003) and active control (p = .035) groups; total cortisol secretion remained decreased in experimental group (p = .004) 30-min postintervention. |
11/13 |
| Yuen et al. (2017) | RCT, P | N = 12. Adults ≥60 years old w/persistent low-back pain >3 months. M age = 67.7 years | In a crossover study design, subjects participated in a single session of Gua sha or hot-pack therapy (active control), followed by a 28-day washout period and then completed the alternative therapy session; both therapies were performed for 15 min | VAS; RMDQ | Salivary biomarkers TNF-α and HO-1 | Pain: Gua sha showed more significant reduction of pain
(p < .001) than hot-pack therapy
(p < .05) on Days 1 and 7
postintervention; RMDQ scores more significantly decreased in
Gua sha group (p < .001) than hot-pack
therapy group (p < .05); VAS scores
correlated with RMDQ scores (r = .37,
p < .05). TNF-α: No statistically significant changes; significant correlation noted with RMDQ score (r = .51, p < .01). HO-1: No statistically significant changes. |
12/13 |
| Biofeedback | |||||||
| Berry et al. (2014) | RCT, P | N = 14 (8 treatment; 6 control). Veterans with diagnosed CP. M age T = 44.5 years | 4 weeks of HRVCB training, including controlled breathing and emotional self-regulation | BPI short form | HRV; cardiac coherence | Pain: Postintervention, treatment group displayed reduction in
pain ratings, t(7) = 6, p <
.001, 95% CI [6.0, 13.7]. Coherence: Treatment group demonstrated increased postintervention cardiac coherence ratio, t(7) = −1.8, p = .05, 95% CI [−0.5, 0.0]. |
8/13 |
Note. ALLO = allopregnanolone/pregnanolone; BDNF = brain-derived neurotrophic factor; BMI = body mass index; BOLD = blood oxygen level–dependent; BPI = Brief Pain Inventory; CBT = cognitive behavioral therapy; CI = confidence interval; CP = chronic pain; CRP = C-reactive protein; EEG = electroencephalogram; DHEA = dehydroepiandrosterone; fMRI = functional magnetic resonance imaging; FMS = fibromyalgia syndrome; HO-1 = heme-oxygenase 1; HRV = heart rate variability; HRVCB = heart rate variability coherence biofeedback; IL = interleukin; INF-γ = interferon gamma; M age = mean age; M age T = mean age treatment group; MBSR = mindfulness-based stress reduction; NPY = neuropeptide Y; NRS = Numeric Rating Scale; P = pilot or feasibility trial; PPS = Pain Perception Scale; PTSD = posttraumatic stress disorder; QA = quality appraisal; QE = quasi-experimental; RCT = randomized controlled trial; RMDQ = Roland–Morris Disability Questionnaire; sgACC = subgenual anterior cingulate cortex; TC = trauma-exposed healthy controls; TNF-α = tumor necrosis factor alpha; UPDRS = Unified Parkinson’s Disease Related Scale; VAS = Visual Analog Scale; vlPFC = ventrolateral and dorsomedial prefrontal cortices.
a Lee et al. (2014) and Cho et al. (2015) report data from the same interventional trial and study sample.
The majority of the studies were conducted in the United States (n = 5), followed by Germany (n = 2), Sweden (n = 2), Hong Kong (n = 1), Italy (n = 1), and the Republic of Korea (n = 1). Of the 12 studies, 7 were quasi-experimental studies and 5 were RCTs. Over half of the studies were described as a pilot and/or feasibility trial (n = 8). The sample sizes of the studies, including control subjects, ranged from 9 (Crisp et al., 2016) to 163 (Grossman et al., 2017). No articles identified in the search included pediatric samples, and the mean age of study participants was greater than 30 years. Across all studies, the majority of participants (approximately 80%) identified as female, and four of the studies (covered in five of the articles) specifically limited their recruitment criteria to include only female participants (Cho et al., 2015; Crisp et al., 2016; Gerdle et al., 2016; Grossman et al., 2017; Lee et al., 2014).
CLBP was the most frequent CP condition studied (n = 6 studies; Ardito et al., 2017; Braden et al., 2016; Cho et al., 2015; Lee et al., 2014; Schmidt et al., 2015; Yuen et al., 2017; Zgierska et al., 2016), followed by fibromyalgia syndrome (FMS; n = 2; Gerdle et al., 2016; Grossman et al., 2017) . In two articles, researchers reported on individuals with CP and an additional primary diagnosis, specifically, posttraumatic stress disorder (Scioli-Salter et al., 2016) and Parkinson’s disease (Törnhage et al., 2013). Additionally, two articles reported the effects of a stress reduction intervention in military service groups: one with active-duty women in the military with chronic pelvic pain (Crisp et al., 2016) and the other a pilot study with veterans experiencing generalized CP (Berry et al., 2014).
The category of nonpharmacologic stress reduction intervention these authors most frequently investigated was mindfulness-based stress reduction (MBSR), as identified in six studies (Ardito et al., 2017; Braden et al., 2016; Crisp et al., 2016; Grossman et al., 2017; Schmidt et al., 2015; Zgierska et al., 2016). Researchers in three studies (discussed in four articles) assessed the effects of physical exercise interventions on CP and biological mechanisms (Cho et al., 2015; Gerdle et al., 2016; Lee et al., 2014; Scioli-Salter, 2016). In two studies, researchers evaluated nonpharmacologic stress reduction interventions that involved the manipulation of tissue and joints, specifically tactile massage (Törnhage et al., 2013) and Gua sha, a traditional integrative therapy that can be used to reduce muscle pain and involves the application of pressure in a stroking sequence until petechiae form (Yuen et al., 2017). Yuen et al. (2017) compared the Gua sha treatment to a hydrocollator hot-pack treatment in a randomized AB/BA crossover study design sequence (i.e., subjects received treatment A then treatment B or vice versa) separated by a 28-day washout period between treatments. The hot-pack therapy included the application of a moist heating pack placed superficially on the low back in the same approximate location of the Gua sha treatment. Finally, a single study investigated the results of a heart rate variability coherence biofeedback intervention, which included the self-regulation techniques of controlled breathing and emotional awareness, on stress and pain in veterans (Berry et al., 2014).
Across the 13 articles, researchers used ten different methods to evaluate pain. For measuring pain severity, they used the Numeric Rating Scale (Ardito et al., 2017; Zgierska et al., 2016), the Brief Pain Inventory (Berry et al, 2014; Crisp et al., 2016; Zgierska et al., 2016), the Oswestry Low Back Pain Scale (Braden et al., 2016), and a Visual Analog Scale (Gerdle et al., 2016; Lee et al., 2014; Schmidt et al., 2015; Yuen et al., 2017). In two of the MBSR studies, researchers examined pain perception using the Pain Perception Scale (Grossman et al., 2017; Schmidt et al., 2015). Scioli-Salter and colleagues (2016) measured pain interference, but did not report results in the article we evaluated for this review. In another two studies, researchers evaluated thermal stimuli in their pain assessment, including (1) pain threshold and tolerance to cold (Scioli-Salter et al., 2016) and (2) pain intensity and unpleasantness in response to heat (Zgierska et al., 2016). Gerdle et al. (2016) also used an algometer to assess the pressure pain threshold prior to the intervention. Further, two more studies evaluated pain-related functional ability outcomes with the Roland–Morris Disability Questionnaire (RMDQ; Cho et al., 2015; Yuen et al., 2017). Törnhage et al. (2013) did not use a pain-specific measure in their study but instead implemented the Unified Parkinson’s Disease Related Scale, which includes two questions regarding Parkinson’s-related pain frequency and severity (Fahn et al., 1987). Of note, only 3 of the 13 articles included self-report measures of stress in their data collection, including (1) the Perceived Stress Scale (Berry et al., 2014; Zgierska et al., 2016) and (2) the Symptoms of Stress Inventory (Cho et al., 2015).
Methods Used for Biological Measurement
Biological samples
In nine of the articles, researchers investigated the effect of a nonpharmacologic stress reduction intervention on specific biochemical substances. In two of these, researchers examined salivary cortisol to directly assess HPA function (Ardito et al., 2017; Törnhage et al., 2013). Participants in the Ardito et al. (2017) study collected saliva samples at 08:00 and 23:00 prior to the intervention start and after the intervention was completed (8-week MBSR program). Törnhage et al. (2013) examined diurnal cortisol rhythms by collecting salivary samples at 08:00, 13:00, 20:00, and 08:00 the following day at five time points during the 8-week intervention. They also collected salivary cortisol immediately prior to, immediately following, and 30 min after the first and eighth interventions. While Ardito et al. (2017) analyzed their salivary cortisol samples with an electrochemiluminescence immunoassay, Törnhage et al. (2013) used a radioimmunoassay technique. Both examined total secretion of cortisol, but only Törnhage et al. (2013) calculated diurnal rhythms and salivary cortisol concentrations over time.
Researchers in four studies focused specifically on assessing inflammatory biomarkers, which are associated with glucocorticoid release in the HPA pathway, although the specimen types varied among studies. Yuen et al. (2017) used an enzyme-linked immunosorbent assay (ELISA) to measure salivary levels of tumor necrosis factor alpha (TNF-α) and heme-oxygenase 1 prior to and twice after (Days 1 and 7) each treatment arm of the AB/BA crossover design. Alternatively, Zgierska et al. (2016) used an electrochemiluminescence assay to measure C-reactive protein (CRP) and multiple inflammatory cytokines (interleukin 1-beta [IL-1β], IL-6, TNF-α, and interferon γ) taken from serum samples at baseline and 8 and 26 weeks from baseline. In the third study, Crisp et al. (2016) examined the pro-inflammatory biomarkers TNF-α and IL-1β in vaginal mucosal secretions to assess cytokine profiles in women with chronic pelvic pain. Specifically, the researchers analyzed swab samples attained prior to the intervention and at Week 8 using bead-based multiplex assays.
In one interventional trial, researchers also investigated the inflammatory biomarkers CRP and TNF-α as well as cortisol (Cho et al., 2015) and pain-specific neuromodulators, specifically brain-derived neurotrophic factor (BDNF) and serotonin (Lee et al., 2014). Researchers collected the serum samples for the results presented in both of these articles before and after the 12-week intervention in a single trial. They used the following four techniques to measure the five targets: immunoturbidimetric assays for the measurement of CRP, ELISA kits for the analysis of TNF-α and BDNF, radioimmunoassay kits for the evaluation of cortisol levels, and high-performance liquid chromatography to measure serotonin (Cho et al., 2015; Lee et al., 2014).
Gerdle et al. (2016) investigated the interstitial concentrations of lactate, pyruvate, glutamate, glucose, and glycerol, which are metabolites that each have a role in cell signaling within the inflammatory and stress cascades. Investigators sampled dialysate from two microdialysis catheters placed in the vastus lateralis muscle, targeting the free nerve endings of nociceptors, every 20 minutes during a 220-min testing period at baseline and after the 15-week exercise intervention. The research team analyzed the interstitial concentrations of dialysate samples from five time points during the testing using a microdialysis analyzer.
Finally, Scioli-Salter et al. (2016) examined plasma levels of nociceptive-specific inhibitory neurohormones, including neuropeptide Y (NPY), allopregnanolone/pregnanolone (ALLO), cortisol, and dehydroepiandrosterone (DHEA). Investigators obtained whole-blood samples at five time points during a single cardiorespiratory exercise intervention: baseline, 5 min prior to the exercise, during peak exercise, and 5 and 30 min after the exercise. To analyze the neurohormone levels, they used variations of radioimmunoassay techniques (cortisol, DHEA, and NPY) and gas chromatography–mass spectrometry (ALLO).
Neurological function
Investigators in two of the studies sought to investigate the impact of stress reduction interventions on neurological function in adults experiencing CP. Braden et al. (2016) examined emotion processing by using functional magnetic resonance imaging (fMRI) to measure changes in neural activity while participants completed an emotional self-awareness task. Each participant completed an fMRI within 2 weeks before and after the stress reduction intervention. During the scans, the participants completed a sadness-induction task in which they were presented with visual and auditory stimuli and asked to report on their emotional state in response. The investigators measured changes in blood oxygen level–dependent (BOLD) signals in the ventrolateral and dorsomedial prefrontal cortices, anterior insula, and anterior cingulate cortex (ACC).
Schmidt et al. (2015) monitored thalamocortical dysrhythmia (TCD) patterns using an electroencephalogram (EEG) to assess whether alterations in neural processing patterns after an MBSR intervention were related to improved pain outcomes. Participants completed an EEG at study enrollment and after the intervention. Researchers evaluated TCD patterns by determining the peak frequency, peak power, center of gravity, and overall power of the EEG data.
Autonomic control
In two studies, investigators evaluated physiological functions. In one trial, Berry et al. (2014) sought to understand whether a biofeedback intervention would increase heart rate variability (HRV) as a measure of cardiac coherence. They assessed the effect of the intervention by monitoring changes in the low-frequency HRV waveforms and calculating the cardiac coherence ratio (peak frequency power/[total frequency power − peak frequency power]), measured in Hertz (Hz), using data collected before and after the 4-week intervention. Grossman et al. (2017) monitored cardiorespiratory function and accelerometry using an ambulatory monitoring system for 24-hr time periods before the intervention, postintervention, and 8 weeks after the completion of the MBSR trial. The research team described different trajectories over time for physiological measures in the intervention and control groups to explore whether autonomic changes were associated with improvements in FMS symptoms.
Outcomes of Stress Reduction Interventions
MBSR
Of the six studies that evaluated the use of MBSR programs in individuals living with CP, four identified significant improvements in pain (Ardito et al., 2017; Braden et al., 2016; Schmidt et al., 2015; Zgierska et al., 2016). Each study reported effect sizes, ranging from small (d = 0.28; Braden et al., 2016) to large (d = 1.15; Schmidt et al., 2015), with the 8-week training programs demonstrating a greater impact on pain severity than the 4-week intervention in one study (Braden et al., 2016). In two of the studies (Ardito et al., 2017; Braden et al., 2016), investigators found a significant change in biological measures; however, only Braden et al. (2016) reported an effect size indicating that the MBSR intervention has a large effect on BOLD signals, particularly in the left subgenual ACC, t(11) = 20.37, p = .0009, d = 1.68.
Physical exercise
In four articles, researchers investigated the effects of various physical activities. In two studies, the physical exercise interventions led to decreased pain (Gerdle et al., 2016; Lee et al., 2014), and in one study, the intervention led to improved functional ability (Cho et al., 2015). However, none of these authors reported effect sizes. In the last of these studies, Scioli-Salter et al. (2016) did not report results from patient-reported pain outcomes. Authors in two of these studies reported that the interventions had an effect on biological measures that were associated with changes in pain sensitivity (Gerdle et al., 2016; Scioli-Salter et al., 2016). Specifically, Gerdle et al. (2016) found that decreased pain intensity in the intervention group was positively correlated with decreased interstitial concentrations of pyruvate and glucose (R 2 = .13, p < .05). Scioli-Salter et al. (2016) showed that 30 min after a single session of peak cardiopulmonary exercise cold pain threshold was positively correlated with NPY levels (r = .66, p < .05) and cold pain tolerance was inversely associated with cortisol (r = −.69, p < .05) and DHEA (r = −.58, p < .05) levels.
Manual therapies
In one study that evaluated manual therapy, researchers noted a more significant reduction in subjective pain scores after participants received Gua sha (p < .001) in comparison to hot-pack therapy (p < .05; Yuen et al., 2017). Pain-related functional ability followed a similar pattern, with a more significant improvement following Gua sha (p < .001) than hot-pack treatment (p < .01). These two pain outcomes were positively correlated (r = .37, p < .05) for the Gua sha intervention. In addition, a reduction in TNF-α was positively correlated with RMDQ scores after Gua sha therapy (r = .51, p < .01). In another study, Törnhage et al. (2013) reported a reduction in concentration and total secretion of cortisol in both the intervention and active control groups immediately following the interventions and at the end of the trial sequence, as shown in Table 1. However, changes in cortisol concentration, secretion, and diurnal rhythm were not sustained long term. The authors did not report any measure of effect size in either of these two articles.
Biofeedback
The intervention Berry et al. (2014) tested led to decreased pain-intensity ratings for veterans living with CP, t(7) = 6, p < .001, 95% confidence interval [CI] [6.0, 13.7]). Further, the intervention group displayed increased cardiac coherence ratios after the intervention, with a 191% improvement in coherence, t(7) = −1.8, p = .05, 95% CI [−0.5, 0.0], indicating that the self-regulation techniques promoted parasympathetic activity. The authors did not report on associations between cardiac coherence and pain ratings.
Discussion
The results of the present review show the potential for implementing a wide range of nonpharmacologic stress reduction interventions in patients with CP. To our knowledge, this is the first systematic review examining biological measures associated with nonpharmacologic stress reduction interventions for clinical CP populations. Although the nonpharmacologic stress reduction interventions we evaluated in the present review were generally associated with improvements in pain outcomes, there was no consistent evidence that the interventions directly targeted the biological mechanisms of pain perception, as the selected biomarkers did not demonstrate repeated associations with changes in pain severity. Previous reviews have also identified mixed results of nonpharmacological stress reduction interventions on CP outcomes (Majeed et al., 2018; Skelly et al., 2018). Methodological differences across the studies in this review (i.e., the variations in measurement of pain, stress, and biological mechanisms; intervention designs) present a considerable challenge for reaching definitive conclusions other than to highlight the pressing need for additional research. Our findings may be used, however, to point to the potential opportunities for future investigations (e.g., replication studies) to ameliorate the limitations of the current evidence.
Sample characteristics and size varied considerably across the studies with only two including more than 50 people in their analyses (Gerdle et al., 2016; Grossman et al., 2017). Less than half (5 of the 13 articles, describing 12 studies) of the publications included descriptions of a power analysis to determine study sample size (Braden et al., 2016; Cho et al., 2015; Gerdle et al., 2016; Grossman et al., 2017; Lee et al., 2014; Törnhage et al., 2013). Additionally, the majority of participants were female, and only three articles included discussions of sociodemographic characteristics other than age or education in their sample descriptions (Crisp et al., 2016; Grossman et al., 2017; Zgierska et al., 2016). Previous research has reported that women are more likely than men to report CP conditions and has identified sex-linked differences in pain perception and sensitivity (Mills et al., 2019). Yet sample homogeneity (e.g., the overrepresentation of female participants) and a lack of clearly described representation of male subgroups or reporting of other demographic characteristics of potential interest further decrease the generalizability of the available findings across different pain populations. In addition, we identified no articles with a pediatric sample (<18 years old) that met our inclusion criteria. More diverse samples are needed to understand the ways in which nonpharmacologic stress reduction interventions might affect pain-related processes while accounting for sex and age variations.
In the five studies that implemented an RCT (Berry et al., 2014; Grossman et al., 2017; Törnhage et al., 2013; Yuen et al., 2017; Zgierska et al., 2016), descriptions of blinding were limited. Due to the nature of mind–body intervention studies, which typically involve a psychosocial component, it is difficult to implement double-blinding procedures in the study design. Utilization of crossover or attention-control designs can reduce threats to study validity (Aycock et al., 2018). Among the studies included in this review, four implemented an attention-control design (Braden et al., 2016; Gerdle et al., 2016; Grossman et al., 2017; Törnhage et al., 2013) and one used a crossover design (Yuen et al., 2017).
Additionally, attrition from the nonpharmacologic stress reduction intervention conditions was a concern across multiple studies, impairing the internal validity of the findings. However, eight of the studies were feasibility or pilot trials. Reasons participants cited for leaving a trial included worsening pain (Ardito et al., 2017; Braden et al., 2016), interference with family or work commitments (Cho et al., 2015; Crisp et al., 2016), and lack of interest (Ardito et al., 2017; Zgierska et al., 2016).
Descriptions of biological measures and methods also varied across the studies. As there is no single standard method for biological measurement in stress reduction interventions, it is difficult to assess the appropriateness of each method applied. Saliva was the most common biological sample collected, possibly due to the noninvasive procedure and the potential for home collection with proper participant instruction. Increased transparency in reporting on the sample collection and processing techniques would improve assessment of reliability across studies. In one study, authors did report inappropriate handling of the biological samples (Crisp et al., 2016), which affects confidence in the interpretation of the reported results.
It is also important to consider the long-term effects of nonpharmacologic stress reduction interventions. Of the studies examined in the present review, four included self-report measures as a long-term follow-up at 8 weeks (Grossman et al., 2017), 18 weeks (Zgierska et al., 2016), 4–5 months (Ardito et al., 2017), and 1 year (Braden et al., 2016) after the completion of the study intervention delivery period. Pain severity remained decreased relative to baseline in a single study (Ardito et al., 2017). Among the studies that did not identify sustained decreases in pain outcomes, the authors discussed attrition and worsening clinical symptoms as factors that might have influenced these findings. Investigators measured biological samples at a follow-up time point in only one study, although they did not find sustained reduction in inflammatory mediators (Zgierska et al., 2016). Authors of a recent review article in which they extensively investigated the long-term effectiveness of nonpharmacologic interventions across multiple CP populations identified similar findings, noting that future research should focus on sustainability of intervention effects (Skelly et al., 2018). However, Skelly et al. (2018) did not include the investigation of biological measures in their review. Therefore, this article adds to these findings by stressing the need for standard protocols to investigate long-term effects of nonpharmacologic interventions and long-term measurement of biomarkers to understand the underlying processes.
At this stage of the science, relatively little is known about the precise stress-related biological factors that modulate pain outcomes or how they may be used to optimize pain management strategies for individual patients. Lack of consistency in the intervention designs within the intervention categories and the biological measures used across studies limits our ability to draw robust conclusions on the effectiveness of the nonpharmacologic stress reduction interventions included in the present review. Further investigation is warranted to understand the role of particular nonpharmacologic stress reduction intervention categories in treating CP, including specific skills and protocols comprising each intervention to facilitate replication and how they relate to mechanisms affecting the experience of CP. Measuring pain trajectories and stress-related biomarkers from the onset of pain would also inform the timing and content of therapeutic intervention delivery.
Limitations
It is important to consider several limitations of the present review to allow for a balanced interpretation of our findings. Exclusion of studies published prior to 2012 may have limited our search results; however, this time frame is reasonable given advancements in biological assays and methods employed. Additionally, we did not include gray literature (e.g., dissertations), which may have contributed to the findings of this review. Although many studies have investigated stress reduction interventions and/or biomarkers in CP populations, identifying studies that met both of these criteria was the single greatest limiting factor for inclusion in this analysis. We did not restrict the type of nonpharmacologic stress reduction intervention, CP, or biomarker a priori, which likely factored into the diversity of methods we identified in our findings. Considering the small number of studies that met our inclusion criteria and the fact that these studies spanned four categories of intervention methods, our ability to draw any general conclusions about the overall effectiveness of stress reduction interventions at a mechanistic level was limited, and addressing that question requires additional research. Finally, although the independent assessments of each article led to moderate-to-high quality assessment (QA) scores (Table 1), the synthesis of the research findings in the present review exposed many limitations in this body of research, as previously discussed. While the JBI QA checklist provides a method for assessing individual studies based on design, the use of additional criteria to evaluate the validity of specific biobehavioral measures and methods used may be necessary when conducting a QA for these types of studies.
Conclusion
Management of CP remains a priority for interdisciplinary health care teams. Precision health approaches that address patient-specific needs have the potential to inform individualized care for patients experiencing CP. This systematic review highlights the paucity of available studies that both describe a nonpharmacologic stress reduction intervention among participants living with CP and also investigate biological measures in these populations. Additional research is needed to fill this gap. In order to advance the science supporting precision health for individuals with CP, future research will need to evaluate the mechanisms of action associated with nonpharmacologic stress reduction interventions and include theoretically grounded biomarkers of the proposed mechanisms. Well-designed and adequately powered studies are needed to identify effective stress reduction strategies directed at improving pain intensity, function, and other pain-related outcomes. Integration of the findings from the current review will facilitate the growing evidence base and improve clinical care through greater standardization in the application of multimodal interventions that can positively impact pain outcomes within a biopsychosocial framework.
Footnotes
Authors’ Note: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: Katherine M. Bernier Carney contributed to conception and design, acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Erin E. Young contributed to analysis and interpretation, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Jessica W. Guite contributed to analysis and interpretation, critically revised manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Angela R. Starkweather contributed to conception and design, acquisition, analysis, and interpretation; critically revised manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The time spent to develop this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under award no. T32NR013456.
ORCID iDs: Katherine M. Bernier Carney  https://orcid.org/0000-0003-2571-8951
https://orcid.org/0000-0003-2571-8951
Angela R. Starkweather  https://orcid.org/0000-0001-7168-0144
https://orcid.org/0000-0001-7168-0144
References
- Ardito R. B., Pirro P. S., Re T. S., Bonapace I., Menardo V., Bruno E., Gianotti L. (2017). Mindfulness-based stress reduction program on chronic low-back pain: A study investigating the impact on endocrine, physical, and psychologic functioning. Journal of Alternative and Complementary Medicine, 23(8), 615–623. 10.1089/acm.2016.0423 [DOI] [PubMed] [Google Scholar]
- Aycock D. M., Hayat M. J., Helvig A., Dunbar S. B., Clark P. C. (2018). Essential considerations in developing attention control groups in behavioral research. Research in Nursing & Health, 41(3), 320–328. 10.1002/nur.21870 [DOI] [PubMed] [Google Scholar]
- Berry M. E., Chapple I. T., Ginsberg J. P., Gleichauf K. J., Meyer J. A., Nagpal M. L. (2014). Non-pharmacological intervention for chronic pain in veterans: A pilot study of heart rate variability biofeedback. Global Advances in Health and Medicine, 3(2), 28–33. 10.7453/gahmj.2013.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden B. B., Pipe T. B., Smith R., Glaspy T. K., Deatherage B. R., Baxter L. C. (2016). Brain and behavior changes associated with an abbreviated 4-week mindfulness-based stress reduction course in back pain patients. Brain and Behavior, 6(3), e00443 10.1002/brb3.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. K., Moon W., Kim J. (2015). Effects of yoga on stress and inflammatory factors in patients with chronic low back pain: A non-randomized controlled study. European Journal of Integrative Medicine, 7(2), 118–123. 10.1016/j.eujim.2014.10.008 [DOI] [Google Scholar]
- Crisp C. D., Hastings-Tolsma M., Jonscher K. R. (2016). Mindfulness-based stress reduction for military women with chronic pelvic pain: A feasibility study. Military Medicine, 181(9), 982–989. 10.7205/MILMED-D-15-00354 [DOI] [PubMed] [Google Scholar]
- Dahlhamer J., Lucas J., Zelaya C., Nahin R., Machey S., DeBar L., Kerns R., Von Korff M., Porter L., Helmick C. (2018). Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morbidity and Mortality Weekly Report, 67, 1001–1006. 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller-Smith O. C., Nicol A. L., Christianson J. A. (2018). Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Frontiers in Cellular Neuroscience, 12, 35 10.3389/fncel.2018.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S., Elton R. L., & UPDRS Program Members. (1987). Unified Parkinson’s Disease Rating Scale In Fahn S., Marsden C. D., Goldstein M., Calne D. B. (Eds.), Recent developments in Parkinson’s disease (Vol. 2, pp. 153–163). Macmillan Healthcare Information. [Google Scholar]
- Fine P. G. (2011). Long-term consequences of chronic pain: Mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Medicine, 12, 998–1004. [DOI] [PubMed] [Google Scholar]
- Fisher E., Law E., Dudeney J., Palermo T. M., Stewart G., Eccleston C. (2018). Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews, 2018(9), CD003968 10.1002/14651858.CD003968.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel R. J. (2004). Comorbidity of chronic pain and mental health disorders: The biopsychosocial perspective. American Psychologist, 59(8), 795–805. 10.1037/0003-066X.59.8.795 [DOI] [PubMed] [Google Scholar]
- Gatchel R. J., Peng Y. B., Peters M. L., Fuchs P. N., Turk D. C. (2007). The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychological Bulletin, 133(4), 581–624. 10.1037/0033-2909.133.4.581 [DOI] [PubMed] [Google Scholar]
- Global Burden of Diseases, Injuries, and Risk Factors Study 2017 Risk Factor Collaborators. (2018). Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. The Lancet, 392(10159), 1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdle B., Ernberg M., Mannerkorpi K., Larsson B., Kosek E., Christidis N., Ghafouri B. (2016). Increased interstitial concentrations of glutamate and pyruvate in vastus lateralis of women with fibromyalgia syndrome are normalized after an exercise intervention—A case-control study. PLoS One, 11(10), e0162010 10.1371/journal.pone.0162010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva N., Defrin R. (2018). Opposite effects of stress on pain modulation depend on the magnitude of individual stress response. Journal of Pain, 19(4), 360–371. [DOI] [PubMed] [Google Scholar]
- Grossman P., Deuring G., Walach H., Schwarzer B., Schmidt S. (2017). Mindfulness-based intervention does not influence cardiac autonomic control or the pattern of physical activity in fibromyalgia during daily life: An ambulatory, multimeasure randomized controlled trial. Clinical Journal of Pain, 33(5), 385–394. 10.1097/AJP.0000000000000420 [DOI] [PubMed] [Google Scholar]
- International Association for the Study of Pain. (n.d.). Unrelieved pain is a major global health care problem. Retrieved January 25, 2019, from https://s3.amazonaws.com/rdcms-iasp/files/production/public/Content/ContentFolders/GlobalYearAgainstPain2/20042005RighttoPainRelief/factsheet.pdf
- Jennings E. M., Okine B. N., Roche M., Finn D. P. (2014). Stress-induced hyperalgesia. Progress in Neurobiology, 121, 1–18. 10.1016/j.pneurobio.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Lee M., Moon W., Kim J. (2014). Effect of yoga on pain, brain-derived neurotrophic factor, and serotonin in premenopausal women with chronic low back pain. Evidence-Based Complementary and Alternative Medicine, 2014, 203173 10.1155/2014/203173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed M. H., Ali A. A., Sudak D. M. (2018). Mindfulness-based interventions for chronic pain: Evidence and applications. Asian Journal of Psychiatry, 32, 79–83. 10.1016/j.ajp.2017.11.025 [DOI] [PubMed] [Google Scholar]
- Mills S., Nicolson K. P., Smith B. H. (2019). Chronic pain: A review of its epidemiology and associated factors in population-based studies. British Journal of Anaesthesia, 123(2), e273–e283. 10.1016/j.bja.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. [DOI] [PubMed] [Google Scholar]
- Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., Currie M., Lisy K., Qureshi R., Mattis P., Mu P. F. (2017). Systematic reviews of etiology and risk In Aromataris E., Munn Z. (Eds.), Joanna Briggs Institute reviewer’s manual (Chapter 7). The Joanna Briggs Institute; https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- Novais A., Monteiro S., Roque S., Correia-Neves M., Sousa N. (2016). How age, sex and genotype shape the stress response. Neurobiology of Stress, 6, 44–56. 10.1016/j.ynstr.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olango W. M., Finn D. P. (2014). Neurobiology of stress-induced hyperalgesia. Current Topics in Behavioral Neuroscience, 20, 251–280. 10.1007/7854_2014_302 [DOI] [PubMed] [Google Scholar]
- Petersen M., La Cour P. (2016). Mindfulness—What works for whom? Referral, feasibility, and user perspectives regarding patients with mixed chronic pain. Journal of Alternative and Complementary Medicine, 22(4), 298–305. 10.1089/acm.2015.0310 [DOI] [PubMed] [Google Scholar]
- Pitcher M. H., Von Korff M., Bushnell M. C., Porter L. (2018). Prevalence and profile of high-impact chronic pain in the United States. Journal of Pain, 20(2), 146–160. 10.1016/j.jpain.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S., Gmeiner S., Schultz C., Löwer M., Kuhn K., Naranjo J. R., Brenneisen C., Hinterberger T. (2015). Mindfulness-based stress reduction (MBSR) as treatment for chronic back pain—An observational study with assessment of thalamocortical dysrhythmia. Forschende Komplementarmedizin, 22(5), 298–303. 10.1159/000440687 [DOI] [PubMed] [Google Scholar]
- Scioli-Salter E., Forman D. E., Otis J. D., Tun C., Allsup K., Marx C. E., Hauger R. L., Shipherd J. C., Higgins D., Tyzik A., Rasmusson A. M. (2016). Potential neurobiological benefits of exercise in chronic pain and posttraumatic stress disorder: Pilot study. Journal of Rehabilitation Research and Development, 53(1), 95–106. 10.1682/JRRD.2014.10.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly A. C., Chou R., Dettori J. R., Turner J. A., Friedly J. L., Rundell S. D., Fu R., Brodt E. D., Wasson N., Winter C., Ferguson A. J. R. (2018). Noninvasive nonpharmacological treatment for chronic pain: A systematic review. U.S. Agency for Healthcare Research and Quality; 10.23970/AHRQEPCCER209 [DOI] [PubMed] [Google Scholar]
- Smith S. M., Vale W. W. (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience, 8(4), 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimbu K., Tavel J. A. (2010). What are biomarkers? Current opinion in HIV and AIDS, 5(6), 463–466. 10.1097/COH.0b013e32833ed177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theadom A., Cropley M., Smith H. E., Feigin V. L., McPherson K. (2015). Mind and body therapy for fibromyalgia. Cochrane Database of Systematic Reviews, 2015(4), CD001980 10.1002/14651858.CD001980.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnhage C. J., Skogar Ö., Borg A., Larsson B., Robertsson L., Andersson L., Backström P., Fall P. A., Hallgren G., Bringer B., Carlsson M., Lennartsson U. B., Sandbjörk H., Lökk J. (2013). Short- and long-term effects of tactile massage on salivary cortisol concentrations in Parkinson’s disease: A randomised controlled pilot study. BMC Complementary and Alternative Medicine, 13, 357 10.1186/1472-6882-13-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede R.-D., Rief W., Barke A., Aziz Q., Bennett M. I., Benoliel R., Cohen M., Evers S., Finnerup N. B., First M. B., Giamberardino M. A., Kaasa S., Korwisi B., Kosek E., Lavand’homme P., Nicholas M., Perrot S., Scholz J., Schug S.…Wang S. J. (2019). Chronic pain as a symptom or a disease: The IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain, 160(1), 19–27. 10.1097/j.pain.0000000000001384 [DOI] [PubMed]
- Treede R.-D., Rief W., Barke A., Aziz Q., Bennett M. I., Benoliel R., Cohen M., Evers S., Finnerup N. B., First M. B., Giamberardino M. A., Kaasa S., Kosek E., Lavand’homme P., Nicholas M., Perrot S., Scholz J., Schug S., Smith B. H.…Wang S. J. (2015). A classification of chronic pain for ICD-11. Pain, 156(6), 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufanaru C., Munn Z., Aromataris E., Campbell J., Hopp L. (2017). Systematic reviews of effectiveness In Aromataris E., Munn Z. (Eds.), Joanna Briggs Institute reviewer’s manual (Chapter 3). The Joanna Briggs Institute; https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- U.S. Department of Health and Human Services. (2016). National pain strategy: A comprehensive population health-level strategy for pain. https://iprcc.nih.gov/sites/default/files/HHSNational_Pain_Strategy_508C.pdf
- Vachon-Presseau E. (2018). Effects of stress on the corticolimbic system: Implications for chronic pain. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 87, 216–223. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E., Roy M., Martel M. O., Caron E., Marin M. F., Chen J., Albouy G., Plante I., Sullivan M. J., Lupien S. J., Rainville P. (2013). The stress model of chronic pain: Evidence from basal cortisol and hippocampal structure and function in humans. Brain: A Journal of Neurology, 136(Pt 3), 815–827. 10.1093/brain/aws371 [DOI] [PubMed] [Google Scholar]
- Yuen J. W. M., Tsang W. W. N., Tse S. H. M., Loo W. T. Y., Chan S. T., Wong D. L. Y., Chung H. H. Y., Tam J. K. K., Choi T. K. S., Chiang V. C. L. (2017). The effects of Gua sha on symptoms and inflammatory biomarkers associated with chronic low back pain: A randomized active-controlled crossover pilot study in elderly. Complementary Therapies in Medicine, 32, 25–32. 10.1016/j.ctim.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Zgierska A. E., Burzinski C. A., Cox J., Kloke J., Stegner A., Cook D. B., Singles J., Mirgain S., Coe C. L., Bačkonja M. (2016). Mindfulness meditation and cognitive behavioral therapy intervention reduces pain severity and sensitivity in opioid-treated chronic low back pain: Pilot findings from a randomized controlled trial. Pain Medicine, 17(10), 1865–1881. 10.1093/pm/pnw006 [DOI] [PMC free article] [PubMed] [Google Scholar]