Trial registration
ChiCTR, ChiCTR2000029758. Registered 12 February 2020 - Retrospectively registered
Dear editors:
The COVID-19 pandemic has spread rapidly around the world and overwhelmed the supply of intensive care beds and ventilators; judicious ICU resource allocation is still one of the major challenges for clinicians and management [1]. The higher incidence of ARDS is the main reason for the burden of ventilator equipment. Early prediction of the occurrence and aggravation of ARDS in the ICU helps clinicians prepare for respiratory support equipment given the absence of effective treatment strategies. Moreover, early selected patients with severe ARDS who do not benefit from conventional treatment might be successfully supported with V-V ECMO [2], which is a relatively scarce critical care resource. Therefore, early prediction of moderate-severe ARDS can help clinicians better allocate scarce ICU resources for COVID-19 crisis.
Neutrophil-to-lymphocyte ratio (NLR) is a simple biomarker of inflammation that can be measured during routine hematology. Previous studies have exhibited that higher NLR was associated with clinical deterioration and mortality for COVID-19 patients [3]. However, it remains unclear to what extent the significance of NLR would predict the occurrence of ARDS and ICU ventilator requirements for the COVID-19 crisis.
Patients diagnosed with severe COVID-19 from 21 hospitals in Sichuan Province between January 16 and March 15 were included in the analysis (ChiCTR2000029758). The maximum value of NLR, PLR, PCT, and CRP during the first 3 days after being diagnosed as severe COVID-19 was included in the analysis. Severe COVID-19 and ARDS were defined according to previous study [4] and Berlin definition [5], respectively. Multivariate logistic regression analysis and the area under the receiver operating characteristic (ROC) curve were used to analyze the ability of NLR in predicting ARDS.
Of totally 81 patients defined as severe COVID-19, 44 were diagnosed as ARDS. The baseline characteristics of the non-ARDS group and ARDS group are listed in Table 1. The area under the ROC curve for ARDS was 0.71, 0.591, 0.494, and 0.625 for NLR, PLR, PCT, and CRP, respectively. We used the median as the cutoff value to divide the patients into two groups. The high NLR group (NLR > 9.8) showed a higher incidence of ARDS (P = 0.005) and higher rate of noninvasive (P = 0.002) and invasive (P = 0.048) mechanical ventilation. Further, we defined moderate-severe ARDS as ARDS patients with oxygenation index less than 150. The area under the ROC curve for moderate-severe ARDS was 0.749, 0.660, 0.531, and 0.635 for NLR, PLR, PCT, and CRP, respectively (Fig. 1); the cutoff value of NLR for moderate-severe ARDS is 11.
Table 1.
Baseline characteristics and clinical outcomes stratified by median NLR value
| Baseline characteristics | Non-ARDSN = 37 | ARDSN = 44 | Pvalues |
| Age | 49 (36.5–62.5) | 53.5(43–70.5) | 0.110 |
| Gender/case (%) | 0.891 | ||
| Male | 23 (62.3%) | 28 (63.6%) | |
| Female | 14 (37.8%) | 16 (36.4%) | |
| BMI (kg/m2) | 23.05 (22.00–27.25) | 24.78(21.29–27.41) | 0.816 |
| Smoking/case (%) | 1 (2.7%) | 2 (4.5%) | 1.000 |
| Comorbidities/case (%) | |||
| Diabetes | 3 (8.1%) | 15 (34.15) | 0.007 |
| Hypertension | 7 (18.9%) | 8 (18.2%) | 0.932 |
| Chronic pulmonary disease | 2 (5.4%) | 9 (20.5%) | 0.049 |
| Cardiovascular disease | 2 (5.4%) | 2 (4.5%) | 1.000 |
| Cerebrovascular disease | 0 (0%) | 3 (6.8%) | 0.246 |
| Renal disease | 1 (2.7%) | 2 (4.5%) | 1.000 |
| Liver disease | 2 (5.4%) | 2 (4.5%) | 1.000 |
| Vital signs | |||
| MAP/mmHg | 94.67 (89.17–100.50) | 97.83(91.75,108.84) | 0.162 |
| Heart rate (beats/min) | 88 (77.5–99) | 92.5 (85.25–104) | 0.175 |
| Respiratory rate (breaths/min) | 20 (20–22.5) | 21 (20–23) | 0.107 |
| Pulse oxygen saturation/% | 96 (93.75–97.25) | 95 (90.25–97) | 0.486 |
| Laboratory findings | |||
| WBC/109/L | 5.43 (4.05–6.59) | 6.47 (3.94–9.62) | 0.122 |
| Hemoglobin/g/L | 141 (127–153.5) | 132 (117.25–146.5) | 0.107 |
| Total bilirubin (μmol/L) | 9 (5.93–15.6) | 9.3 (6.65–14.3) | 0.927 |
| AST (IU/L) | 30.5 (19–39.75) | 29.15 (15.75–57.68) | 0.764 |
| ALT (IU/L) | 30 (25–39.8) | 35 (25.75–51.6) | 0.221 |
| Creatinine (μmol/L) | 71.75 (54.35–79.75) | 69.2 (54.63–80.53) | 0.980 |
| PT/s | 12.7 (12.5–13.98) | 13.1 (12.6–13.8) | 0.787 |
| APTT/s | 32.75 (29.1–40.13) | 31.3 (28.8–35.5) | 0.246 |
| NLR/% | 6.4 (3.75–13.1) | 13.55 (6.05–24.13) | 0.002 |
| Clinical outcomes | Low NLRN = 41 | High NLRN = 40 | Pvalue |
| Respiratory support | |||
| High-flow nasal cannula | 15 (36.6%) | 16 (40%) | 0.752 |
| Noninvasive ventilation | 5 (12.2%) | 17 (42.5%) | 0.002 |
| Invasive ventilation | 2 (4.9%) | 8 (20%) | 0.048 |
| ARDS | |||
| Mild-moderate ARDS | 11 (26.8%) | 11 (27.5%) | 0.946 |
| Moderate-severe ARDS | 5 (12.2%) | 11 (42.5%) | 0.002 |
Data are presented as interquartile range or number (percentage)
BMI body mass index, MAP mean arterial pressure, WBC white blood cell, AST aspartate aminotransferase, ALT alanine aminotransferase, PT prothrombin time, APTT activated partial thromboplastin time, NLR neutrophil-to-lymphocyte ratio, ARDS acute respiratory distress syndrome
Fig. 1.
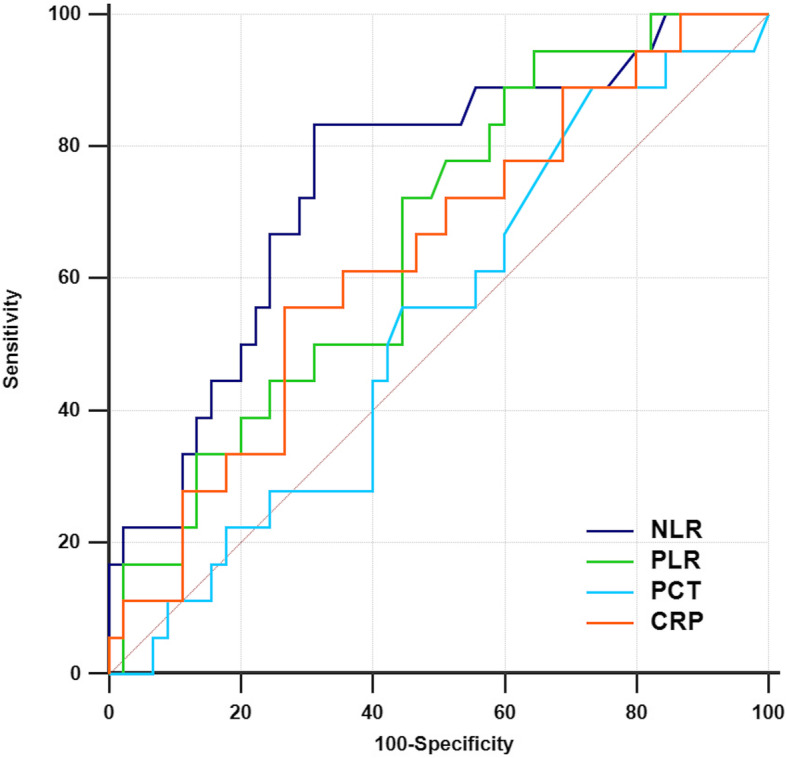
Moderate-severe ARDS prediction biomarkers in severe COVID-19 patients: NLR (0.749, 95% CI 0.624–0.850), PLR (0.660, 95% CI 0.530–0.775), PCT (0.531, 95% CI 0.401–0.658), and CRP (0.635, 95% CI 0.504–0.752)
Our data revealed that NLR could be a valuable biomarker to recognize severe COVID-19 patients with moderate-severe ARDS, which facilitated clinicians to give effective respiratory supporting strategies and quickly find out moderate-severe ARDS patients who are at high indication for V-V ECMO.
Because of the mismatch of the oxygenation and lung function [6], a comprehensive consideration of immune indicators would improve early prediction for COVID-19 patients with “atypical” ARDS [6]. NLR is an extremely common laboratory test wherein the initial NLR value can be used to identify high-risk patients with moderate-severe ARDS, with the optimal threshold value of 11. This biomarker may be helpful in assessing the allocation of respiratory equipment in ICU patients and early assessment of ECMO. However, further clinical studies are needed to evaluate the benefits of NLR in ARDS.
Acknowledgements
We would like to thank all the medical workers involved in the rescue and the staff for collection of the data in Sichuan. We would like to thank all the investigators of the study of 2019 novel coronavirus pneumonia-infected critically ill patients in Sichuan province (SUNRISE).
Abbreviations
- COVID-19
Coronavirus disease 2019
- ARDS
Acute respiratory distress syndrome
- ICU
Intensive care unit
- WHO
World Health Organization
- ECMO
Extracorporeal membrane oxygenation
- V-V ECMO
Veno-venous extracorporeal membrane oxygenation
- NLR
Neutrophil-to-lymphocyte ratio
- PLR
Platelet-to-lymphocyte ratio
- PCT
Procalcitonin
- CRP
C-reactive protein
- ROC
Receiver operating characteristic
Authors’ contributions
AJM, JLC, MLD, and JY designed the study. MLD and YK participated in the rescue work on the clinical frontline. XLL and YK organized and managed the data and its quality. JLC and AJM collected the data, performed the statistical analysis, and drafted the manuscript with JY. All authors participated in the data interpretation. All authors read the manuscript carefully and approved the final version.
Funding
This project was supported by Project of Novel Coronavirus Pneumonia in West China Hospital (HX2019nCoV027).
Availability of data and materials
The datasets used for the analysis in the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the West China Hospital of Sichuan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aijia Ma, Jiangli Chen, and Jing Yang contributed to the work equally and should be regarded as co-first authors.
Contributor Information
Aijia Ma, Email: maaaijiaicu@gmail.com.
Jiangli Cheng, Email: 2581715118@qq.com.
Jing Yang, Email: yjingscu@163.com.
Meiling Dong, Email: dml332639860@163.com.
Xuelian Liao, Email: xuelianliao@hotmail.com.
Yan Kang, Email: Kangyan@scu.edu.cn.
References
- 1.Liew MF, Siow WT, MacLaren G, See KC. Preparing for COVID-19: early experience from an intensive care unit in Singapore. Crit care (London) 2020;24(1):83. doi: 10.1186/s13054-020-2814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramanathan K, Antognini D, Combes A, Paden M, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8(5):518–526. doi: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;S0163-4453(20)30208-5. 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed]
- 4.Liao X, Chen H, Wang B, Li Z, et al. Critical care for patients with severe COVID-2019 in Sichuan Province, China——a provincial cohort study.medRxiv 2020.03.22.20041277; 10.1101/2020.03.22.20041277.
- 5.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. Jama. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 Does Not Lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–300. 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used for the analysis in the current study are available from the corresponding author on reasonable request.


