Abstract
Introduction
Sepsis biomarkers can have important diagnostic, therapeutic, and prognostic functions. In a previous review, we identified 3370 references reporting on 178 different biomarkers related to sepsis. In the present review, we evaluate the progress in the research of sepsis biomarkers.
Methods
Using the same methodology as in our previous review, we searched the PubMed database from 2009 until September 2019 using the terms “Biomarker” AND “Sepsis.” There were no restrictions by age or language, and all studies, clinical and experimental, were included.
Results
We retrieved a total of 5367 new references since our previous review. We identified 258 biomarkers, 80 of which were new compared to our previous list. The majority of biomarkers have been evaluated in fewer than 5 studies, with 81 (31%) being assessed in just a single study. Apart from studies of C-reactive protein (CRP) or procalcitonin (PCT), only 26 biomarkers have been assessed in clinical studies with more than 300 participants. Forty biomarkers have been compared to PCT and/or CRP for their diagnostic value; 9 were shown to have a better diagnostic value for sepsis than either or both of these biomarkers. Forty-four biomarkers have been evaluated for a role in answering a specific clinical question rather than for their general diagnostic or prognostic properties in sepsis.
Conclusions
The number of biomarkers being identified is still increasing although at a slower rate than in the past. Most of the biomarkers have not been well-studied; in particular, the clinical role of these biomarkers needs to be better evaluated.
Keywords: Procalcitonin, C-reactive protein, Diagnosis, Prognosis, Infection, Validation
Introduction
Biomarkers have been evaluated for several applications in patients with sepsis including diagnosis of infection, prognostication, and therapeutic guidance. Sepsis is a common and severe condition [1, 2], responsible for high mortality and morbidity rates and also for reduced quality of life [1–4]. Sepsis biomarkers may provide information beyond what is available using other metrics and could therefore help inform clinical decision-making and potentially improve patient management. For example, more timely and appropriate antibiotic therapy could be administered and unnecessary antibiotics avoided if biomarkers were available that could accurately diagnose sepsis early. Similarly, biomarkers could help physicians monitor the effectiveness of therapeutic decisions and adjust treatment if necessary [5]. Many potential sepsis biomarkers have been proposed, procalcitonin (PCT) and C-reactive protein (CRP) being the most frequently studied. The Surviving Sepsis Campaign guidelines for the management of sepsis mention that sepsis biomarkers can complement clinical evaluation [6], but in the Sepsis-3 definition consensus, the role of biomarkers in sepsis diagnosis remains undefined [7].
In 2010, we published a literature review of biomarkers that had been studied for their potential diagnostic or prognostic role in sepsis [8]. We concluded that none of the 178 biomarkers identified had “sufficient specificity or sensitivity to be routinely employed in clinical practice” [8]. In this narrative review, we evaluate the progress that has been made in identifying new sepsis biomarkers since that report and reappraise the utility of such research in the management of patients with sepsis.
Methods
We searched the Medline database via the PubMed portal between February 2009 and September 2019 using (“biomarker” AND “sepsis”) as keywords to identify all studies that evaluated a biomarker in sepsis. There were no restrictions by age or language, and all studies, clinical and experimental, were included. The reference lists from all relevant retrieved manuscripts were further reviewed in order to identify additional studies. For each identified biomarker, the PubMed database was searched again using the biomarker name and the keyword “biomarker.”
Newly found biomarkers were added to our previous database. Details related to the methodology used in each study were collected, namely (1) type of study (mono- vs. multicenter, prospective vs. retrospective, experimental vs. clinical), (2) study population (intensive care unit [ICU], emergency room, other population), (3) number of studied subjects, (4) reference non-sepsis population, and (5) purpose of study or use of biomarker being tested (diagnostic, prognostic, other clinical roles). Results of receiver operating characteristic (ROC) curve analysis were noted where this technique was used to assess biomarker specificity and sensitivity. The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool [9] was used to assess the methodological quality of the studies that included more than 300 patients and performed ROC analysis. For each biomarker, the main pathophysiological role (Additional file 1, Figure S1) was recorded. We also reported separately biomarkers that had been compared with PCT and/or CRP.
Results
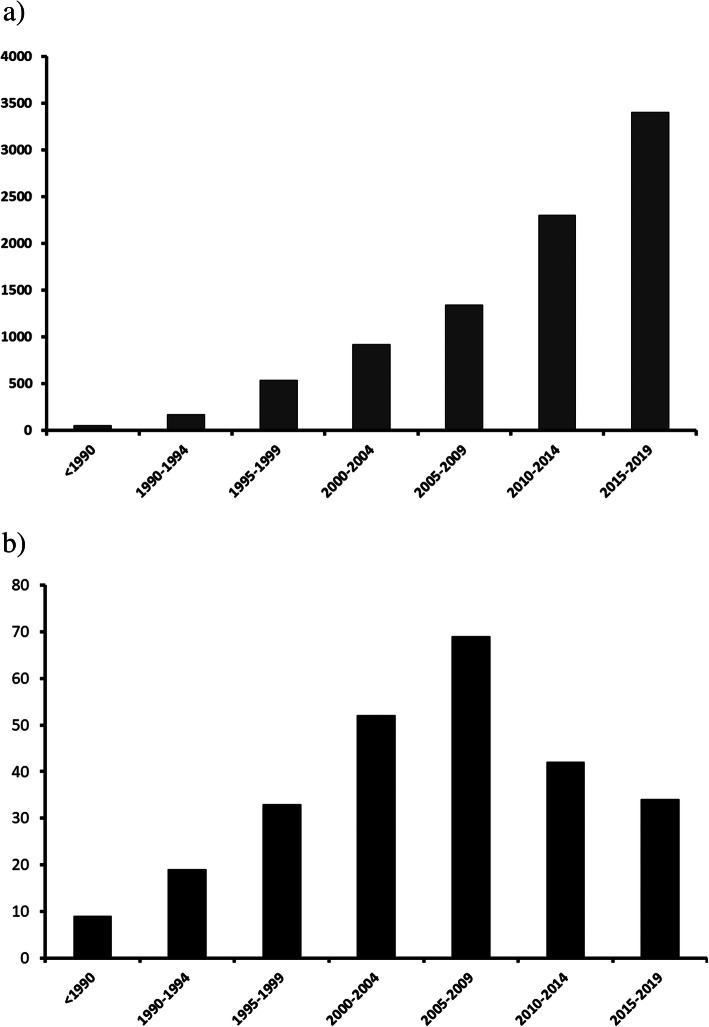
A total of 5367 studies met our search criteria for the period 2009 to 2019 compared with the 3370 studies retrieved in our previous study [3]. A total of 80 new biomarkers (54 assessed in clinical studies, 23 in clinical and experimental studies, and 3 in only experimental studies) were added to the list of 178 biomarkers that had previously been identified. Despite a steady increase in the number of published studies related to sepsis biomarkers over time, the number of publications reporting new biomarkers has decreased since our prior review (Fig. 1).
Fig. 1.
Changes over time in the a number of references meeting our search criteria and b number of new biomarkers referred to in identified references
The full list of biomarkers with selected references and major findings are shown in Additional file 1, Tables S1–9. Of the 258 biomarkers, 69 (27%) were assessed primarily for their diagnostic value, 100 (39%) for their prognostic value, and 89 (34%) for both diagnostic and prognostic purposes. A validation population was used in just 12 studies. Most of the biomarkers (n = 216 [84%]) have been assessed in fewer than five studies, and 81 (31%) have been studied only once. CRP and PCT are the biomarkers that have been studied most frequently, followed by interleukin (IL)-6, presepsin, and CD64 in 31, 25, and 21 studies, respectively.
Apart from CRP and PCT, only 26 biomarkers have been evaluated in studies that enrolled more than 300 patients (Tables 1 and 2). In 15 of these 24 studies (63%), sepsis was defined using either the 1992 ACCP/SCCM [34] or the 2001 International Sepsis Definitions Conference [35] definitions. In one study, the Sepsis-3 definition [7] was used. Other studies used definitions based on clinical signs compatible with sepsis or positive blood cultures. Of the 10 biomarkers evaluated for their diagnostic value in more than 300 patients, 6 (60%) were evaluated using receiver operating characteristic (ROC) curve analysis; the area under the curve (AUC) was > 0.8 for just three of the biomarkers (for inter-alpha inhibitor proteins [11], CD64 [13], and IL-6 [18]). Of the 18 biomarkers evaluated for their prognostic value in more than 300 patients, mortality was the primary study endpoint for 14 (78%); prediction of circulatory failure or organ dysfunction and failure of antibiotic therapy were the primary endpoints in the other studies. ROC curve analysis was used in the analysis of 9 of the 18 biomarkers (50%): the AUC for predicting mortality was > 0.8 only for pro-adrenomedullin, with a high specificity (specificity, 92%; sensitivity, 75%). In two studies, combining a sepsis biomarker with a severity score improved the predictive value (urokinase plasminogen activator receptor [uPAR] + APACHE II AUC, 0.83 [19]; adrenomedullin + Mortality in Emergency Department Sepsis (MEDS) score AUC, 0.81 [27]). All the studies that evaluated more than 300 patients and used ROC analysis had a high risk of bias because a pre-specified abnormal biomarker value was used (Additional file, Table S10).
Table 1.
Sepsis biomarkers, except for C-reactive protein (CRP) and procalcitonin (PCT), that have been evaluated for their diagnostic value in clinical studies with more than 300 subjects
| Biomarker [ref] | No. of patients | Sepsis definition | Study population | Reference group | Sensitivity/specificity (%) | AUC |
|---|---|---|---|---|---|---|
| Interleukin (IL)-27 [10] | 702 | Positive blood cultures | Pediatric ICU patients with infection | Non-infected critical care patients | 84/63 | 0.75 |
| Inter-alpha inhibitor proteins [11] | 573 | Positive blood cultures | Neonates with sepsis | Neonates with risk factors for sepsis | 89/99 | 0.9 |
| Group II phospholipase A2 [12] | 525 | ACCP 1992 | ED patients with sepsis | ED patients with suspected infection (with and without SIRS) | NR (logistic regression analysis) | NR |
| Bactericidal/permeability increasing protein [12] | 525 | ACCP 1992 | ED with sepsis | ED patients with suspected infection (with and without SIRS) | NR (logistic regression analysis) | NR |
| CD64 [13] | 468 | International Sepsis Definitions Conference 2001 | Non-selected ICU population with sepsis | ICU patients admitted without sepsis | 89/87 | 0.94 |
| Selenoprotein P [14] | 378 | ACCP 1992 | Non-selected population with sepsis or septic shock | Healthy individuals | NR (no test) | NR |
| Lipopolysaccharide-binding protein [15] | 327 | ACCP 1992 | Surgical patients without sepsis at admission | Surgical patients with SIRS without sepsis | 60/62 | 0.66 |
| Syndecan-1 [16] | 512 | International Sepsis Definitions Conference 2001 | Trauma patients (4 h after admission) without sepsis | Trauma patients without sepsis | NR (logistic regression analysis) | NR |
| Presepsin [17] | 440 | Sepsis-3 | ICU patients with sepsis | ICU patients without sepsis | 89/59 | 0.76 |
| IL-6 [18] | 306 | SIRS and organ dysfunction, systolic blood pressure < 90 mmHg, or lactate ≥ 4 mmol/L plus infection | ED patients with suspected sepsis | ED patients with SIRS and organ dysfunction, systolic blood pressure < 90 mmHg, or lactate ≥ 4 mmol/L without infection | NR | 0.86 |
ED emergency department, ICU intensive care unit, COPD chronic obstructive pulmonary disease, SIRS systemic inflammatory response syndrome, NR not reported, AUC area under the receiver operating characteristic curve
Table 2.
Sepsis biomarkers, except for C-reactive protein (CRP) and procalcitonin (PCT), that have been evaluated for their prognostic value in clinical studies with more than 300 subjects
| Biomarker [ref] | No. of patients | Sepsis definition | Study population | Main finding | Sensitivity/specificity (%) | AUC |
|---|---|---|---|---|---|---|
| Urokinase plasminogen activator receptor (uPAR) [19] | 1914 | International Sepsis Definitions Conference 2001 | Critically ill patients and patients hospitalized in internal medicine ward | Levels ≥ 12 ng/mL predicted fatal outcome within 30 days | NR/> 70% |
0.708 0.83 (when combined with APACHE II score) |
| Plasminogen activator inhibitor (PAI) 1 [20] | 1790 | ACCP 1992 | Septic patients with disseminated intravascular coagulation (DIC) | Levels > 90 ng/mL predict fatal outcome within 30 days | NR (Kaplan-Meier survival functions) | NR |
| Interleukin (IL)-12 [21] | 1444 | Proven peritonitis or mediastinitis and systemic inflammation signs | Surgical patients | Pre-surgery IL-12-synthesizing capability was low in patients who had fatal sepsis after operation | NR | 0.72 |
| Thrombomodulin [22] | 1103 | ACCP 1992 | Critically ill patients with sepsis | Levels > 14 ng/mL can predict circulatory failure or death—gray zone between 7 and 14 ng/mL | NR (logistic regression analysis) | NR |
| Syndecan-1 [22] | 1103 | ACCP 1992 | Critically ill patients with sepsis | Levels > 240 ng/mL can predict circulatory failure or death—gray zone between 70 and 240 ng/mL | NR (logistic regression analysis) | NR |
| Fibrinogen [23] | 1103 | ACCP 1992 | Critically ill patients with sepsis | Levels < 200 mg/dL related to increased risk of fatal outcome | NR (logistic regression analysis) | NR |
| Antithrombin activity [23] | 1103 | ACCP 1992 | Critically ill patients with sepsis | Decrease in activity > 50% related to increased risk of fatal outcome | NR (logistic regression analysis) | NR |
| Brain natriuretic peptide (BNP) [24] | 1000 | International Sepsis Definition Conference 2001 | ED patients | Levels > 113 pg/mL can predict fatal outcome within 28 days | 86/55 | 0.73 |
| Angiopoietin-2 [25] | 931 | NR | Critically ill patients with ARDS | Persistently increased levels related to fatal outcome within 90 days | NR (logistic regression analysis) | NR |
| Prothrombin time (PT) [26] | 840 | Suspected infection plus ≥ 3 signs of systematic inflammatory response | Critically ill patients with sepsis | Increase in PT time within first 7 days of sepsis was higher in patients who died within 28 days | NR (no test) | NR |
| Adrenomedullin [27] | 837 | International Sepsis Definitions Conference 2001 | ED patients sepsis | Levels < 34.4 ng/L predicted fatal outcome within 30 days | 86/61 |
0.77 0.81 (when combined with Mortality in Emergency Department Sepsis (MEDS) score) |
| Pro-adrenomedullin [28] | 896 | Clinical suspicion of infection | ED patients with sepsis | Levels ≥ 1.6 nmol/L predicted fatal outcome within 28 days | 75/92 | 0.89 |
| Heparin-binding protein [29] | 759 | Suspected infection and at least one clinical sign of systematic inflammatory response | ED patients with sepsis | Levels > 30 ng/mL predicted any organ dysfunction development within 72 h | 78/76 (cross-tabulation analysis) | NR |
| D-dimer [30] | 684 | International Sepsis Definitions Conference 2001 | Emergency department patients with sepsis | Higher in non-survivors than survivors within 28 days | NR | 0.68 |
| Troponin [31] | 598 | ACCP 1992 | Critically ill patients | Levels > 0.06 ng/mL independent prognostic marker for 28-day mortality | NR (logistic regression analysis) | NR |
| YKL-40 [32] | 502 | ACCP 1992 | Critically ill patients | Levels ≤ 505 ng/mL predicted survival in 90 days | 53/76 | 0.64 |
| CD64 [13] | 468 | International Sepsis Definition Conference 2001 | Critically ill patients | Sustained elevated levels were related to non-appropriate antibiotic therapy | 93/48 | 0.74 |
| Cell-free DNA [33] | 481 | International Sepsis Definitions Conference 2001 | ED patients | Levels > 1.6 μg/mL predicted short-term fatal outcome | 70/76 | 0.77 |
ARDS acute respiratory distress syndrome, NR not reported, IL interleukin, SOFA sequential organ failure assessment, AUC area under the receiver operating characteristic curve
Forty biomarkers have been compared with CRP and/or PCT for their diagnostic value (Table 3); 9 were shown to have better diagnostic value and 11 improved the diagnostic value of CRP and/or PCT when used in combination with one of these two biomarkers. In 10 of the 23 studies in which these results were reported (43%), patients with systemic inflammatory response syndrome (SIRS) without infection were selected as the reference group; two studies used patients after major surgery as the reference group. A validation group of healthy volunteers was used in 5 studies (22%).
Table 3.
Sepsis biomarkers that were compared with procalcitonin (PCT) and/or C-reactive protein (CRP) for sepsis diagnosis
| Biomarker | Study group | Reference group | Comment [refs] |
|---|---|---|---|
| Diagnostic performance similar to or worse than that of PCT and/or CRP | |||
| Cell-free DNA (cfDNA) | ICU patients with sepsis | ICU patients with SIRS | No better than PCT [36, 37] |
| Copeptin | ED patients with sepsis | ED patients with SIRS | No better than PCT [38] |
| ICAM-1 | Patients with necrotic pancreatitis | Patients with sterile necrosis | No better than PCT [39] |
| Lipopolysaccharide-binding protein | ED patients with sepsis | ED patients with infection | No better than PCT [40] |
| Non-critically ill patients with sepsis | Non-critically ill patients with infection | No better than PCT [41] | |
| Children with neutropenia and clinical sepsis and/or bacteremia | Children with febrile neutropenia without infection | No better than PCT [42] | |
| Patients with proven bacterial lower respiratory infection | Patients with proven viral lower respiratory infection | No better than CRP [43] | |
| Patients treated in internal medicine ward | Healthy control | No better than PCT [27] | |
| Pancreatic stone protein | ED patients with sepsis | ED patients without infection | No better than PCT [44] |
| sCD22 | Surgical patients with infection after major operation | Surgical patients with SIRS but without infection | Equal value to PCT [45] |
| Interleukin (IL)-2 | ICU patients with sepsis | ICU patients with SIRS without infection | No better than CRP [46] |
| IL-1β | Neonates with infection and sepsis | Neonates with infection without sepsis | No better than CRP [47] |
| RANTES | Neonates with infection | Healthy neonates | No better than CRP [48] |
| Neopterin | ICU patients with sepsis | ICU patients without sepsis | Less accurate than PCT [49, 50] |
| Macrophage migration inhibitory factor (MIF) | Patients with infection in medical ward or ED | No bacterial infection | No better than PCT [51] |
| Adrenomedullin | Neutropenic patients with sepsis | Neutropenic patients with fever and clinically documented infection | No better than PCT [52] |
| Pro-adrenomedullin | Sepsis with organ dysfunction and or shock | Patients admitted to coronary unit without infection | No better than PCT [53] |
| High-mobility group-box 1 protein (HMGB1) | Infected patients admitted in the ward | Healthy individuals | No better than CRP or PCT [54] |
| IL-8 | Neutropenic children with blood culture positive, and/or fever periods with a documented clinical sepsis and/or local infection | Neutropenic children with fever and no infection | No better than CRP [55] |
| IL-10 | Patients with bacteremia and SIRS, | Patients with SIRS without bacteremia | Comparable with PCT [56] |
| Endocan | Critically ill patients with sepsis and organ dysfunction | Critically ill patients with infection and SIRS | Comparable with PCT [57] |
| Pro-atrial natriuretic peptide (ANP) | Burned patients that received antibiotics and had either microbiological confirmation of infection or antibiotics leaded to an improvement in clinical situation | Burned patients without infection | Comparable with PCT [58] |
| Pentraxin 3 | Mechanically ventilated patients with ventilator associated pneumonia | Mechanically ventilated patient > 48 h without VAP | No better than CRP [59] |
| Hematological patients with bacteremia and/or septic shock | Hematological patients with fever without infection | No better than CRP [60] | |
| Better diagnostic value than PCT and/or CRP | |||
| Thromboelastometry lysis index | Patients with severe sepsis | Patients after operation without sepsis | Better than PCT [61] |
| Decoy receptor 3 | ICU patients with sepsis | ICU patients with SIRS | Positive when PCT was negative [62] |
| Group II phospholipase A2 (PLA2-II) | ED patients with sepsis and organ dysfunction | ED patients with SIRS without infection | Better than CRP [63] |
| Hepcidin | Infants with sepsis and or bacteremia | Infants with SIRS and not sepsis | Better than CRP [64] |
| sCD163 | Patients with sepsis admitted to ICU | Patients with SIRS without sepsis | Better than PCT [65] |
| CD64 | ICU patients with sepsis | ICU patients without sepsis | Better than PCT and CRP [66] |
| Patients with ventilator associated pneumonia and sepsis | Patients with ventilator associated pneumonia without sepsis | Better than PCT and CRP [67] | |
| Serum amyloid A | Full term infants with sepsis | Full term infants with risk for sepsis but without sepsis | Earlier increase in neonates with early onset sepsis than CRP [68] |
| Heparin-binding protein | Patients with sepsis for less than 48 h | Patients with infection without sepsis | Better than CRP and PCT [69] |
| Delta-like canonical Notch ligand 1 (DLL1) | Patients with abdominal infection or surgical site associated infection | Surgical patients, trauma patients without infection, and healthy volunteers | Better than CRP and PCT [70] |
| Conflicting findings | |||
| IL-6 | Critically ill patients with sepsis | Patients with SIRS without infection | IL-6 was not found to have lower diagnostic utility compared to PCT (meta-analysis) [71] |
| Cirrhotic patients with infection at admission to ICU | Cirrhotic patients without sepsis | IL-6 was found to increase earlier than PCT in cirrhotic patients [72] | |
| sCD25 | ED patients with infection | ED patients with suspected infection but finally infection excluded | Equal diagnostic value to PCT for diagnosis of infection in ED [44] |
| Patients admitted in ICU with infection and SIRS | Patients with SIRS without sepsis | Better performance than PCT to identify Sepsis I at ICU admission [73] | |
| Calprotectin | ICU patients with infection | ICU patients without sepsis | Better than CRP and PCT [74] |
| Patients after major operation who developed sepsis | Patients after major operation who did not develop sepsis | Similar value to PCT [75] | |
| IL-27 | Critically ill children with sepsis | Children with SIRS without infection | Better than PCT [76] |
| ICU patients with sepsis | ICU patients without sepsis | No better than PCT [77] | |
| sTREM | ICU patients with sepsis | ICU patients with SIRS | Better than PCT [78] |
| ICU patients with sepsis | ICU patients with SIRS | No better than PCT and CRP [79] | |
| Presepsin (CD14) | ED patients with sepsis | ED patients with at least two criteria of SIRS without sepsis | Better than PCT in diagnosis of sepsis in ED [80] |
| Critically ill patients with sepsis and organ dysfunction | Critically ill patients without infection | No better than PCT regardless of the presence or not of AKI [17] | |
| Neonates with SIRS and positive blood cultures | Neonates with SIRS with negative blood cultures | Better than PCT [81] | |
| Better performance when combined with PCT and/or CRP | |||
| IL-6 | Neonates with infection within the first week of life | Neonates with suspicion of infection but finally excluded within the first week of sepsis | Combination with CRP in neonates with suspected sepsis [82] |
| CD64 | Neonates with sepsis | Healthy controls | Combination with PCT and CRP for diagnosis of neonatal sepsis [83] |
| Leptin | Patients with community acquired pneumonia with sepsis or complicated intraabdominal infection | SIRS without infection, healthy controls | Combination with CRP [84] |
| Pro-adrenomedullin | Septic patients | Patients with SIRS without sepsis | Combination to PCT [53, 85] |
| suPAR | Septic patients admitted to ICU | Critically ill patients with SIRS without infection and healthy controls | Combination with PCT for diagnosis of sepsis on day 1 of sepsis [86] |
| CD11b | Patients with Gram (+) infection | Patients with Gram (−) infection | Combination with CRP for differentiation from Gram (−) infection [87] |
| Fibrinogen | Neutropenic patients with sepsis | Neutropenic patients with fever without infection | Combination with CRP for diagnosis of sepsis [88] |
| BNP and antithrombin | Neutropenic patients with fever and bacteremia | Neutropenic patients with fever without infection | Combination with PCT for diagnosis of Gram (−) bacteremia [88] |
| IL-27 | Pediatric patients with sepsis | Pediatric patients with SIRS without infection | Improvement of diagnostic accuracy of PCT for diagnosis of sepsis [77, 89] |
| α-2 macroglobulin | Surgical patients with sepsis | Surgical patients with SIRS without sepsis | Combination with PCT to exclude sepsis in surgical patients [90] |
| Decoy receptor 3 and uPAR | Patients with sepsis | Patients with SIRS without infection, healthy volunteers | Combination with PCT for diagnosis of sepsis [91] |
sTREM soluble triggering receptor expressed on myeloid cells, RANTES regulated on activation, normal T-cell expressed, and secreted
Forty-four biomarkers were tested in 55 clinical studies for their use in answering specific, clinically relevant questions rather than simply for diagnosis and/or prognosis of sepsis in general (Table 4): 20 were assessed for use to diagnose infection in specific groups of critically ill patients where diagnosis may be difficult based on clinical evaluation and laboratory values, 8 were assessed for diagnosis of ARDS or associated endothelial damage in patients with sepsis, 6 were tested for their ability to identify specific infections or type of microorganism, 6 were studied for use in the diagnosis of disseminated intravascular coagulation, 4 were assessed for use in deciding which patients with hematological malignancy or neutropenia had a low risk of infection, 3 were assessed for their ability to diagnose infection before any clinical symptoms, 2 were evaluated for use in assessing the risk of delirium or encephalopathy in patients with sepsis, and 1 was assessed to differentiate between sepsis and graft rejection.
Table 4.
Some examples of biomarkers that have been assessed for use in specific clinical situations
| Situation | Biomarker |
|---|---|
| To diagnose infection in patients with a particular pathology/condition | |
| After cardiac surgery | Endocan [92], CD64 [93], pancreatic stone protein [94] |
| After major surgery | Peptidoglycan [95], elastase [96], leptin [84], calprotectin [75], a proliferation-inducing ligand [97], α-2 macroglobulin [89], lipopolysaccharide-binding protein [15] |
| COPD | Pentraxin 3 [98] |
| Cirrhosis | Interleukin (IL)-6 [72] |
| Trauma | IL-10 [99], NT-proCNP [100], P-selectin [101] |
| Catheter-related infections | Citrulline [102] |
| Infants with necrotic enterocolitis | IP-10 [103] |
| Neutropenic patients | Lipopolysaccharide-binding protein [104], pro-adrenomedullin [105] |
| Burns | IL-8 [106], MIF [107] |
| Autoimmune diseases | CD64 [108] |
| To diagnose specific types of infection | |
| Gram (−) vs. Gram (+) | Fibrin degradation products [109], lipopolysaccharide-binding protein [104], CD11b [87] |
| Virus vs. bacterial infection or co-infection | Transforming growth factor (TGF-β) [110], tumor necrosis factor (TNF)-α [111] |
| VAP | suPAR [112] |
| Diagnosis of specific conditions | |
| Sepsis vs. graft rejection | Lysozyme [113] |
| Diagnosis of ARDS | Club cell secretory protein (CC)-16 [114], surfactant protein [114] |
| Vascular leakage risk in ARDS | von Willebrand factor [115], angiopoietins (1 and 2) [25], IL-8 [116], syndecan-1 [117], HMGB-1 [118] |
| Recovery from ARDS—endothelial repair | sRAGE [119] |
| Identification of low risk of infection in hematological/oncological patients | IL-6 [120, 121], IL-8 [120–122], MCP-1 [55], IL-5 [123] |
| Identification of infection before clinical symptoms | IL-6 [124], IL-ra [125], soluble protein C receptors [126] |
| Risk of encephalopathy/delirium | VCAM [127], neuron-specific enolase [128] |
| Disseminated intravascular coagulation | P-selectin [129], protein C [130], microparticles [131], matrix-metalloproteinases [132], thrombin-antithrombin complex [133], a2PI [134] |
COPD chronic obstructive pulmonary disease, ARDS acute respiratory distress syndrome, TNF tumor necrosis factor, VAP ventilator-associated pneumonia, NT-ProCNP N-terminal pro-C-type natriuretic peptide, MIF macrophage migration inhibitory factor, VCAM vascular cell adhesion molecule, IP interferon-gamma-inducible protein, sUPAR soluble urokinase plasminogen receptor, IL-1ra IL-1 receptor antagonist, MCP monocyte chemoattractant protein, sRAGE soluble receptor for advanced glycation end products, HMGB high-mobility group-box 1 protein
Discussion
Our literature search illustrates that although new biomarkers have been proposed, little real progress has been made in identifying biomarkers with clinical significance. Using a similar method of searching for sepsis biomarkers to that of our previous study, we noted that the number of publications related to sepsis biomarkers has increased considerably over the years. The proportion of new biomarkers being identified has decreased, but this may reflect publication bias with journals becoming more selective in deciding what merits publication as the volume of these studies increases. Because of the complexity of the sepsis response with multiple mediators, and the improved sensitivity of many tests enabling identification of smaller concentrations of substances than in the past, it is likely that our list of biomarkers will expand further in the future. However, the potential utility of creating an ever-expanding list of potential biomarkers without a more rigorous framework to evaluate them is questionable. An improved methodological approach is needed in order to assess the utility of sepsis biomarkers in daily clinical practice.
Accurate evaluation of the possible clinical utility of a biomarker requires assessment in a large number of patients [5], but we identified only a few biomarkers that have been assessed in studies of more than 300 patients. Moreover, many of the biomarkers have been assessed in only a limited number of clinical studies and one third in just a single study. Patients with sepsis represent a very heterogeneous population, and potential biomarkers need to be assessed in studies with a significant number of patients to ensure random distribution of risk factors that may affect the results of the study (e.g., age, organ dysfunction, type of infection, comorbidities). However, the number of patients enrolled in a study is not the only factor to consider when evaluating the potential role of a sepsis biomarker, and of note, none of the large multicenter studies were able to draw conclusions about the biomarker under study that could change clinical practice.
There was considerable diversity in the methods used to assess sepsis-related biomarkers. Most biomarkers were proposed as being useful for diagnosing sepsis simply because they were increased or decreased to a larger extent in septic than in non-septic patients or healthy individuals. Many studies have assessed the sensitivity and specificity of the biomarker for sepsis diagnosis, but identification of sepsis was often based on the commonly used constellation of non-specific clinical and laboratory findings; in the absence of a “gold standard” diagnostic tool, this method cannot conclusively demonstrate the value of the biomarker with respect to diagnosing sepsis. Other parameters, including positive and negative predictive value or likelihood ratios, can provide greater insight into how well a biomarker performs but these were rarely provided [135]. Similarly, many biomarkers have been used to evaluate sepsis severity using all-cause mortality as the primary endpoint. Importantly, the majority of the studies that evaluated sepsis biomarkers using this method showed only a limited value; it seems highly unlikely that mortality in septic patients is related to only one pathophysiologic process that could be reflected by abnormal levels of a biomarker. Furthermore, the need for another prognostic test can be questioned because clinical data and other laboratory test results, including blood lactate levels, can already reflect severity and the risk of death in septic patients [136]. Prognostic biomarkers may be useful to triage patients in special environments, such as in the emergency room, when the information provided can help clinicians to decide whether hospitalization is necessary and, if so, on the ICU or on the regular floor. However, in a multicenter trial (TRIAGE III) in which emergency room physicians were asked to incorporate the prognostic information portrayed by abnormal uPAR levels into their triage decisions, there was no effect on mortality rates compared to standard practice without uPAR levels [137].
To be of value in clinical practice, a biomarker must be shown to provide an answer to a specific, clinically relevant question, rather than just having diagnostic or prognostic value in general. We identified just 55 studies in which a sepsis biomarker was shown to have a potentially useful role by answering a specific clinical question. For example, biomarkers that could identify specific types of infection may help in guiding a more targeted antibiotic therapy, and a biomarker able to identify septic patients at risk of ARDS may influence fluid management in such patients, reducing risks of fluid overload. Further study needs to better evaluate the potential utility and beneficial effects on outcomes of using biomarkers to answer specific clinical questions.
We attempted to categorize the various biomarkers according to their pathophysiological role, although for many it was not possible to identify a clear role, and some have multiple roles. Only a few biomarkers were found to have a role specifically related to sepsis pathophysiology rather than to a more general inflammatory reaction, including presepsin (the N-terminal fragment of the macrophage lipopolysaccharide [LPS] receptor), LPS-binding protein (LBP), bactericidal/permeability increasing protein, peptidoglycan, thrombomodulin, and anti-endotoxin core antibodies. Such biomarkers may help transform our understanding of sepsis from a “physiological syndrome to a group of distinct biochemical disorders” [138] and advance our search for adjunctive sepsis therapies.
CRP and PCT are by far the most widely used and studied biomarkers. Both increase transiently during sepsis, but long enough to allow for their detection, reflecting a real-time response. Although PCT is considered superior to CRP in many studies [139, 140], it is not a definitive test for diagnosing sepsis because PCT levels can also be increased in other conditions [141]. PCT, similar to CRP, may be more useful to rule out sepsis than to diagnose it [142–144], and the combination of these two biomarkers may improve their ability to exclude sepsis [145]. Studying the time course of these biomarkers may also be helpful to evaluate an individual patient’s response to therapy. Changes in serum CRP levels during the first 48 h after antibiotic initiation can help evaluate the response to initial antimicrobial therapy [146]. Likewise, a PCT-based algorithm may help reduce antibiotic exposure in septic patients without compromising clinical outcomes [147, 148]. However, not all studies have shown the same positive effect [149], suggesting that the effectiveness of PCT-based algorithms may depend on the physician’s experience and the clinical setting. Some biomarkers have been compared to PCT and CRP, most for their diagnostic value. A few were shown to be superior to PCT and/or CRP for this purpose, for example, presepsin and CD64 [66, 67, 150].
Measuring several biomarkers concurrently may be useful to overcome the limitations of any single biomarker. Combining biomarkers that are involved in different sepsis-related pathways may be particularly attractive. A seven-biomarker panel including cellular markers and interleukins correctly identified 89% of patients with ventilator-associated pneumonia (VAP) and 100% of patients without VAP [151]. Similarly, a combination of several sepsis-related biomarkers (PCT, presepsin, galectin-3, and soluble suppression of tumorigenicity 2) was found to have better prognostic value than PCT alone [152]. However, it is not clear from the existing literature whether the biomarkers included in such panels should be selected based on pathophysiological or other criteria. The combination of a biomarker panel with clinical information may be particularly useful in the diagnosis of sepsis or in the risk stratification of patients with sepsis [153].
The study has some limitations that should be acknowledged. First, although we performed an extensive search, we cannot be sure that some studies were not missed. Nevertheless, the large number of sepsis biomarkers that we retrieved suggests that we managed to identify the majority of the biomarkers that have been studied. Second, we included studies over a long period of time, during which the definition of sepsis has changed so that it is difficult to make comparisons. Third, it is difficult to compare different biomarkers because the methods used to evaluate the biomarkers and to define sepsis and the populations studied varied across the studies.
Conclusions
Since our original search, many additional sepsis-related biomarkers have been identified. However, the precise roles of most biomarkers in the management of septic patients have not been well defined, and of the many biomarkers that have been studied, only a few have been evaluated in large or repeated studies. As such, it is not possible to draw any reliable conclusions about which compounds could be considered as the most “promising” candidates. Even the biomarkers that had an AUC > 0.8 for diagnosis or prognosis, making them potentially more interesting for further study, were evaluated in studies with a high risk of bias. Moreover, while there are multiple putative biomarkers, rarely have they been compared against each other to determine how they differ in what they are measuring, and which does it better. Almost all studies report a single marker in isolation, but given the complexity of sepsis, surely these markers are not biologically independent, so how can we know which is best to use?
It is therefore important to develop a more rigorous, standardized methodology to assess sepsis biomarkers and identify those that can provide valuable, clinically relevant information. Such an approach could include the following factors:
- What is the question being asked?
- Greater likelihood of infection leading to administration of empiric antibiotics or performance of a diagnostic test (e.g., carcinoembryonic antigen [CEA] levels are used to detect early recurrence in patients with colon cancer, and so guide further investigations)
- Resolution of infection and therefore safety in stopping antibiotics
- Increased likelihood of benefiting from specific interventions, such as steroids or a biologic agent
- Increased risk of adverse outcome not apparent by other evidence
- Ensuring random distribution of risk factors in a randomized controlled trial
- How is the study designed?
- What is the control group
- Which patients and how many are being studied
- How are outcomes adjudicated
- Is there a validation cohort
- Uniform techniques to evaluate results—sensitivity, specificity, positive and negative predictive values, likelihood ratios, and ROC analysis
Is the marker biologically plausible, and what do alterations tell us about the pathobiology of disease in this patient?
Consideration of these factors and their application to sepsis biomarker research may help identify new biomarkers with real clinical utility. Continuing to produce reports of novel biomarkers without developing a more rigorous framework to evaluate them and establishing a recognized purpose is futile: it is time for a reappraisal of the possible roles of biomarkers in sepsis.
Supplementary information
Additional file 1: Figure S1. Biomarkers of sepsis: Time for a reappraisal Pierrakos et al. Table S1. Cytokine/chemokine biomarkers identified in the literature search. Table S2. Receptor biomarkers identified in the literature search. Table S3. Cell marker biomarkers identified in the literature search. Table S4. Coagulation-related biomarkers identified in the literature search. Table S5. Microcirculation related biomarkers identified in the literature search. Table S6. Vasodilation-related biomarkers identified in the literature search. Table S7. Biomarkers of organ dysfunction in sepsis identified in the literature search. Table S8. Acute phase proteins used as biomarkers in sepsis identified in the literature search. Table S9. Diverse sepsis biomarkers identified in the literature search. Table S10. QUADAS-2 score [1145] for quality assessment for the studies that included >300 patients where ROC curve analysis was used.
Abbreviations
- AUC
Area under the curve
- CRP
C-reactive protein
- ICU
Intensive care unit
- IL
Interleukin
- LBP
Lipopolysaccharide-binding protein
- LPS
Lipopolysaccharide
- MEDS
Mortality in Emergency Department Sepsis score
- PCT
Procalcitonin
- ROC
Receiver operating characteristic
- uPAR
Urokinase plasminogen activator receptor
Authors’ contributions
CP conceived the study, performed the literature search, drafted the manuscript, and approved the submitted version of the manuscript. DV helped perform the literature search, revised the manuscript for critical content, and approved the submitted version of the manuscript. MB helped perform the literature search, revised the manuscript for critical content, and approved the submitted version of the manuscript. JCM revised the manuscript for critical content and approved the submitted version of the manuscript. JLV conceived the study, revised the manuscript for critical content, and approved the submitted version of the manuscript.
Funding
None
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
JLV is the Editor-in-Chief of Critical Care and has no other conflicts of interest.
The other authors declare that they have no relevant financial interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13054-020-02993-5.
References
- 1.Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 2.Machado FR, Cavalcanti AB, Bozza FA, Ferreira EM, Angotti Carrara FS, Sousa JL, et al. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis. 2017;17:1180–1189. doi: 10.1016/S1473-3099(17)30322-5. [DOI] [PubMed] [Google Scholar]
- 3.Widmann CN, Heneka MT. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014;13:630–636. doi: 10.1016/S1474-4422(14)70017-1. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay E, Vincent JL, Angus DC, Arabi YM, Brochard L, Brett SJ, et al. Recovery after critical illness: putting the puzzle together-a consensus of 29. Crit Care. 2017;21:296. doi: 10.1186/s13054-017-1887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 7.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 10.Hanna WJ, Berrens Z, Langner T, Lahni P, Wong HR. Interleukin-27: a novel biomarker in predicting bacterial infection among the critically ill. Crit Care. 2015;19:378. doi: 10.1186/s13054-015-1095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaaban H, Singh K, Huang J, Siryaporn E, Lim YP, Padbury JF. The role of inter-alpha inhibitor proteins in the diagnosis of neonatal sepsis. J Pediatr. 2009;154:620–622. doi: 10.1016/j.jpeds.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Uusitalo-Seppala R, Peuravuori H, Koskinen P, Vahlberg T, Rintala EM. Role of plasma bactericidal/permeability-increasing protein, group IIA phospholipase A (2), C-reactive protein, and white blood cell count in the early detection of severe sepsis in the emergency department. Scand J Infect Dis. 2012;44:697–704. doi: 10.3109/00365548.2012.677061. [DOI] [PubMed] [Google Scholar]
- 13.Dimoula A, Pradier O, Kassengera Z, Dalcomune D, Turkan H, Vincent JL. Serial determinations of neutrophil CD64 expression for the diagnosis and monitoring of sepsis in critically ill patients. Clin Infect Dis. 2014;58:820–829. doi: 10.1093/cid/cit936. [DOI] [PubMed] [Google Scholar]
- 14.Hollenbach B, Morgenthaler NG, Struck J, Alonso C, Bergmann A, Kohrle J, et al. New assay for the measurement of selenoprotein P as a sepsis biomarker from serum. J Trace Elem Med Biol. 2008;22:24–32. doi: 10.1016/j.jtemb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Sakr Y, Burgett U, Nacul FE, Reinhart K, Brunkhorst F. Lipopolysaccharide binding protein in a surgical intensive care unit: a marker of sepsis? Crit Care Med. 2008;36:2014–2022. doi: 10.1097/CCM.0b013e31817b86e3. [DOI] [PubMed] [Google Scholar]
- 16.Wei S, Gonzalez Rodriguez E, Chang R, Holcomb JB, Kao LS, Wade CE. Elevated syndecan-1 after trauma and risk of sepsis: a secondary analysis of patients from the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial. J Am Coll Surg. 2018;227:587–595. doi: 10.1016/j.jamcollsurg.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura Y, Hoshino K, Kiyomi F, Kawano Y, Mizunuma M, Tanaka J, et al. Comparison of accuracy of presepsin and procalcitonin concentrations in diagnosing sepsis in patients with and without acute kidney injury. Clin Chim Acta. 2019;490:200–206. doi: 10.1016/j.cca.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Henning DJ, Hall MK, Watsjold BK, Bhatraju PK, Kosamo S, Shapiro NI, et al. Interleukin-6 improves infection identification when added to physician judgment during evaluation of potentially septic patients. Am J Emerg Med. 2019. 10.1016/j.ajem.2019.158361. Epub ahead of print. [DOI] [PubMed]
- 19.Giamarellos-Bourboulis EJ, Norrby-Teglund A, Mylona V, Savva A, Tsangaris I, Dimopoulou I, et al. Risk assessment in sepsis: a new prognostication rule by APACHE II score and serum soluble urokinase plasminogen activator receptor. Crit Care. 2012;16:R149. doi: 10.1186/cc11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madoiwa S, Nunomiya S, Ono T, Shintani Y, Ohmori T, Mimuro J, et al. Plasminogen activator inhibitor 1 promotes a poor prognosis in sepsis-induced disseminated intravascular coagulation. Int J Hematol. 2006;84:398–405. doi: 10.1532/IJH97.05190. [DOI] [PubMed] [Google Scholar]
- 21.Novotny AR, Emmanuel K, Ulm K, Bartels H, Siewert JR, Weighardt H, et al. Blood interleukin 12 as preoperative predictor of fatal postoperative sepsis after neoadjuvant radiochemotherapy. Br J Surg. 2006;93:1283–1289. doi: 10.1002/bjs.5404. [DOI] [PubMed] [Google Scholar]
- 22.Johansen ME, Johansson PI, Ostrowski SR, Bestle MH, Hein L, Jensen AL, et al. Profound endothelial damage predicts impending organ failure and death in sepsis. Semin Thromb Hemost. 2015;41:16–25. doi: 10.1055/s-0034-1398377. [DOI] [PubMed] [Google Scholar]
- 23.Matsubara T, Yamakawa K, Umemura Y, Gando S, Ogura H, Shiraishi A, et al. Significance of plasma fibrinogen level and antithrombin activity in sepsis: a multicenter cohort study using a cubic spline model. Thromb Res. 2019;181:17–23. doi: 10.1016/j.thromres.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Li C. Prognostic significance of brain natriuretic peptide obtained in the ED in patients with SIRS or sepsis. Am J Emerg Med. 2009;27:701–706. doi: 10.1016/j.ajem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med. 2012;40:1731–1737. doi: 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinasewitz GT, Yan SB, Basson B, Comp P, Russell JA, Cariou A, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569] Crit Care. 2004;8:R82–R90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YX, Li CS. Prognostic value of adrenomedullin in septic patients in the ED. Am J Emerg Med. 2013;31:1017–1021. doi: 10.1016/j.ajem.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Saeed K, Wilson DC, Bloos F, Schuetz P, van der Does Y, Melander O, et al. The early identification of disease progression in patients with suspected infection presenting to the emergency department: a multi-centre derivation and validation study. Crit Care. 2019;23:40. doi: 10.1186/s13054-019-2329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linder A, Christensson B, Herwald H, Bjorck L, Akesson P. Heparin-binding protein: an early marker of circulatory failure in sepsis. Clin Infect Dis. 2009;49:1044–1050. doi: 10.1086/605563. [DOI] [PubMed] [Google Scholar]
- 30.Rodelo JR, De la Rosa G, Valencia ML, Ospina S, Arango CM, Gomez CI, et al. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30:1991–1999. doi: 10.1016/j.ajem.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 31.John J, Woodward DB, Wang Y, Yan SB, Fisher D, Kinasewitz GT, et al. Troponin-I as a prognosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270–275. doi: 10.1016/j.jcrc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Kornblit B, Hellemann D, Munthe-Fog L, Bonde J, Strom JJ, Madsen HO, et al. Plasma YKL-40 and CHI3L1 in systemic inflammation and sepsis-experience from two prospective cohorts. Immunobiology. 2013;218:1227–1234. doi: 10.1016/j.imbio.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Rannikko J, Seiskari T, Huttunen R, Tarkiainen I, Jylhava J, Hurme M, et al. Plasma cell-free DNA and qSOFA score predict 7-day mortality in 481 emergency department bacteraemia patients. J Intern Med. 2018;284:418–426. doi: 10.1111/joim.12766. [DOI] [PubMed] [Google Scholar]
- 34.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 35.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 36.Garnacho-Montero J, Huici-Moreno MJ, Gutierrez-Pizarraya A, Lopez I, Marquez-Vacaro JA, Macher H, et al. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit Care. 2014;18:R116. doi: 10.1186/cc13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreira VG, Prieto B, Rodriguez JS, Alvarez FV. Usefulness of cell-free plasma DNA, procalcitonin and C-reactive protein as markers of infection in febrile patients. Ann Clin Biochem. 2010;47:253–258. doi: 10.1258/acb.2010.009173. [DOI] [PubMed] [Google Scholar]
- 38.Battista S, Audisio U, Galluzzo C, Maggiorotto M, Masoero M, Forno D, et al. Assessment of diagnostic and prognostic role of copeptin in the clinical setting of sepsis. Biomed Res Int. 2016;2016:3624730. doi: 10.1155/2016/3624730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandi Y, Farkas G, Takacs T, Boda K, Lonovics J. Diagnostic relevance of procalcitonin, IL-6, and sICAM-1 in the prediction of infected necrosis in acute pancreatitis. Int J Pancreatol. 2000;28:41–49. doi: 10.1385/IJGC:28:1:41. [DOI] [PubMed] [Google Scholar]
- 40.Garcia de Guadiana RL, Albaladejo Oton MD, Rebello Acebes S, Esteban TP, Hernando HA, Jimenez SE, et al. Diagnostic accuracy of lipopolysaccharide-binding protein for sepsis in patients with suspected infection in the emergency department. Ann Clin Biochem. 2018;55:143–148. doi: 10.1177/0004563217694378. [DOI] [PubMed] [Google Scholar]
- 41.Ratzinger F, Schuardt M, Eichbichler K, Tsirkinidou I, Bauer M, Haslacher H, et al. Utility of sepsis biomarkers and the infection probability score to discriminate sepsis and systemic inflammatory response syndrome in standard care patients. PLoS One. 2013;8:e82946. doi: 10.1371/journal.pone.0082946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitanovski L, Jazbec J, Hojker S, Derganc M. Diagnostic accuracy of lipopolysaccharide-binding protein for predicting bacteremia/clinical sepsis in children with febrile neutropenia: comparison with interleukin-6, procalcitonin, and C-reactive protein. Support Care Cancer. 2014;22:269–277. doi: 10.1007/s00520-013-1978-1. [DOI] [PubMed] [Google Scholar]
- 43.ten Oever J, Tromp M, Bleeker-Rovers CP, Joosten LA, Netea MG, Pickkers P, et al. Combination of biomarkers for the discrimination between bacterial and viral lower respiratory tract infections. J Inf Secur. 2012;65:490–495. doi: 10.1016/j.jinf.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Garcia de Guadiana-Romualdo L, Berger M, Jimenez-Santos E, Rebollo-Acebes S, Jimenez-Sanchez R, Esteban-Torrella P, et al. Pancreatic stone protein and soluble CD25 for infection and sepsis in an emergency department. Eur J Clin Invest. 2017;47:297–304. doi: 10.1111/eci.12732. [DOI] [PubMed] [Google Scholar]
- 45.Jiang YN, Cai X, Zhou HM, Jin WD, Zhang M, Zhang Y, et al. Diagnostic and prognostic roles of soluble CD22 in patients with Gram-negative bacterial sepsis. Hepatobiliary Pancreat Dis Int. 2015;14:523–529. doi: 10.1016/s1499-3872(15)60394-0. [DOI] [PubMed] [Google Scholar]
- 46.BalcI C, Sungurtekin H, Gurses E, Sungurtekin U, Kaptanoglu B. Usefulness of procalcitonin for diagnosis of sepsis in the intensive care unit. Crit Care. 2003;7:85–90. doi: 10.1186/cc1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayazi P, Mahyar A, Daneshi MM, Jahanihashemi H, Esmailzadehha N, Mosaferirad N. Comparison of serum IL-1beta and C reactive protein levels in early diagnosis and management of neonatal sepsis. Infez Med. 2014;22:296–301. [PubMed] [Google Scholar]
- 48.Stojewska M, Wasek-Buko M, Jakub B, Wisniewska-Ulfig D, Goleniowska-Krol A, Szymanska A, et al. Evaluation of serum chemokine RANTES concentration as a biomarker in the diagnosis of early-onset severe infections in neonates. Postepy Hig Med Dosw (Online) 2016;70:272–279. doi: 10.5604/17322693.1198990. [DOI] [PubMed] [Google Scholar]
- 49.Sapa A, Rak A, Wybieralska M, Machon J, Krzywonos-Zawadzka A, Zawadzki K, et al. Diagnostic usefulness of sCD163, procalcitonin and neopterin for sepsis risk assessment in critically ill patients. Adv Clin Exp Med. 2017;26:101–108. doi: 10.17219/acem/63251. [DOI] [PubMed] [Google Scholar]
- 50.Ruokonen E, Ilkka L, Niskanen M, Takala J. Procalcitonin and neopterin as indicators of infection in critically ill patients. Acta Anaesthesiol Scand. 2002;46:398–404. doi: 10.1034/j.1399-6576.2002.460412.x. [DOI] [PubMed] [Google Scholar]
- 51.Kofoed K, Andersen O, Kronborg G, Tvede M, Petersen J, Eugen-Olsen J, et al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care. 2007;11:R38. doi: 10.1186/cc5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demirkaya M, Tugcu D, Akcay A, Aydogan G, Akici F, Salcioglu Z, et al. Adrenomedullin--a new marker in febrile neutropenia: comparison with CRP and procalcitonin. Pediatr Hematol Oncol. 2015;32:482–489. doi: 10.3109/08880018.2015.1057310. [DOI] [PubMed] [Google Scholar]
- 53.Enguix-Armada A, Escobar-Conesa R, Garcia-De La Torre A, De La Torre-Prados MV. Usefulness of several biomarkers in the management of septic patients: C-reactive protein, procalcitonin, presepsin and mid-regional pro-adrenomedullin. Clin Chem Lab Med. 2016;54:163–168. doi: 10.1515/cclm-2015-0243. [DOI] [PubMed] [Google Scholar]
- 54.Gaini S, Koldkjaer OG, Moller HJ, Pedersen C, Pedersen SS. A comparison of high-mobility group-box 1 protein, lipopolysaccharide-binding protein and procalcitonin in severe community-acquired infections and bacteraemia: a prospective study. Crit Care. 2007;11:R76. doi: 10.1186/cc5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Maghraby SM, Moneer MM, Ismail MM, Shalaby LM, El-Mahallawy HA. The diagnostic value of C-reactive protein, interleukin-8, and monocyte chemotactic protein in risk stratification of febrile neutropenic children with hematologic malignancies. J Pediatr Hematol Oncol. 2007;29:131–136. doi: 10.1097/MPH.0b013e3180308770. [DOI] [PubMed] [Google Scholar]
- 56.Matera G, Puccio R, Giancotti A, Quirino A, Pulicari MC, Zicca E, et al. Impact of interleukin-10, soluble CD25 and interferon-gamma on the prognosis and early diagnosis of bacteremic systemic inflammatory response syndrome: a prospective observational study. Crit Care. 2013;17:R64. doi: 10.1186/cc12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mihajlovic D, Brkic S, Lendak D, Mikic AN, Draskovic B, Mitic G. Endothelial biomarkers in the light of new sepsis definition. Biomark Med. 2019;13:341–351. doi: 10.2217/bmm-2018-0282. [DOI] [PubMed] [Google Scholar]
- 58.Gille J, Schmidt J, Kremer T, Sablotzki A. Evaluation of MR-proANP and copeptin for sepsis diagnosis after burn injury. J Crit Care. 2019;52:149–155. doi: 10.1016/j.jcrc.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 59.Lin Q, Fu F, Shen L, Zhu B. Pentraxin 3 in the assessment of ventilator-associated pneumonia: an early marker of severity. Heart Lung. 2013;42:139–145. doi: 10.1016/j.hrtlng.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Vanska M, Koivula I, Hamalainen S, Pulkki K, Nousiainen T, Jantunen E, et al. High pentraxin 3 level predicts septic shock and bacteremia at the onset of febrile neutropenia after intensive chemotherapy of hematologic patients. Haematologica. 2011;96:1385–1389. doi: 10.3324/haematol.2011.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adamzik M, Eggmann M, Frey UH, Gorlinger K, Brocker-Preuss M, Marggraf G, et al. Comparison of thromboelastometry with procalcitonin, interleukin 6, and C-reactive protein as diagnostic tests for severe sepsis in critically ill adults. Crit Care. 2010;14:R178. doi: 10.1186/cc9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao L, Yang B, Zhang H, Ou Q, Lin Y, Zhang M, et al. DcR3, a new biomarker for sepsis, correlates with infection severity and procalcitonin. Oncotarget. 2018;9:10934–10944. doi: 10.18632/oncotarget.23736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan TL, Goh YY. The role of group IIA secretory phospholipase A2 (sPLA2-IIA) as a biomarker for the diagnosis of sepsis and bacterial infection in adults-a systematic review. PLoS One. 2017;12:e0180554. doi: 10.1371/journal.pone.0180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu TW, Tabangin M, Kusano R, Ma Y, Ridsdale R, Akinbi H. The utility of serum hepcidin as a biomarker for late-onset neonatal sepsis. J Pediatr. 2013;162:67–71. doi: 10.1016/j.jpeds.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Feng L, Zhou X, Su LX, Feng D, Jia YH, Xie LX. Clinical significance of soluble hemoglobin scavenger receptor CD163 (sCD163) in sepsis, a prospective study. PLoS One. 2012;7:e38400. doi: 10.1371/journal.pone.0038400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jamsa J, Ala-Kokko T, Huotari V, Ohtonen P, Savolainen ER, Syrjala H. Neutrophil CD64, C-reactive protein, and procalcitonin in the identification of sepsis in the. J Crit Care. 2018;43:139–142. doi: 10.1016/j.jcrc.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 67.Muzlovic I, Ihan A, Stubljar D. CD64 index on neutrophils can diagnose sepsis and predict 30-day survival in subjects after ventilator-associated pneumonia. J Infect Dev Ctries. 2016;10:260–268. doi: 10.3855/jidc.6532. [DOI] [PubMed] [Google Scholar]
- 68.Arnon S, Litmanovitz I, Regev RH, Bauer S, Shainkin-Kestenbaum R, Dolfin T. Serum amyloid A: an early and accurate marker of neonatal early-onset sepsis. J Perinatol. 2007;27:297–302. doi: 10.1038/sj.jp.7211682. [DOI] [PubMed] [Google Scholar]
- 69.Zhou Y, Liu Z, Huang J, Li G, Li F, Cheng Y, et al. Usefulness of the heparin-binding protein level to diagnose sepsis and septic shock according to Sepsis-3 compared with procalcitonin and C reactive protein: a prospective cohort study in China. BMJ Open. 2019;9:e026527. doi: 10.1136/bmjopen-2018-026527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hildebrand D, Decker SO, Koch C, Schmitt FCF, Ruhrmann S, Schneck E, et al. Host-derived delta-like canonical Notch ligand 1 as a novel diagnostic biomarker for bacterial sepsis - results from a combinational secondary analysis. Front Cell Infect Microbiol. 2019;9:267. doi: 10.3389/fcimb.2019.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma L, Zhang H, Yin YL, Guo WZ, Ma YQ, Wang YB, et al. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine. 2016;88:126–135. doi: 10.1016/j.cyto.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 72.Lin S, Huang Z, Wang M, Weng Z, Zeng D, Zhang Y, et al. Interleukin-6 as an early diagnostic marker for bacterial sepsis in patients with liver cirrhosis. J Crit Care. 2015;30:732–738. doi: 10.1016/j.jcrc.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 73.Llewelyn MJ, Berger M, Gregory M, Ramaiah R, Taylor AL, Curdt I, et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit Care. 2013;17:R60. doi: 10.1186/cc12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jonsson N, Nilsen T, Gille-Johnson P, Bell M, Martling CR, Larsson A, et al. Calprotectin as an early biomarker of bacterial infections in critically ill patients: an exploratory cohort assessment. Crit Care Resusc. 2017;19:205–213. [PubMed] [Google Scholar]
- 75.Huang L, Li J, Han Y, Zhao S, Zheng Y, Sui F, et al. Serum calprotectin expression as a diagnostic marker for sepsis in postoperative intensive care unit patients. J Interf Cytokine Res. 2016;36:607–616. doi: 10.1089/jir.2016.0037. [DOI] [PubMed] [Google Scholar]
- 76.Wong HR, Cvijanovich NZ, Hall M, Allen GL, Thomas NJ, Freishtat RJ, et al. Interleukin-27 is a novel candidate diagnostic biomarker for bacterial infection in critically ill children. Crit Care. 2012;16:R213. doi: 10.1186/cc11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong HR, Liu KD, Kangelaris KN, Lahni P, Calfee CS. Performance of interleukin-27 as a sepsis diagnostic biomarker in critically ill adults. J Crit Care. 2014;29:718–722. doi: 10.1016/j.jcrc.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Z, Wang H, Liu J, Chen B, Li G. Serum soluble triggering receptor expressed on myeloid cells-1 and procalcitonin can reflect sepsis severity and predict prognosis: a prospective cohort study. Mediat Inflamm. 2014;2014:641039. doi: 10.1155/2014/641039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jedynak M, Siemiatkowski A, Milewski R, Mroczko B, Szmitkowski M. Diagnostic effectiveness of soluble triggering receptor expressed on myeloid cells-1 in sepsis, severe sepsis and septic shock. Arch Med Sci. 2019;15:713–721. doi: 10.5114/aoms.2018.73090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu B, Chen YX, Yin Q, Zhao YZ, Li CS. Diagnostic value and prognostic evaluation of Presepsin for sepsis in an emergency department. Crit Care. 2013;17:R244. doi: 10.1186/cc13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iskandar A, Arthamin MZ, Indriana K, Anshory M, Hur M, Di Somma S. Comparison between presepsin and procalcitonin in early diagnosis of neonatal sepsis. J Matern Fetal Neonatal Med. 2019;32:3903–3908. doi: 10.1080/14767058.2018.1475643. [DOI] [PubMed] [Google Scholar]
- 82.Dollner H, Vatten L, Austgulen R. Early diagnostic markers for neonatal sepsis: comparing C-reactive protein, interleukin-6, soluble tumour necrosis factor receptors and soluble adhesion molecules. J Clin Epidemiol. 2001;54:1251–1257. doi: 10.1016/s0895-4356(01)00400-0. [DOI] [PubMed] [Google Scholar]
- 83.Yang AP, Liu J, Yue LH, Wang HQ, Yang WJ, Yang GH. Neutrophil CD64 combined with PCT, CRP and WBC improves the sensitivity for the early diagnosis of neonatal sepsis. Clin Chem Lab Med. 2016;54:345–351. doi: 10.1515/cclm-2015-0277. [DOI] [PubMed] [Google Scholar]
- 84.Grigoras I, Branisteanu DD, Ungureanu D, Rusu D, Ristescu I. Early dynamics of leptin plasma level in surgical critically ill patients. a prospective comparative study. Chirurgia (Bucur) 2014;109:66–72. [PubMed] [Google Scholar]
- 85.Angeletti S, Battistoni F, Fioravanti M, Bernardini S, Dicuonzo G. Procalcitonin and mid-regional pro-adrenomedullin test combination in sepsis diagnosis. Clin Chem Lab Med. 2013;51:1059–1067. doi: 10.1515/cclm-2012-0595. [DOI] [PubMed] [Google Scholar]
- 86.Zeng M, Chang M, Zheng H, Li B, Chen Y, He W, et al. Clinical value of soluble urokinase-type plasminogen activator receptor in the diagnosis, prognosis, and therapeutic guidance of sepsis. Am J Emerg Med. 2016;34:375–380. doi: 10.1016/j.ajem.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 87.Nuutila J, Jalava-Karvinen P, Hohenthal U, Laitinen I, Kotilainen P, Rajamaki A, et al. CRP/CD11b ratio: a novel parameter for detecting gram-positive sepsis. Hum Immunol. 2009;70:237–243. doi: 10.1016/j.humimm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 88.Juutilainen A, Hamalainen S, Pulkki K, Kuittinen T, Nousiainen T, Jantunen E, et al. Biomarkers for bacteremia and severe sepsis in hematological patients with neutropenic fever: multivariate logistic regression analysis and factor analysis. Leuk Lymphoma. 2011;52:2349–2355. doi: 10.3109/10428194.2011.597904. [DOI] [PubMed] [Google Scholar]
- 89.Kelly BJ, Lautenbach E, Nachamkin I, Coffin SE, Gerber JS, Fuchs BD, et al. Combined biomarkers discriminate a low likelihood of bacterial infection among surgical intensive care unit patients with suspected sepsis. Diagn Microbiol Infect Dis. 2016;85:109–115. doi: 10.1016/j.diagmicrobio.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suri M, Thirupuram S, Sharma VK. Diagnostic and prognostic utility of C-reactive protein, alpha-1-antitrypsin and alpha-2-macroglobulin in neonatal sepsis: a comparative account. Indian Pediatr. 1991;28:1159–1164. [PubMed] [Google Scholar]
- 91.Kim S, Fotiadu A, Kotoula V. Increased expression of soluble decoy receptor 3 in acutely inflamed intestinal epithelia. Clin Immunol. 2005;115:286–294. doi: 10.1016/j.clim.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 92.Perrotti A, Chenevier-Gobeaux C, Ecarnot F, Barrucand B, Lassalle P, Dorigo E, et al. Relevance of endothelial cell-specific molecule 1 (endocan) plasma levels for predicting pulmonary infection after cardiac surgery in chronic kidney disease patients: the Endolung Pilot Study. Cardiorenal Med. 2017;8:1–8. doi: 10.1159/000479337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Djebara S, Biston P, Fosse E, Daper A, Joris M, Boudjeltia KZ, et al. Time course of CD64, a leukocyte activation marker, during cardiopulmonary bypass surgery. Shock. 2017;47:158–164. doi: 10.1097/SHK.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 94.Klein HJ, Csordas A, Falk V, Slankamenac K, Rudiger A, Schonrath F, et al. Pancreatic stone protein predicts postoperative infection in cardiac surgery patients irrespective of cardiopulmonary bypass or surgical technique. PLoS One. 2015;10:e0120276. doi: 10.1371/journal.pone.0120276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimizu T, Endo Y, Tabata T, Mori T, Hanasawa K, Tsuchiya M, et al. Diagnostic and predictive value of the silkworm larvae plasma test for postoperative infection following gastrointestinal surgery. Crit Care Med. 2005;33:1288–1295. doi: 10.1097/01.ccm.0000165810.97971.dd. [DOI] [PubMed] [Google Scholar]
- 96.Duswald KH, Jochum M, Schramm W, Fritz H. Released granulocytic elastase: an indicator of pathobiochemical alterations in septicemia after abdominal surgery. Surgery. 1985;98:892–899. [PubMed] [Google Scholar]
- 97.Lendak DF, Mihajlovic DM, Novakov-Mikic AS, Boban JM, Ubavic M, Brkic SV. APRIL and sTACI could be predictors of multiorgan dysfunction syndrome in sepsis. Virulence. 2018;9:946–953. doi: 10.1080/21505594.2018.1462636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thulborn SJ, Dilpazir M, Haldar K, Mistry V, Brightling CE, Barer MR, et al. Investigating the role of pentraxin 3 as a biomarker for bacterial infection in subjects with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1199–1205. doi: 10.2147/COPD.S123528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lausevic Z, Vukovic G, Stojimirovic B, Trbojevic-Stankovic J, Resanovic V, Lausevic M. Kinetics of C-reactive protein, interleukin-6 and -10, and phospholipase A2-II in severely traumatized septic patients. Vojnosanit Pregl. 2010;67:893–897. doi: 10.2298/vsp1011893l. [DOI] [PubMed] [Google Scholar]
- 100.Bahrami S, Pelinka L, Khadem A, Maitzen S, Hawa G, van Griensven M, et al. Circulating NT-proCNP predicts sepsis in multiple-traumatized patients without traumatic brain injury. Crit Care Med. 2010;38:161–166. doi: 10.1097/CCM.0b013e3181b78a06. [DOI] [PubMed] [Google Scholar]
- 101.Simons RK, Hoyt DB, Winchell RJ, Rose RM, Holbrook T. Elevated selectin levels after severe trauma: a marker for sepsis and organ failure and a potential target for immunomodulatory therapy. J Trauma. 1996;41:653–662. doi: 10.1097/00005373-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 102.Hull MA, Jones BA, Zurakowski D, Raphael B, Lo C, Jaksic T, et al. Low serum citrulline concentration correlates with catheter-related bloodstream infections in children with intestinal failure. JPEN J Parenter Enteral Nutr. 2011;35:181–187. doi: 10.1177/0148607110381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ng PC, Li K, Chui KM, Leung TF, Wong RP, Chu WC, et al. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr Res. 2007;61:93–98. doi: 10.1203/01.pdr.0000250207.95723.96. [DOI] [PubMed] [Google Scholar]
- 104.Oude Nijhuis CSM, Vellenga E, Daenen SMGJ, van der Graaf WTA, Gietema JA, Groen HJM, et al. Lipopolysaccharide-binding protein: a possible diagnostic marker for Gram-negative bacteremia in neutropenic cancer patients. Intensive Care Med. 2003;29:2157–2161. doi: 10.1007/s00134-003-2026-2. [DOI] [PubMed] [Google Scholar]
- 105.Al Shuaibi M, Bahu RR, Chaftari AM, Al Wohoush I, Shomali W, Jiang Y, et al. Pro-adrenomedullin as a novel biomarker for predicting infections and response to antimicrobials in febrile patients with hematologic malignancies. Clin Infect Dis. 2013;56:943–950. doi: 10.1093/cid/cis1029. [DOI] [PubMed] [Google Scholar]
- 106.Kraft R, Herndon DN, Finnerty CC, Cox RA, Song J, Jeschke MG. Predictive value of IL-8 for sepsis and severe infections after burn injury: a clinical study. Shock. 2015;43:222–227. doi: 10.1097/SHK.0000000000000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grieb G, Simons D, Piatkowski A, Bernhagen J, Steffens G, Pallua N. Macrophage migration inhibitory factor-a potential diagnostic tool in severe burn injuries? Burns. 2010;36:335–342. doi: 10.1016/j.burns.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 108.Ajmani S, Singh H, Chaturvedi S, Mishra R, Rai MK, Jain A, et al. Utility of neutrophil CD64 and serum TREM-1 in distinguishing bacterial infection from disease flare in SLE and ANCA-associated vasculitis. Clin Rheumatol. 2019;38:997–1005. doi: 10.1007/s10067-018-4334-5. [DOI] [PubMed] [Google Scholar]
- 109.Deitcher SR, Eisenberg PR. Elevated concentrations of cross-linked fibrin degradation products in plasma. An early marker of gram-negative bacteremia. Chest. 1993;103:1107–1112. doi: 10.1378/chest.103.4.1107. [DOI] [PubMed] [Google Scholar]
- 110.Rendon-Ramirez EJ, Ortiz-Stern A, Martinez-Mejia C, Salinas-Carmona MC, Rendon A, Mata-Tijerina VL, et al. TGF-beta blood levels distinguish between influenza A (H1N1)pdm09 virus sepsis and sepsis due to other forms of community-acquired pneumonia. Viral Immunol. 2015;28:248–254. doi: 10.1089/vim.2014.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hall MW, Geyer SM, Guo CY, Panoskaltsis-Mortari A, Jouvet P, Ferdinands J, et al. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med. 2013;41:224–236. doi: 10.1097/CCM.0b013e318267633c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sunnetcioglu A, Sunnetcioglu M, Adiyaman F, Binici I, Soyoral L. Could soluble urokinase plasminogen receptor (suPAR) be used as a diagnostic biomarker for ventilator-associated pneumonia? Clin Respir J. 2017;11:925–930. doi: 10.1111/crj.12438. [DOI] [PubMed] [Google Scholar]
- 113.Jones JW, Jr, Su S, Jones MB, Heniford BT, McIntyre K, Granger DK. Serum lysozyme activity can differentiate infection from rejection in organ transplant recipients. J Surg Res. 1999;84:134–137. doi: 10.1006/jsre.1999.5628. [DOI] [PubMed] [Google Scholar]
- 114.Ware LB, Koyama T, Zhao Z, Janz DR, Wickersham N, Bernard GR, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17:R253. doi: 10.1186/cc13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rubin DB, Wiener-Kronish JP, Murray JF, Green DR, Turner J, Luce JM, et al. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990;86:474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu XW, Ma T, Cai Q, Wang L, Song HW, Liu Z. Elevation of serum PARK7 and IL-8 levels is associated with acute lung injury in patients with severe sepsis/septic shock. J Intensive Care Med. 2019;34:662–668. doi: 10.1177/0885066617709689. [DOI] [PubMed] [Google Scholar]
- 117.Smart L, Bosio E, Macdonald SPJ, Dull R, Fatovich DM, Neil C, et al. Glycocalyx biomarker syndecan-1 is a stronger predictor of respiratory failure in patients with sepsis due to pneumonia, compared to endocan. J Crit Care. 2018;47:93–98. doi: 10.1016/j.jcrc.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 118.Zheng YJ, Xu WP, Ding G, Gao YH, Wang HR, Pan SM. Expression of HMGB1 in septic serum induces vascular endothelial hyperpermeability. Mol Med Rep. 2016;13:513–521. doi: 10.3892/mmr.2015.4536. [DOI] [PubMed] [Google Scholar]
- 119.Jabaudon M, Futier E, Roszyk L, Chalus E, Guerin R, Petit A, et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med. 2011;39:480–488. doi: 10.1097/CCM.0b013e318206b3ca. [DOI] [PubMed] [Google Scholar]
- 120.de Bont ES, Vellenga E, Swaanenburg JC, Fidler V, Visser-van Brummen PJ, Kamps WA. Plasma IL-8 and IL-6 levels can be used to define a group with low risk of septicaemia among cancer patients with fever and neutropenia. Br J Haematol. 1999;107:375–380. doi: 10.1046/j.1365-2141.1999.01707.x. [DOI] [PubMed] [Google Scholar]
- 121.Diepold M, Noellke P, Duffner U, Kontny U, Berner R. Performance of interleukin-6 and interleukin-8 serum levels in pediatric oncology patients with neutropenia and fever for the assessment of low-risk. BMC Infect Dis. 2008;8:28. doi: 10.1186/1471-2334-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spasova MI, Terzieva DD, Tzvetkova TZ, Stoyanova AA, Mumdzhiev IN, Yanev IB, et al. Interleukin-6, interleukin-8, interleukin-10, and C-reactive protein in febrile neutropenia in children with malignant diseases. Folia Med (Plovdiv ) 2005;47:46–52. [PubMed] [Google Scholar]
- 123.Aquino VM, Cost C, Gomez A, Bowers DC, Ramilo O, Ahmad N, et al. Predictive value of interleukin-5 and monocyte chemotactic protein-1 for bacteremia in children with febrile neutropenia. J Pediatr Hematol Oncol. 2012;34:e241–e245. doi: 10.1097/MPH.0b013e31824e498d. [DOI] [PubMed] [Google Scholar]
- 124.Magudumana MO, Ballot DE, Cooper PA, Trusler J, Cory BJ, Viljoen E, et al. Serial interleukin 6 measurements in the early diagnosis of neonatal sepsis. J Trop Pediatr. 2000;46:267–271. doi: 10.1093/tropej/46.5.267. [DOI] [PubMed] [Google Scholar]
- 125.Kuster H, Weiss M, Willeitner AE, Detlefsen S, Jeremias I, Zbojan J, et al. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet. 1998;352:1271–1277. doi: 10.1016/S0140-6736(98)08148-3. [DOI] [PubMed] [Google Scholar]
- 126.Vassiliou AG, Kotanidou A, Mastora Z, Maniatis NA, Albani P, Jahaj E, et al. Elevated soluble endothelial protein C receptor levels at ICU admission are associated with sepsis development. Minerva Anestesiol. 2015;81:125–134. [PubMed] [Google Scholar]
- 127.Su CM, Cheng HH, Tsai TC, Hsiao SY, Tsai NW, Chang WN, et al. Elevated serum vascular cell adhesion molecule-1 is associated with septic encephalopathy in adult community-onset severe sepsis patients. Biomed Res Int. 2014;2014:598762. doi: 10.1155/2014/598762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anderson BJ, Reilly JP, Shashaty MGS, Palakshappa JA, Wysoczanski A, Dunn TG, et al. Admission plasma levels of the neuronal injury marker neuron-specific enolase are associated with mortality and delirium in sepsis. J Crit Care. 2016;36:18–23. doi: 10.1016/j.jcrc.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mosad E, Elsayh KI, Eltayeb AA. Tissue factor pathway inhibitor and P-selectin as markers of sepsis-induced non-overt disseminated intravascular coagulopathy. Clin Appl Thromb Hemost. 2011;17:80–87. doi: 10.1177/1076029609344981. [DOI] [PubMed] [Google Scholar]
- 130.Koyama K, Madoiwa S, Nunomiya S, Koinuma T, Wada M, Sakata A, et al. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit Care. 2014;18:R13. doi: 10.1186/cc13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Delabranche X, Boisrame-Helms J, Asfar P, Berger A, Mootien Y, Lavigne T, et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med. 2013;39:1695–1703. doi: 10.1007/s00134-013-2993-x. [DOI] [PubMed] [Google Scholar]
- 132.Sivula M, Hastbacka J, Kuitunen A, Lassila R, Tervahartiala T, Sorsa T, et al. Systemic matrix metalloproteinase-8 and tissue inhibitor of metalloproteinases-1 levels in severe sepsis-associated coagulopathy. Acta Anaesthesiol Scand. 2015;59:176–184. doi: 10.1111/aas.12423. [DOI] [PubMed] [Google Scholar]
- 133.Psuja P, Zozulinska M, Turowiecka Z, Cieslikowski W, Vinazzer H, Zawilska K. Plasma markers of hypercoagulability in patients with serious infections and risk of septic shock. Clin Appl Thromb Hemost. 2002;8:225–230. doi: 10.1177/107602960200800305. [DOI] [PubMed] [Google Scholar]
- 134.Koyama K, Madoiwa S, Tanaka S, Koinuma T, Wada M, Sakata A, et al. Evaluation of hemostatic biomarker abnormalities that precede platelet count decline in critically ill patients with sepsis. J Crit Care. 2013;28:556–563. doi: 10.1016/j.jcrc.2012.10.069. [DOI] [PubMed] [Google Scholar]
- 135.Vetter TR, Schober P, Mascha EJ. Diagnostic testing and decision-making: beauty is not just in the eye of the beholder. Anesth Analg. 2018;127:1085–1091. doi: 10.1213/ANE.0000000000003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pletcher MJ, Pignone M. Evaluating the clinical utility of a biomarker: a review of methods for estimating health impact. Circulation. 2011;123:1116–1124. doi: 10.1161/CIRCULATIONAHA.110.943860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schultz M, Rasmussen LJH, Andersen MH, Stefansson JS, Falkentoft AC, Alstrup M, et al. Use of the prognostic biomarker suPAR in the emergency department improves risk stratification but has no effect on mortality: a cluster-randomized clinical trial (TRIAGE III) Scand J Trauma Resusc Emerg Med. 2018;26:69. doi: 10.1186/s13049-018-0539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marshall JC, Reinhart K. Biomarkers of sepsis. Crit Care Med. 2009;37:2290–2298. doi: 10.1097/CCM.0b013e3181a02afc. [DOI] [PubMed] [Google Scholar]
- 139.Simon L, Saint-Louis P, Amre DK, Lacroix J, Gauvin F. Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr Crit Care Med. 2008;9:407–413. doi: 10.1097/PCC.0b013e31817285a6. [DOI] [PubMed] [Google Scholar]
- 140.Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003;31:1737–1741. doi: 10.1097/01.CCM.0000063440.19188.ED. [DOI] [PubMed] [Google Scholar]
- 141.Park JH, Kim DH, Jang HR, Kim MJ, Jung SH, Lee JE, et al. Clinical relevance of procalcitonin and C-reactive protein as infection markers in renal impairment: a cross-sectional study. Crit Care. 2014;18:640. doi: 10.1186/s13054-014-0640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hattori T, Nishiyama H, Kato H, Ikegami S, Nagayama M, Asami S, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol. 2014;141:43–51. doi: 10.1309/AJCP4GV7ZFDTANGC. [DOI] [PubMed] [Google Scholar]
- 143.Kono T, Otsuka M, Ito M, Misawa M, Hoshioka A, Suzuki M, et al. Negative C-reactive protein in children with bacterial infection. Pediatr Int. 1999;41:496–499. doi: 10.1046/j.1442-200x.1999.01126.x. [DOI] [PubMed] [Google Scholar]
- 144.Chan YL, Liao HC, Tsay PK, Chang SS, Chen JC, Liaw SJ. C-reactive protein as an indicator of bacterial infection of adult patients in the emergency department. Chang Gung Med J. 2002;25:437–445. [PubMed] [Google Scholar]
- 145.Han JH, Nachamkin I, Coffin SE, Gerber JS, Fuchs B, Garrigan C, et al. Use of a combination biomarker algorithm to identify medical intensive care unit patients with suspected sepsis at very low likelihood of bacterial infection. Antimicrob Agents Chemother. 2015;59:6494–6500. doi: 10.1128/AAC.00958-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Povoa P, Teixeira-Pinto AM, Carneiro AH. C-reactive protein, an early marker of community-acquired sepsis resolution: a multi-center prospective observational study. Crit Care. 2011;15:R169. doi: 10.1186/cc10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van der Does Y, Limper M, Jie KE, Schuit SCE, Jansen H, Pernot N, et al. Procalcitonin-guided antibiotic therapy in patients with fever in a general emergency department population: a multicentre non-inferiority randomized clinical trial (HiTEMP study) Clin Microbiol Infect. 2018;24:1282–1289. doi: 10.1016/j.cmi.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 148.Wirz Y, Meier MA, Bouadma L, Luyt CE, Wolff M, Chastre J, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care. 2018;22:191. doi: 10.1186/s13054-018-2125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang DT, Yealy DM, Filbin MR, Brown AM, Chang CH, Doi Y, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379:236–249. doi: 10.1056/NEJMoa1802670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One. 2015;10:e0129450. doi: 10.1371/journal.pone.0129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Grover V, Pantelidis P, Soni N, Takata M, Shah PL, Wells AU, et al. A biomarker panel (Bioscore) incorporating monocytic surface and soluble TREM-1 has high discriminative value for ventilator-associated pneumonia: a prospective observational study. PLoS One. 2014;9:e109686. doi: 10.1371/journal.pone.0109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim H, Hur M, Moon HW, Yun YM, Di Somma S. Multi-marker approach using procalcitonin, presepsin, galectin-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann Intensive Care. 2017;7:27. doi: 10.1186/s13613-017-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mearelli F, Fiotti N, Giansante C, Casarsa C, Orso D, De Helmersen M, et al. Derivation and validation of a biomarker-based clinical algorithm to rule out sepsis from noninfectious systemic inflammatory response syndrome at emergency department admission: a multicenter prospective study. Crit Care Med. 2018;46:1421–1429. doi: 10.1097/CCM.0000000000003206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Biomarkers of sepsis: Time for a reappraisal Pierrakos et al. Table S1. Cytokine/chemokine biomarkers identified in the literature search. Table S2. Receptor biomarkers identified in the literature search. Table S3. Cell marker biomarkers identified in the literature search. Table S4. Coagulation-related biomarkers identified in the literature search. Table S5. Microcirculation related biomarkers identified in the literature search. Table S6. Vasodilation-related biomarkers identified in the literature search. Table S7. Biomarkers of organ dysfunction in sepsis identified in the literature search. Table S8. Acute phase proteins used as biomarkers in sepsis identified in the literature search. Table S9. Diverse sepsis biomarkers identified in the literature search. Table S10. QUADAS-2 score [1145] for quality assessment for the studies that included >300 patients where ROC curve analysis was used.
Data Availability Statement
Not applicable.